 Found 3414 hits with Last Name = 'ha' and Initial = 'sb'
Found 3414 hits with Last Name = 'ha' and Initial = 'sb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B2 bradykinin receptor
(RAT) | BDBM50370083
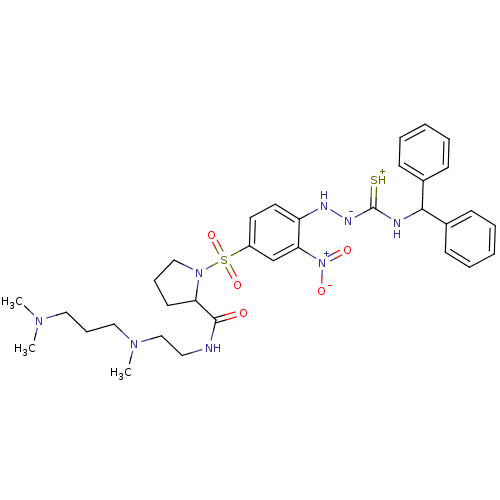
(CHEMBL1907651)Show SMILES CN(C)CCCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C33H44N8O5S2/c1-38(2)20-11-21-39(3)23-19-34-32(42)29-16-10-22-40(29)48(45,46)27-17-18-28(30(24-27)41(43)44)36-37-33(47)35-31(25-12-6-4-7-13-25)26-14-8-5-9-15-26/h4-9,12-15,17-18,24,29,31,36H,10-11,16,19-23H2,1-3H3,(H3,34,35,37,42,47) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131
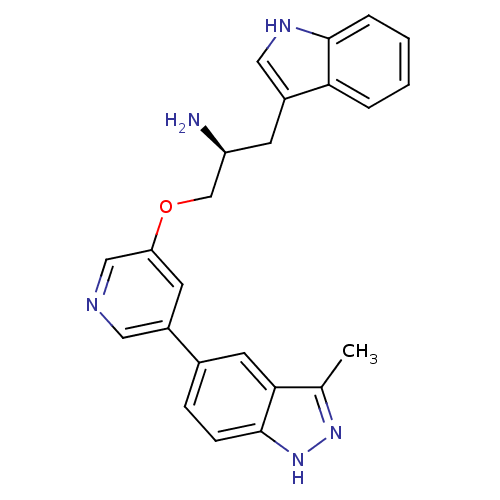
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01467
BindingDB Entry DOI: 10.7270/Q24Q801K |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370077
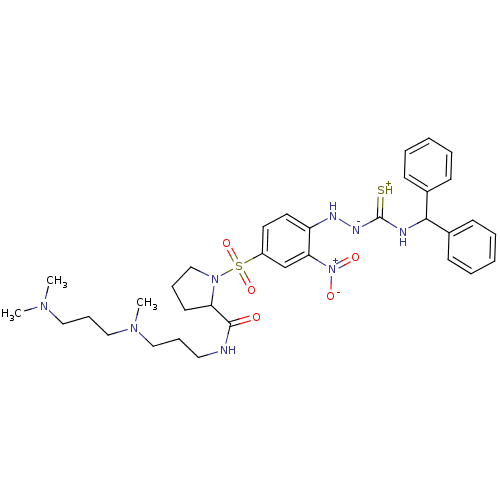
(CHEMBL1907652)Show SMILES CN(C)CCCN(C)CCCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H46N8O5S2/c1-39(2)21-12-23-40(3)22-11-20-35-33(43)30-17-10-24-41(30)49(46,47)28-18-19-29(31(25-28)42(44)45)37-38-34(48)36-32(26-13-6-4-7-14-26)27-15-8-5-9-16-27/h4-9,13-16,18-19,25,30,32,37H,10-12,17,20-24H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50113263
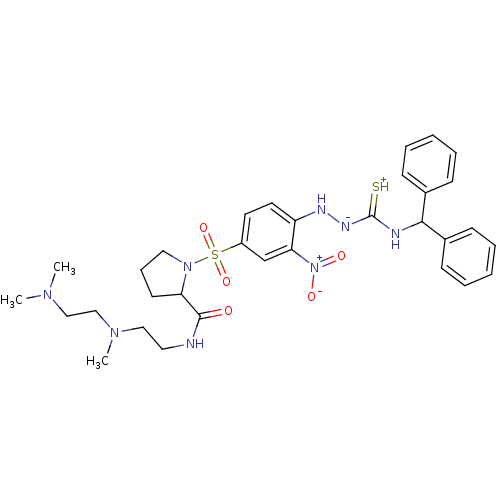
((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H3,33,34,36,41,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50113263

((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H3,33,34,36,41,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50409527
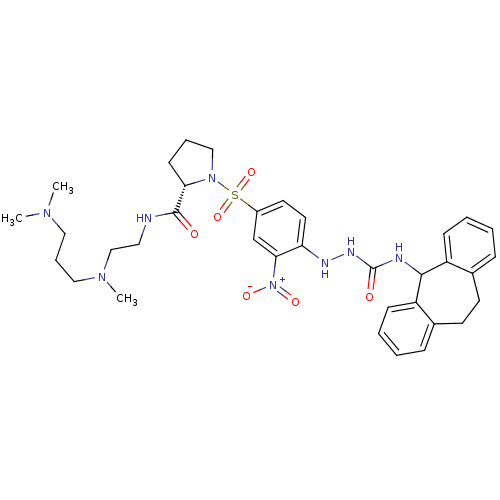
(CHEMBL2112283)Show SMILES CN(C)CCCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C35H46N8O6S/c1-40(2)20-9-21-41(3)23-19-36-34(44)31-14-8-22-42(31)50(48,49)27-17-18-30(32(24-27)43(46)47)38-39-35(45)37-33-28-12-6-4-10-25(28)15-16-26-11-5-7-13-29(26)33/h4-7,10-13,17-18,24,31,33,38H,8-9,14-16,19-23H2,1-3H3,(H,36,44)(H2,37,39,45)/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50281137
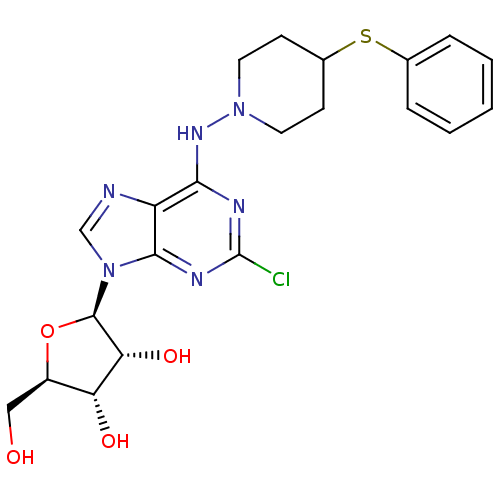
((2R,3R,4S,5R)-2-[2-Chloro-6-(4-phenylsulfanyl-pipe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCC(CC3)Sc3ccccc3)nc(Cl)nc12 Show InChI InChI=1S/C21H25ClN6O4S/c22-21-24-18(26-27-8-6-13(7-9-27)33-12-4-2-1-3-5-12)15-19(25-21)28(11-23-15)20-17(31)16(30)14(10-29)32-20/h1-5,11,13-14,16-17,20,29-31H,6-10H2,(H,24,25,26)/t14-,16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50409529
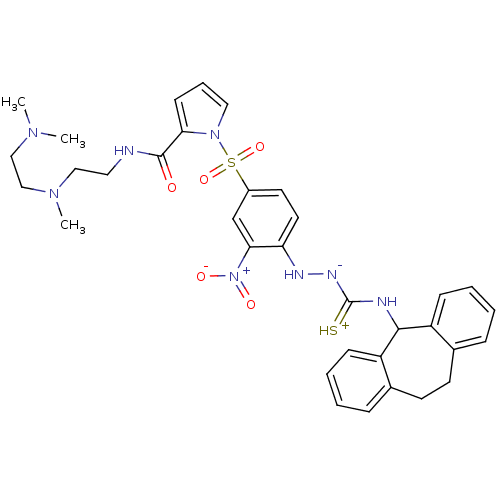
(CHEMBL2112221)Show SMILES CN(C)CCN(C)CCNC(=O)c1cccn1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H40N8O5S2/c1-39(2)21-22-40(3)20-18-35-33(43)30-13-8-19-41(30)49(46,47)26-16-17-29(31(23-26)42(44)45)37-38-34(48)36-32-27-11-6-4-9-24(27)14-15-25-10-5-7-12-28(25)32/h4-13,16-17,19,23,32,37H,14-15,18,20-22H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
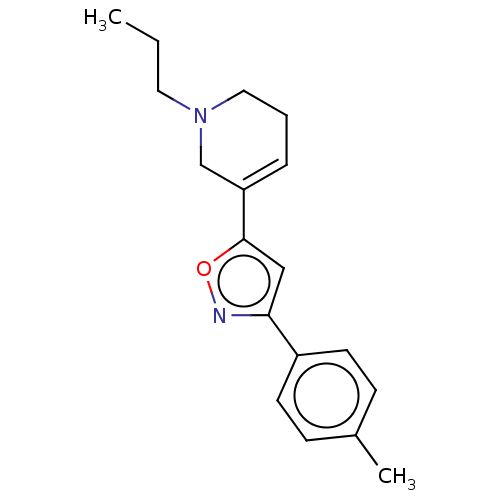
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366484

(CHEMBL610971)Show SMILES OC[C@H](Cc1ccccc1)Nc1nc(Cl)nc2n(cnc12)C1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H22ClN5O5/c20-19-23-16(22-11(7-26)6-10-4-2-1-3-5-10)13-17(24-19)25(9-21-13)18-15(29)14(28)12(8-27)30-18/h1-5,9,11-12,14-15,18,26-29H,6-8H2,(H,22,23,24)/t11-,12+,14+,15+,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50290900
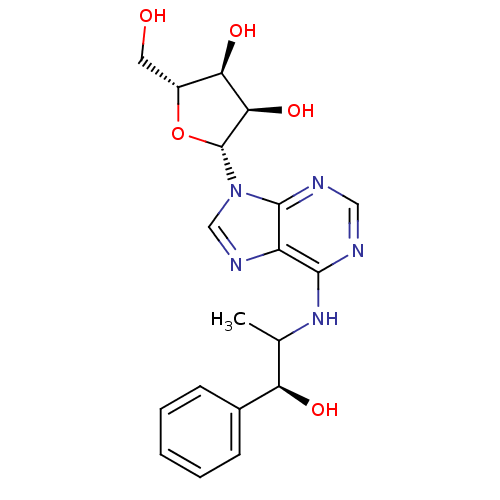
((2R,3S,4R,5R)-2-Hydroxymethyl-5-[6-((S)-2-hydroxy-...)Show SMILES CC(Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)[C@@H](O)c1ccccc1 Show InChI InChI=1S/C19H23N5O5/c1-10(14(26)11-5-3-2-4-6-11)23-17-13-18(21-8-20-17)24(9-22-13)19-16(28)15(27)12(7-25)29-19/h2-6,8-10,12,14-16,19,25-28H,7H2,1H3,(H,20,21,23)/t10?,12-,14-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50409528
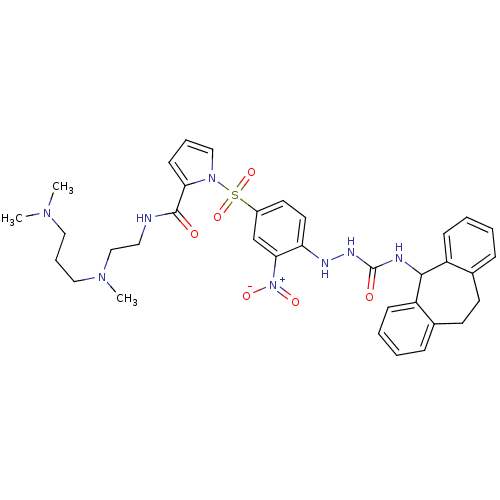
(CHEMBL2112220)Show SMILES CN(C)CCCN(C)CCNC(=O)c1cccn1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C35H42N8O6S/c1-40(2)20-9-21-41(3)23-19-36-34(44)31-14-8-22-42(31)50(48,49)27-17-18-30(32(24-27)43(46)47)38-39-35(45)37-33-28-12-6-4-10-25(28)15-16-26-11-5-7-13-29(26)33/h4-8,10-14,17-18,22,24,33,38H,9,15-16,19-21,23H2,1-3H3,(H,36,44)(H2,37,39,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50080400
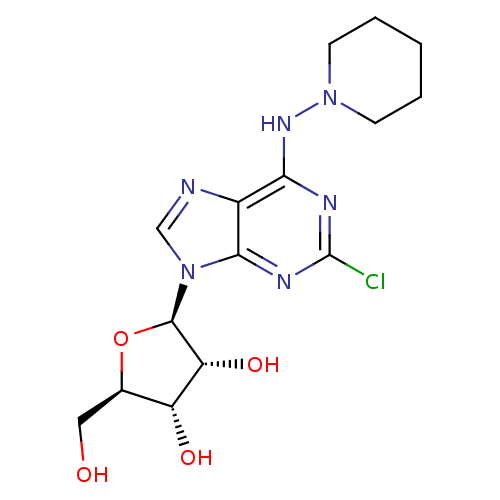
((2R,3R,4S,5R)-2-[2-Chloro-6-(piperidin-1-ylamino)-...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCCCC3)nc(Cl)nc12 Show InChI InChI=1S/C15H21ClN6O4/c16-15-18-12(20-21-4-2-1-3-5-21)9-13(19-15)22(7-17-9)14-11(25)10(24)8(6-23)26-14/h7-8,10-11,14,23-25H,1-6H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50150078
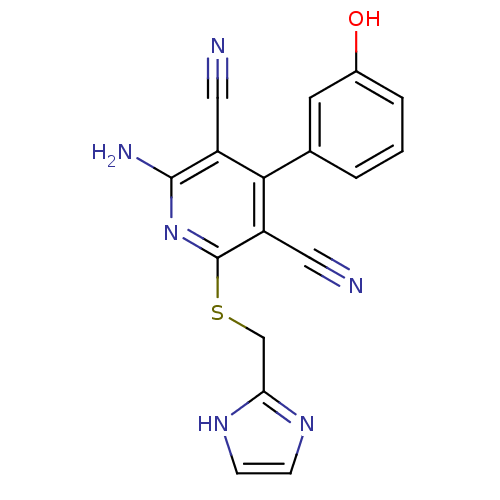
(2-Amino-4-(3-hydroxy-phenyl)-6-(1H-imidazol-2-ylme...)Show SMILES Nc1nc(SCc2ncc[nH]2)c(C#N)c(-c2cccc(O)c2)c1C#N Show InChI InChI=1S/C17H12N6OS/c18-7-12-15(10-2-1-3-11(24)6-10)13(8-19)17(23-16(12)20)25-9-14-21-4-5-22-14/h1-6,24H,9H2,(H2,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from recombinant human adenosine A1 receptor expressed in CHO cell membrane by by scintillation counting method |
J Med Chem 59: 5922-8 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00552
BindingDB Entry DOI: 10.7270/Q2P84DV8 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370080
(CHEMBL1907656)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2Cc3ccccc3Cc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H44N8O5S2/c1-39(2)19-20-40(3)18-16-35-33(43)31-13-8-17-41(31)49(46,47)27-14-15-29(32(23-27)42(44)45)37-38-34(48)36-30-22-25-10-5-4-9-24(25)21-26-11-6-7-12-28(26)30/h4-7,9-12,14-15,23,30-31,37H,8,13,16-22H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50281138
((2R,3R,4S,5R)-2-[2-Chloro-6-(morpholin-4-ylamino)-...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCOCC3)nc(Cl)nc12 Show InChI InChI=1S/C14H19ClN6O5/c15-14-17-11(19-20-1-3-25-4-2-20)8-12(18-14)21(6-16-8)13-10(24)9(23)7(5-22)26-13/h6-7,9-10,13,22-24H,1-5H2,(H,17,18,19)/t7-,9-,10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370081
(CHEMBL1907654)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2Cl)c2ccccc2Cl)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H40Cl2N8O5S2/c1-39(2)19-20-40(3)18-16-35-31(43)28-13-8-17-41(28)49(46,47)22-14-15-27(29(21-22)42(44)45)37-38-32(48)36-30(23-9-4-6-11-25(23)33)24-10-5-7-12-26(24)34/h4-7,9-12,14-15,21,28,30,37H,8,13,16-20H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50569815
(CHEMBL4873623)Show SMILES CCC(Oc1cccc(Cl)c1Cl)c1nc(N)nc(n1)N1CCN(C)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50569820
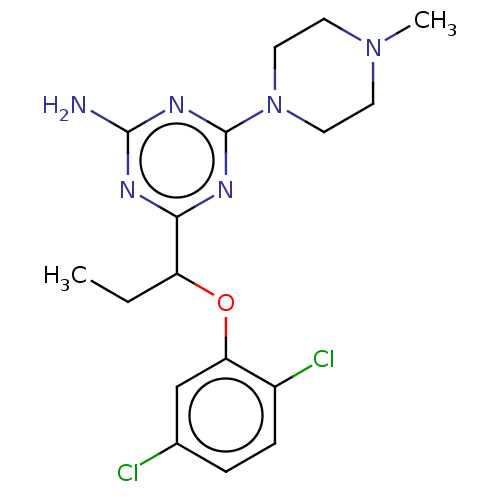
(CHEMBL4860275)Show SMILES CCC(Oc1cc(Cl)ccc1Cl)c1nc(N)nc(n1)N1CCN(C)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370078
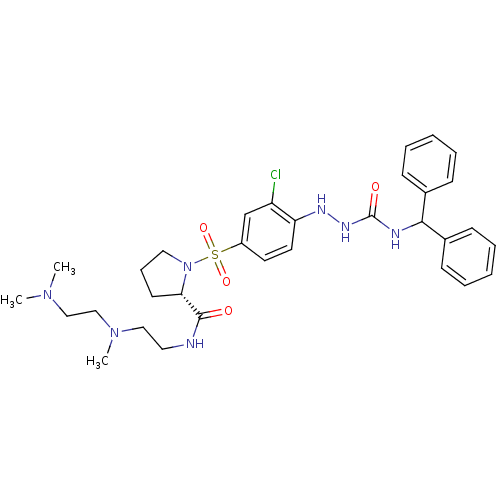
(CHEMBL1907653)Show SMILES CN(C)CCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC(c2ccccc2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C32H42ClN7O4S/c1-38(2)21-22-39(3)20-18-34-31(41)29-15-10-19-40(29)45(43,44)26-16-17-28(27(33)23-26)36-37-32(42)35-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,29-30,36H,10,15,18-22H2,1-3H3,(H,34,41)(H2,35,37,42)/t29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50281135
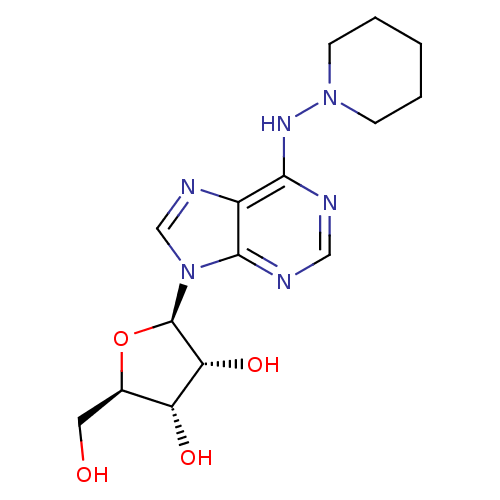
((2R,3S,4R,5R)-2-Hydroxymethyl-5-[6-(piperidin-1-yl...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCCCC3)ncnc12 Show InChI InChI=1S/C15H22N6O4/c22-6-9-11(23)12(24)15(25-9)21-8-18-10-13(16-7-17-14(10)21)19-20-4-2-1-3-5-20/h7-9,11-12,15,22-24H,1-6H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50281138

((2R,3R,4S,5R)-2-[2-Chloro-6-(morpholin-4-ylamino)-...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCOCC3)nc(Cl)nc12 Show InChI InChI=1S/C14H19ClN6O5/c15-14-17-11(19-20-1-3-25-4-2-20)8-12(18-14)21(6-16-8)13-10(24)9(23)7(5-22)26-13/h6-7,9-10,13,22-24H,1-5H2,(H,17,18,19)/t7-,9-,10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Adenosine A1 receptor |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50290902
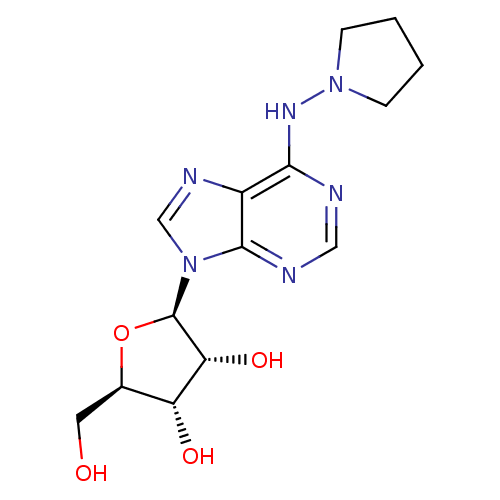
((2R,3S,4R,5R)-2-Hydroxymethyl-5-[6-(pyrrolidin-1-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCCC3)ncnc12 Show InChI InChI=1S/C14H20N6O4/c21-5-8-10(22)11(23)14(24-8)20-7-17-9-12(15-6-16-13(9)20)18-19-3-1-2-4-19/h6-8,10-11,14,21-23H,1-5H2,(H,15,16,18)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50290902

((2R,3S,4R,5R)-2-Hydroxymethyl-5-[6-(pyrrolidin-1-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NN3CCCC3)ncnc12 Show InChI InChI=1S/C14H20N6O4/c21-5-8-10(22)11(23)14(24-8)20-7-17-9-12(15-6-16-13(9)20)18-19-3-1-2-4-19/h6-8,10-11,14,21-23H,1-5H2,(H,15,16,18)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409527

(CHEMBL2112283)Show SMILES CN(C)CCCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C35H46N8O6S/c1-40(2)20-9-21-41(3)23-19-36-34(44)31-14-8-22-42(31)50(48,49)27-17-18-30(32(24-27)43(46)47)38-39-35(45)37-33-28-12-6-4-10-25(28)15-16-26-11-5-7-13-29(26)33/h4-7,10-13,17-18,24,31,33,38H,8-9,14-16,19-23H2,1-3H3,(H,36,44)(H2,37,39,45)/t31-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50606915
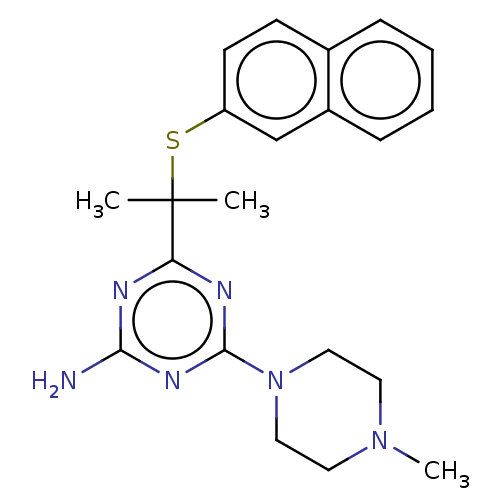
(CHEMBL5219240)Show SMILES CN1CCN(CC1)c1nc(N)nc(n1)C(C)(C)Sc1ccc2ccccc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50516529

(CHEMBL4520796)Show InChI InChI=1S/C19H28N6O/c1-13(2)15-6-5-14(3)11-16(15)26-12-17-21-18(20)23-19(22-17)25-9-7-24(4)8-10-25/h5-6,11,13H,7-10,12H2,1-4H3,(H2,20,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370080

(CHEMBL1907656)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2Cc3ccccc3Cc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H44N8O5S2/c1-39(2)19-20-40(3)18-16-35-33(43)31-13-8-17-41(31)49(46,47)27-14-15-29(32(23-27)42(44)45)37-38-34(48)36-30-22-25-10-5-4-9-24(25)21-26-11-6-7-12-28(26)30/h4-7,9-12,14-15,23,30-31,37H,8,13,16-22H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150078

(2-Amino-4-(3-hydroxy-phenyl)-6-(1H-imidazol-2-ylme...)Show SMILES Nc1nc(SCc2ncc[nH]2)c(C#N)c(-c2cccc(O)c2)c1C#N Show InChI InChI=1S/C17H12N6OS/c18-7-12-15(10-2-1-3-11(24)6-10)13(8-19)17(23-16(12)20)25-9-14-21-4-5-22-14/h1-6,24H,9H2,(H2,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from recombinant human adenosine A2A receptor expressed in HEK293 cell membrane by scintillation counting method |
J Med Chem 59: 5922-8 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00552
BindingDB Entry DOI: 10.7270/Q2P84DV8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50252344
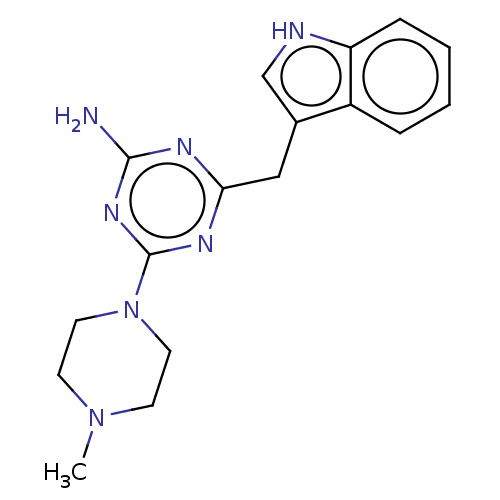
(CHEMBL4086515)Show InChI InChI=1S/C17H21N7/c1-23-6-8-24(9-7-23)17-21-15(20-16(18)22-17)10-12-11-19-14-5-3-2-4-13(12)14/h2-5,11,19H,6-10H2,1H3,(H2,18,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366489

(CHEMBL611882)Show SMILES OC[C@H](Cc1ccccc1)Nc1nc(I)nc2n(cnc12)C1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H22IN5O5/c20-19-23-16(22-11(7-26)6-10-4-2-1-3-5-10)13-17(24-19)25(9-21-13)18-15(29)14(28)12(8-27)30-18/h1-5,9,11-12,14-15,18,26-29H,6-8H2,(H,22,23,24)/t11-,12+,14+,15+,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50516522
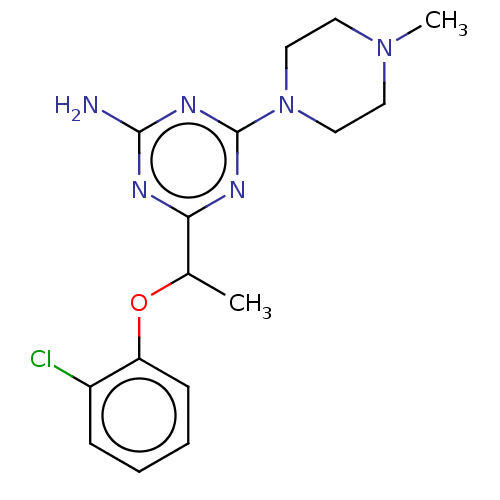
(CHEMBL4447416)Show InChI InChI=1S/C16H21ClN6O/c1-11(24-13-6-4-3-5-12(13)17)14-19-15(18)21-16(20-14)23-9-7-22(2)8-10-23/h3-6,11H,7-10H2,1-2H3,(H2,18,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370081

(CHEMBL1907654)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2Cl)c2ccccc2Cl)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H40Cl2N8O5S2/c1-39(2)19-20-40(3)18-16-35-31(43)28-13-8-17-41(28)49(46,47)22-14-15-27(29(21-22)42(44)45)37-38-32(48)36-30(23-9-4-6-11-25(23)33)24-10-5-7-12-26(24)34/h4-7,9-12,14-15,21,28,30,37H,8,13,16-20H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50606915

(CHEMBL5219240)Show SMILES CN1CCN(CC1)c1nc(N)nc(n1)C(C)(C)Sc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114645
BindingDB Entry DOI: 10.7270/Q2SQ94GB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M4 (CHRM4) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409529

(CHEMBL2112221)Show SMILES CN(C)CCN(C)CCNC(=O)c1cccn1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H40N8O5S2/c1-39(2)21-22-40(3)20-18-35-33(43)30-13-8-19-41(30)49(46,47)26-16-17-29(31(23-26)42(44)45)37-38-34(48)36-32-27-11-6-4-9-24(27)14-15-25-10-5-7-12-28(25)32/h4-13,16-17,19,23,32,37H,14-15,18,20-22H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366485
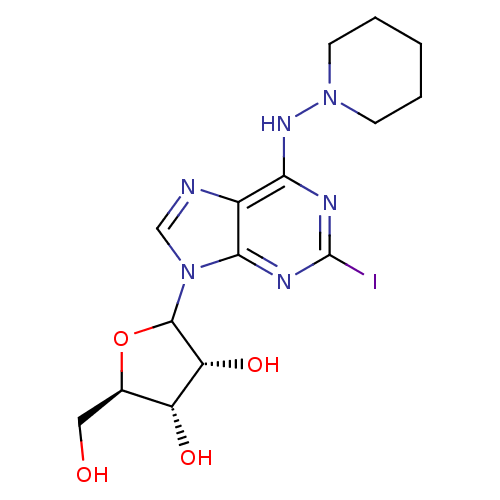
(CHEMBL610970)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NN3CCCCC3)nc(I)nc12 |r| Show InChI InChI=1S/C15H21IN6O4/c16-15-18-12(20-21-4-2-1-3-5-21)9-13(19-15)22(7-17-9)14-11(25)10(24)8(6-23)26-14/h7-8,10-11,14,23-25H,1-6H2,(H,18,19,20)/t8-,10-,11-,14?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H](R)-PIA displacement. |
Bioorg Med Chem Lett 7: 3085-3090 (1997)
Article DOI: 10.1016/S0960-894X(97)10177-9
BindingDB Entry DOI: 10.7270/Q2SQ90W2 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM22985
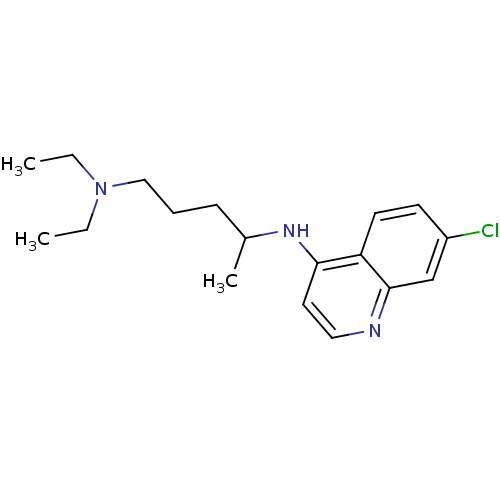
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370077

(CHEMBL1907652)Show SMILES CN(C)CCCN(C)CCCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H46N8O5S2/c1-39(2)21-12-23-40(3)22-11-20-35-33(43)30-17-10-24-41(30)49(46,47)28-18-19-29(31(25-28)42(44)45)37-38-34(48)36-32(26-13-6-4-7-14-26)27-15-8-5-9-16-27/h4-9,13-16,18-19,25,30,32,37H,10-12,17,20-24H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50189099
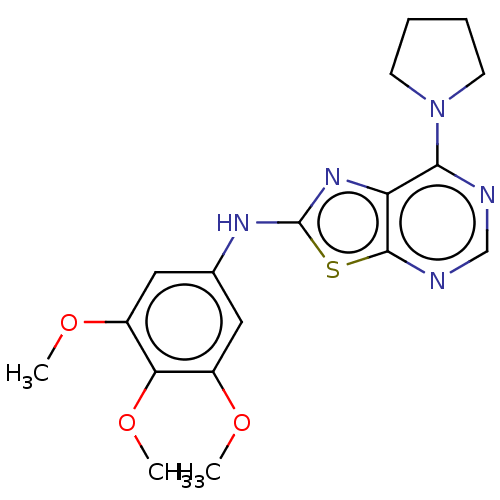
(CHEMBL3828479)Show InChI InChI=1S/C18H21N5O3S/c1-24-12-8-11(9-13(25-2)15(12)26-3)21-18-22-14-16(23-6-4-5-7-23)19-10-20-17(14)27-18/h8-10H,4-7H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from recombinant human adenosine A2A receptor expressed in CHO cell membrane after 3 hrs by microbeta scintillation counting... |
J Med Chem 59: 5922-8 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00552
BindingDB Entry DOI: 10.7270/Q2P84DV8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50150078

(2-Amino-4-(3-hydroxy-phenyl)-6-(1H-imidazol-2-ylme...)Show SMILES Nc1nc(SCc2ncc[nH]2)c(C#N)c(-c2cccc(O)c2)c1C#N Show InChI InChI=1S/C17H12N6OS/c18-7-12-15(10-2-1-3-11(24)6-10)13(8-19)17(23-16(12)20)25-9-14-21-4-5-22-14/h1-6,24H,9H2,(H2,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-ABMECA from recombinant human adenosine A3 receptor expressed in HEK293 cell membrane by scintillation counting method |
J Med Chem 59: 5922-8 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00552
BindingDB Entry DOI: 10.7270/Q2P84DV8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data