

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057000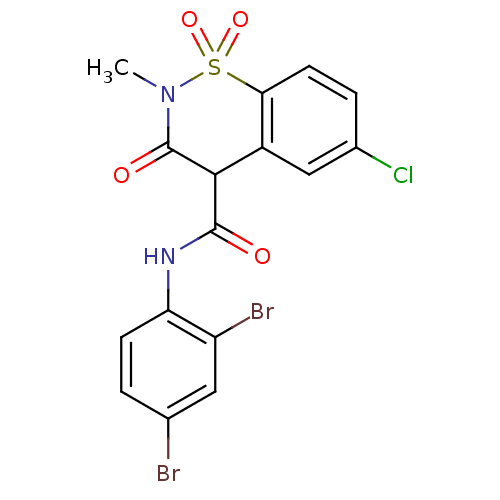 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057004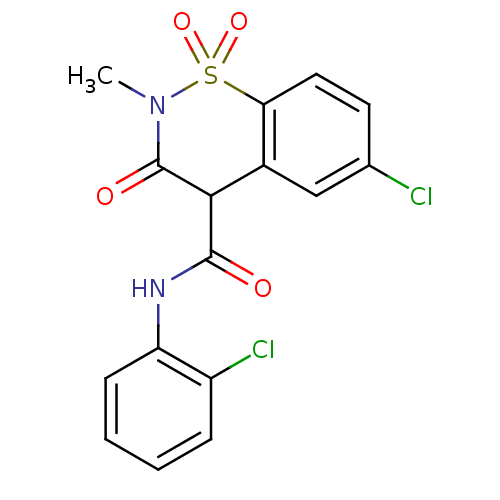 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056994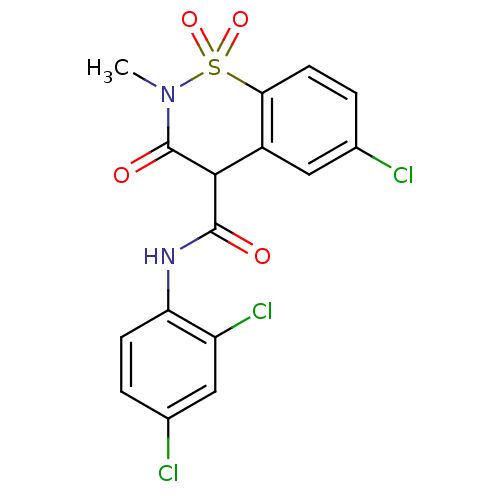 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050527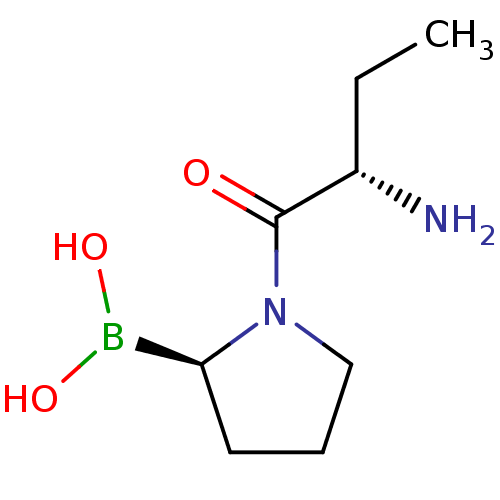 (Boronic acid derivative | CHEMBL66032 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050521 ((2-Dihydroxyborane-pyrrolidin-1-yl)-pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050517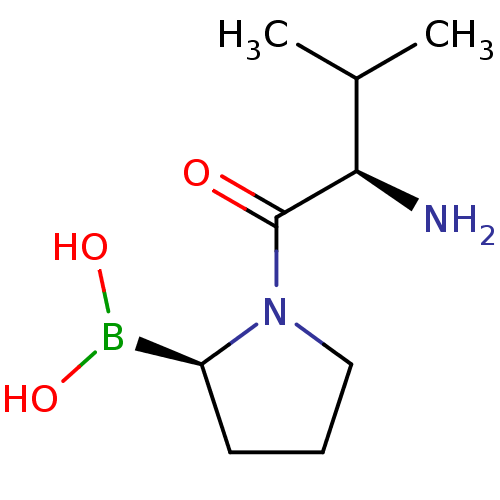 (Boronic acid derivative | CHEMBL305170 | N-alkyl G...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50369128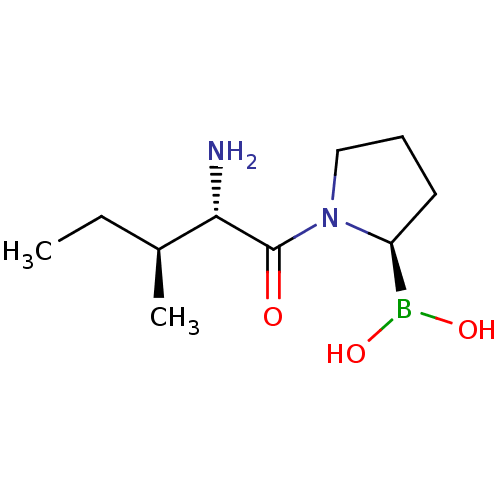 (CHEMBL1790483 | US11096924, DASH-inhibitors 4316 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050514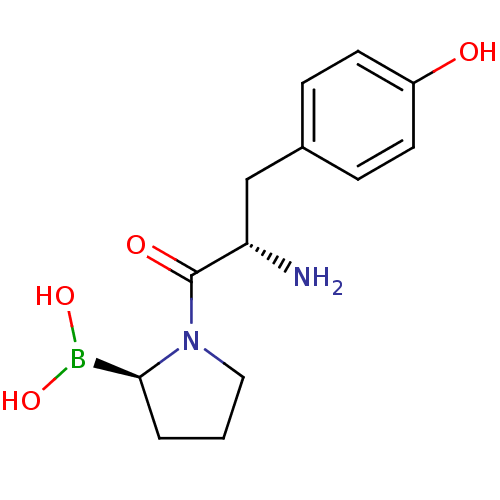 (Boronic acid derivative | CHEMBL304007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057004 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050528 (Boronic acid derivative | CHEMBL63698 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056997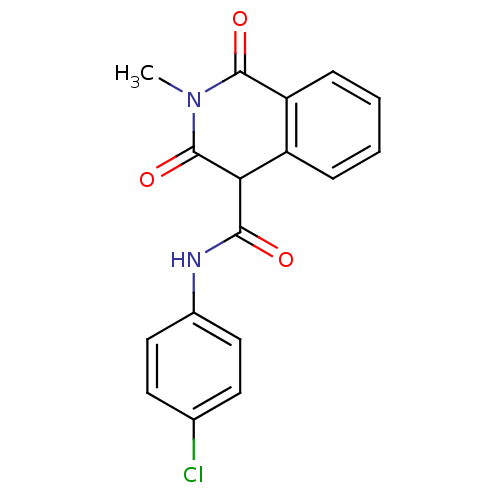 (3-Hydroxy-2-methyl-1-oxo-1,2-dihydro-isoquinoline-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057001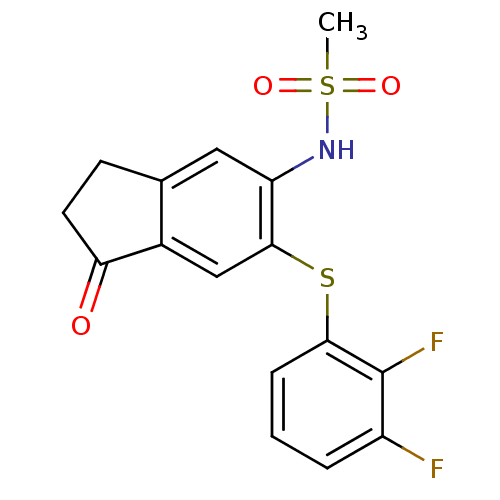 (CHEMBL361563 | N-[6-(2,3-Difluoro-phenylsulfanyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human Prostaglandin G/H synthase 2 at 10 ug/mL expressed as the mean percent inhibition of control PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050520 (Boronic acid derivative | CHEMBL63406 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050524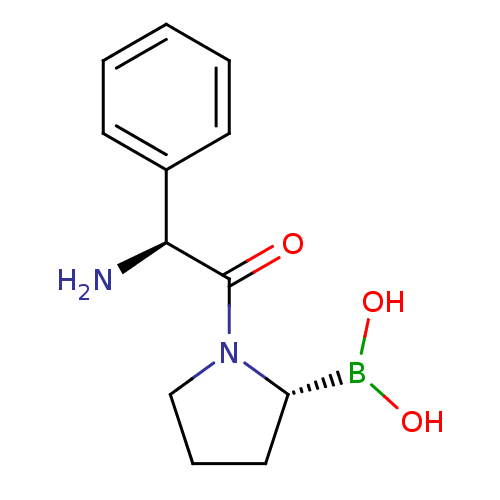 (Boronic acid derivative | CHEMBL291428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056995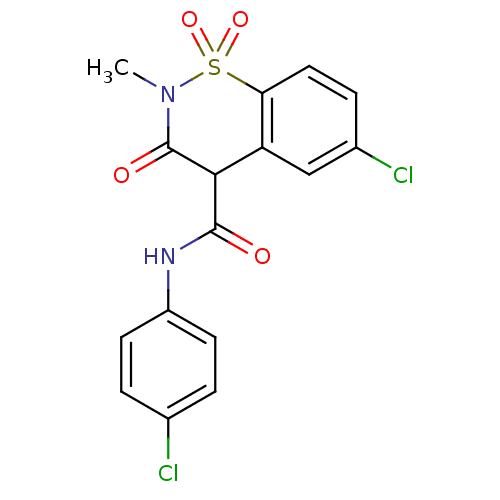 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050515 (Boronic acid derivative | CHEMBL63652 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056994 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029616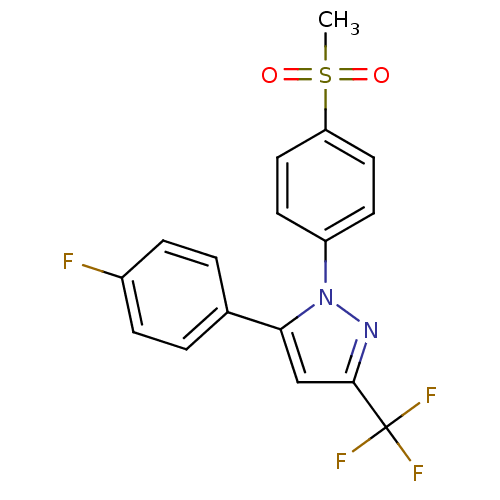 (5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human Prostaglandin G/H synthase 2 at 10 ug/mL expressed as the mean percent inhibition of control PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050516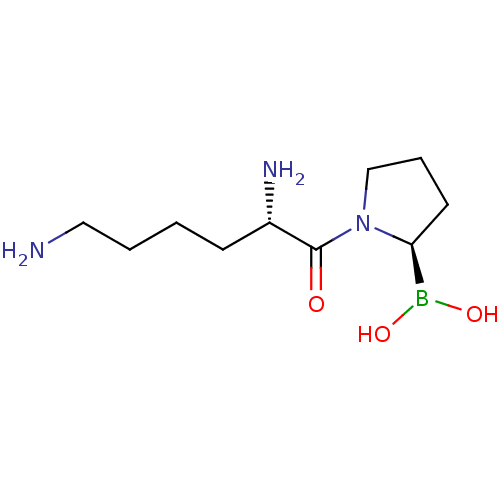 (Boronic acid derivative | CHEMBL63726 | US11096924...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056997 (3-Hydroxy-2-methyl-1-oxo-1,2-dihydro-isoquinoline-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057000 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057006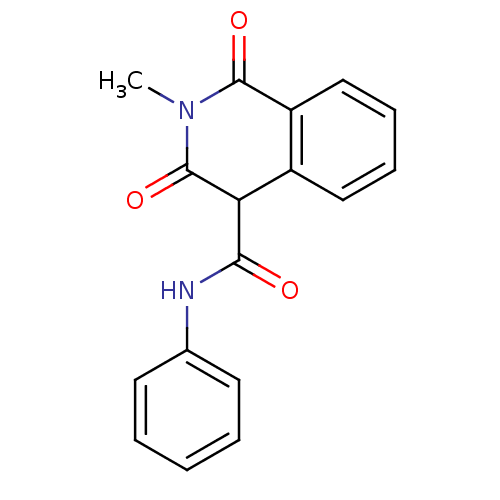 (3-Hydroxy-2-methyl-1-oxo-1,2-dihydro-isoquinoline-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50369129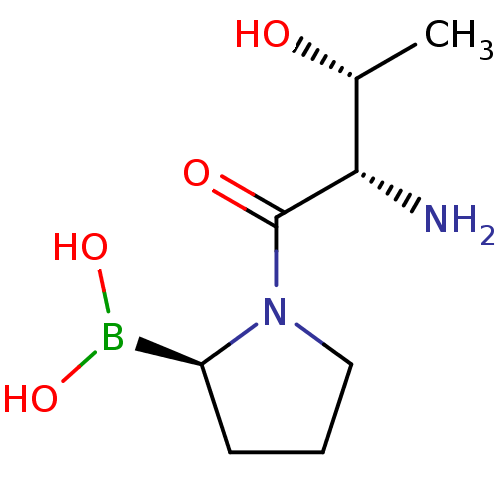 (CHEMBL1790478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057005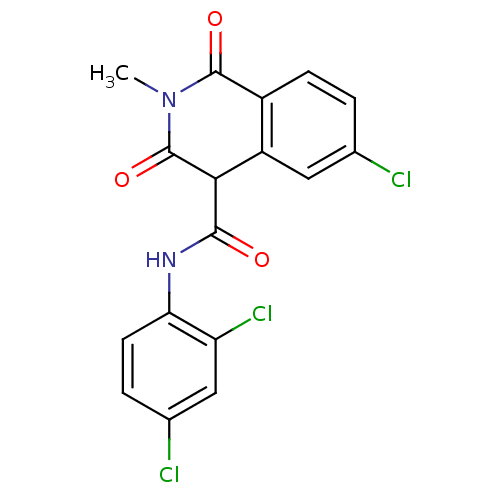 (6-Chloro-3-hydroxy-2-methyl-1-oxo-1,2-dihydro-isoq...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056996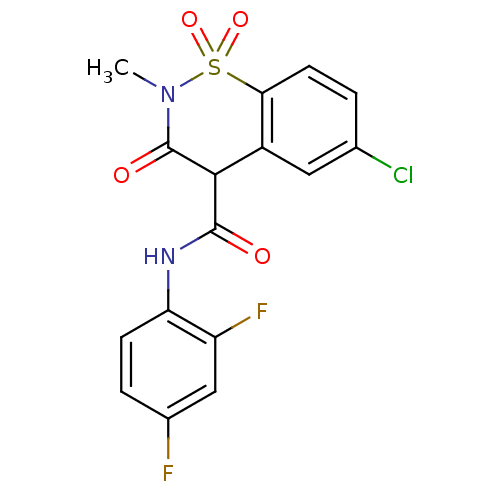 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050526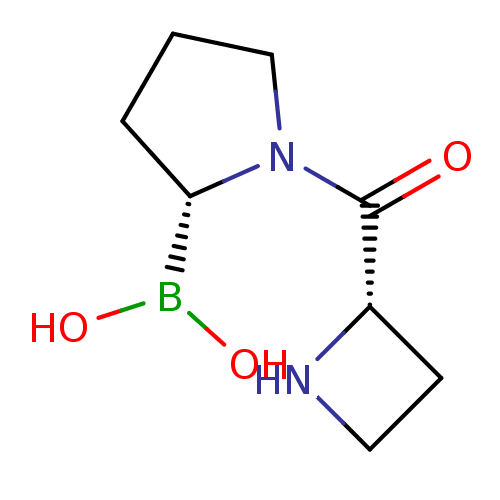 (Boronic acid derivative | CHEMBL292342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056996 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057006 (3-Hydroxy-2-methyl-1-oxo-1,2-dihydro-isoquinoline-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056995 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057002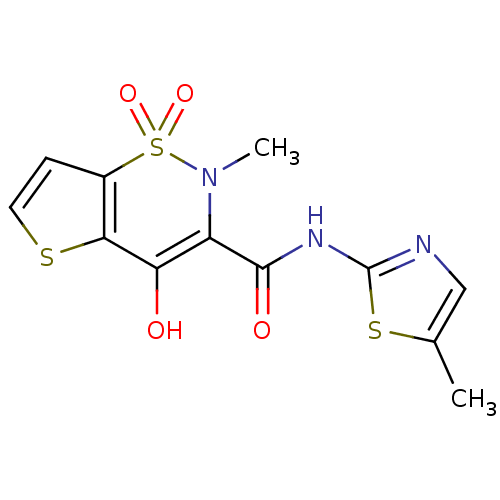 (4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production (... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057005 (6-Chloro-3-hydroxy-2-methyl-1-oxo-1,2-dihydro-isoq...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50369129 (CHEMBL1790478) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050521 ((2-Dihydroxyborane-pyrrolidin-1-yl)-pyrrolidin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057003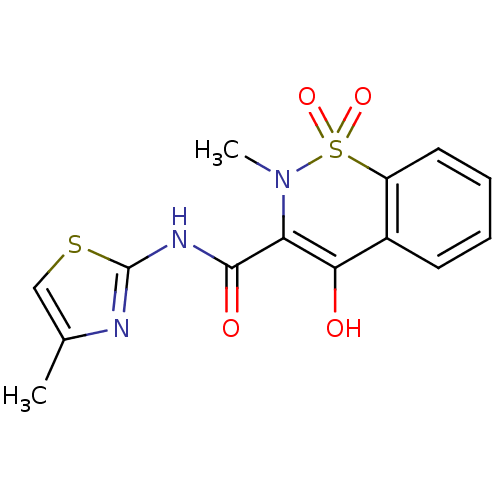 (4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Prolyl endopeptidase | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM85245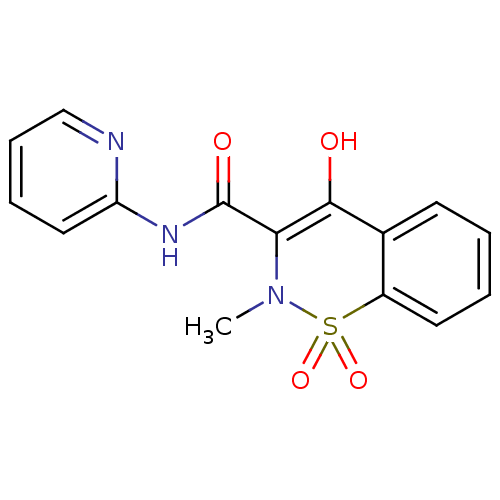 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE-2 production('... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050512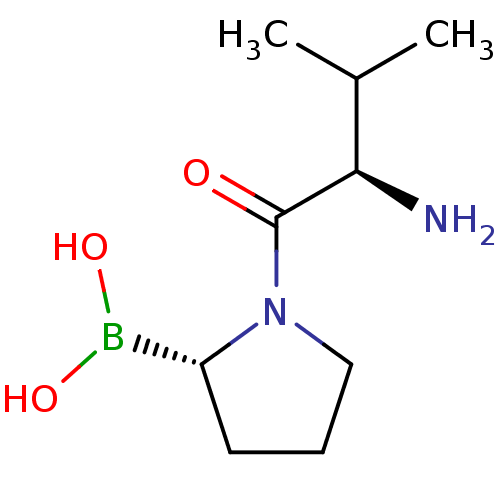 (Boronic acid derivative | CHEMBL65406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50369129 (CHEMBL1790478) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Prolyl endopeptidase | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057003 (4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056999 (CHEMBL56367 | nimesulide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human Prostaglandin G/H synthase 2 at 10 ug/mL expressed as the mean percent inhibition of control PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057001 (CHEMBL361563 | N-[6-(2,3-Difluoro-phenylsulfanyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human Prostaglandin G/H synthase 1 at 10 ug/mL expressed as the mean percent inhibition of control PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050511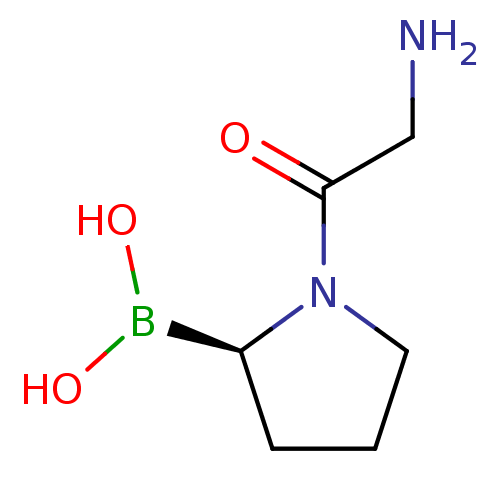 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050519 (Boronic acid derivative | CHEMBL303131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of Dipeptidylpeptidase IV. | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050512 (Boronic acid derivative | CHEMBL65406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of Dipeptidylpeptidase II | J Med Chem 39: 2087-94 (1996) Article DOI: 10.1021/jm950732f BindingDB Entry DOI: 10.7270/Q29Z95K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50057002 (4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 1 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |