

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for ability to compete with dansylamide for binding to human erythrocyte carbonic-anhydrase-II (HCA-II) | J Med Chem 37: 240-7 (1994) BindingDB Entry DOI: 10.7270/Q2N29XKD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012229 (5-Thiomorpholin-4-ylmethyl-thieno[2,3-b]thiophene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50017725 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for ability to compete with dansylamide for binding to human erythrocyte carbonic-anhydrase-II (HCA-II) | J Med Chem 37: 240-7 (1994) BindingDB Entry DOI: 10.7270/Q2N29XKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029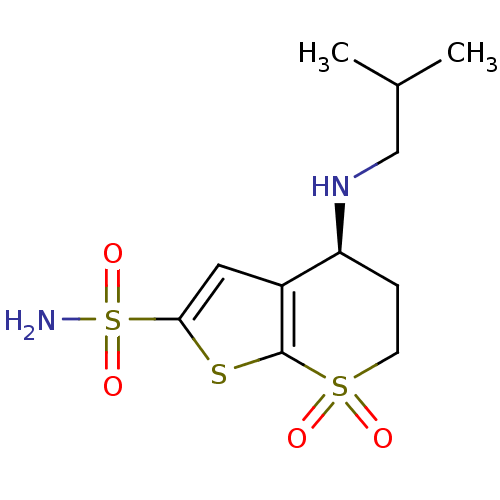 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for ability to compete with dansylamide for binding to human erythrocyte carbonic-anhydrase-II (HCA-II) | J Med Chem 37: 240-7 (1994) BindingDB Entry DOI: 10.7270/Q2N29XKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124914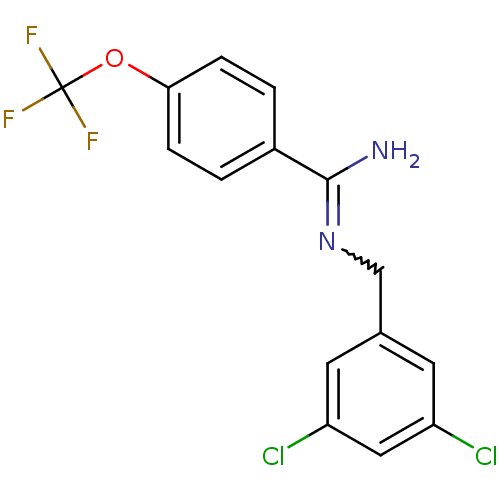 (CHEMBL161180 | N-(3,5-Dichloro-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077035 (1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124885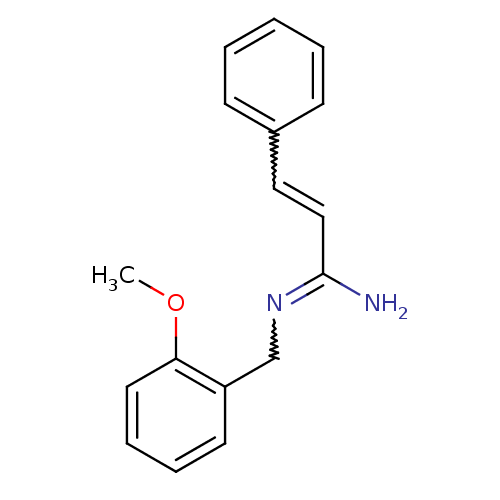 ((E)-N-(2-Methoxy-benzyl)-3-phenyl-acrylamidine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077032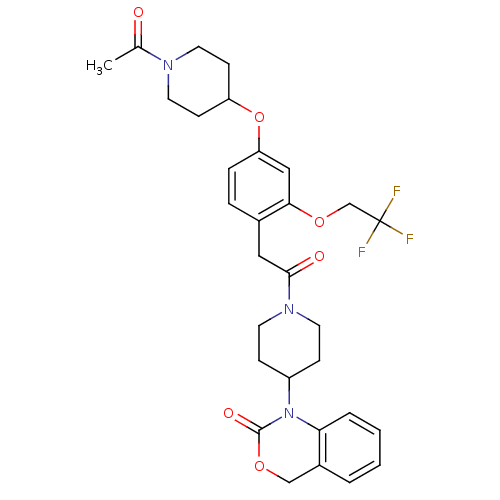 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064709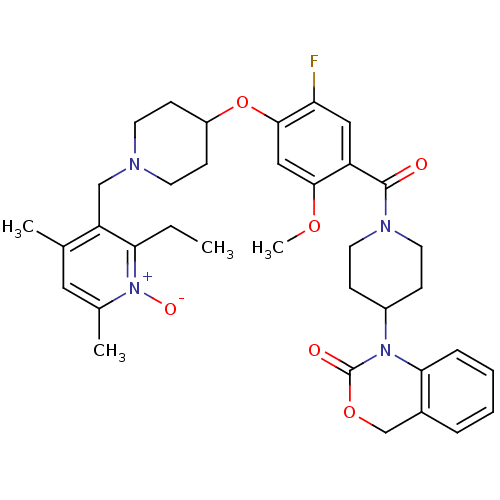 (1-(1-{4-[1-(2-Ethyl-4,6-dimethyl-1-oxy-pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064738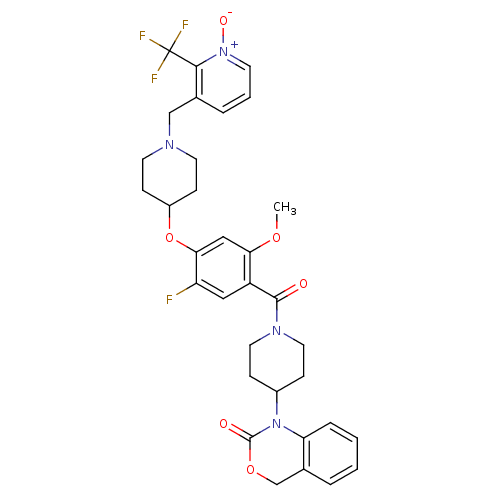 (1-(1-{5-Fluoro-2-methoxy-4-[1-(1-oxy-2-trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077035 (1-(1-{2-[4-Morpholin-4-yl-2-(2,2,2-trifluoro-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012211 (5-{[Bis-(2-methoxy-ethyl)-amino]-methyl}-thieno[3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50043900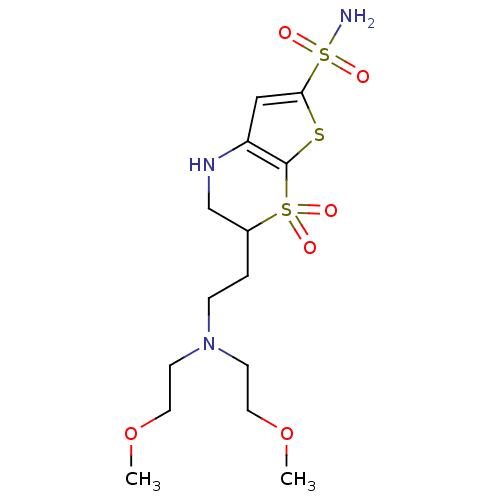 (3-{2-[Bis-(2-methoxy-ethyl)-amino]-ethyl}-4,4-diox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for ability to compete with dansylamide for binding to human erythrocyte carbonic-anhydrase-II (HCA-II) | J Med Chem 37: 240-7 (1994) BindingDB Entry DOI: 10.7270/Q2N29XKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012231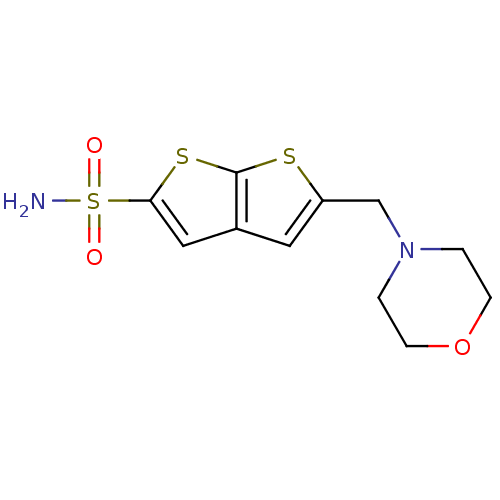 (5-Morpholin-4-ylmethyl-thieno[2,3-b]thiophene-2-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064734 (1-(1-{5-Fluoro-2-methoxy-4-[1-(2,4,6-trimethyl-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077041 (1-(1-{2-[4-(1-Cyclopropylmethyl-piperidin-4-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064735 (1-(1-{4-[1-(2,4-Dimethyl-1-oxy-pyridin-3-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064733 (1-(1-{5-Fluoro-2-methoxy-4-[1-(2-methyl-1-oxy-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077041 (1-(1-{2-[4-(1-Cyclopropylmethyl-piperidin-4-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50043913 (3-(2-Hydroxy-ethyl)-4,4-dioxo-1,2,3,4-tetrahydro-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for ability to compete with dansylamide for binding to human erythrocyte carbonic-anhydrase-II (HCA-II) | J Med Chem 37: 240-7 (1994) BindingDB Entry DOI: 10.7270/Q2N29XKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50004551 (5-Morpholin-4-ylmethyl-thieno[2,3-b]furan-2-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dansylamide at human carbonic anhydrase II | J Med Chem 35: 3027-33 (1992) BindingDB Entry DOI: 10.7270/Q2GX49H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50124909 (CHEMBL159744 | N-(3,5-Dimethyl-benzyl)-4-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of NMDA receptor-specific [3H]-ifenprodil binding to recombinant human NMDA receptor, NR2B subtype expressed in L cells | Bioorg Med Chem Lett 13: 697-700 (2003) BindingDB Entry DOI: 10.7270/Q2FX78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064719 (1-(1-{5-Fluoro-2-methoxy-4-[1-(1-oxy-4-trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012209 (5-{[Bis-(2-methoxy-ethyl)-amino]-methyl}-thieno[2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064741 (1-(1-{2-Methoxy-4-[1-(1-oxy-2-trifluoromethyl-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003252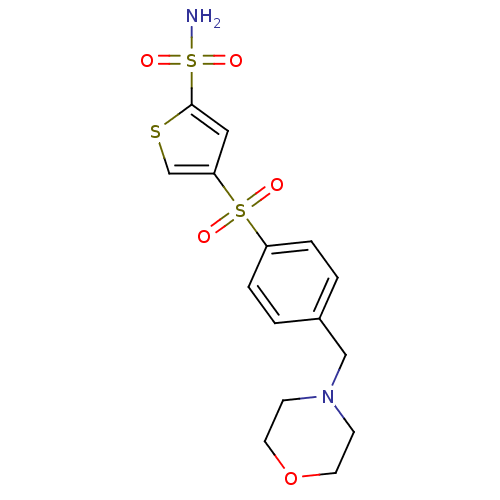 (4-(4-Morpholin-4-ylmethyl-benzenesulfonyl)-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077037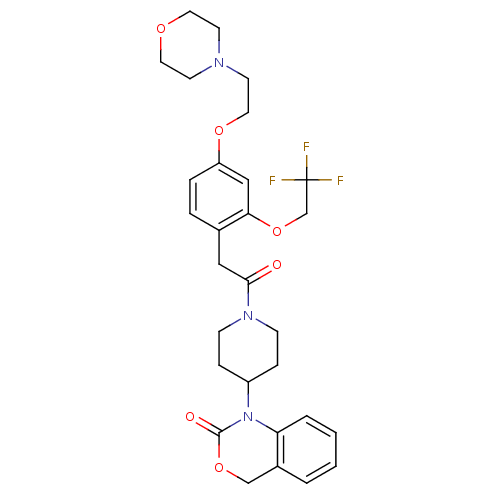 (1-(1-{2-[4-(2-Morpholin-4-yl-ethoxy)-2-(2,2,2-trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077032 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012227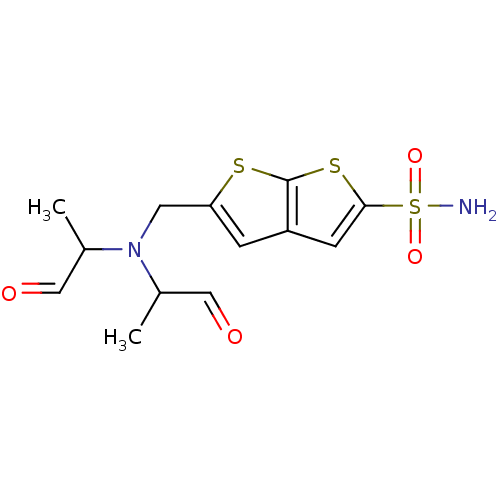 (5-{[Bis-(1-methyl-2-oxo-ethyl)-amino]-methyl}-thie...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for in vitro binding affinity against human Carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064725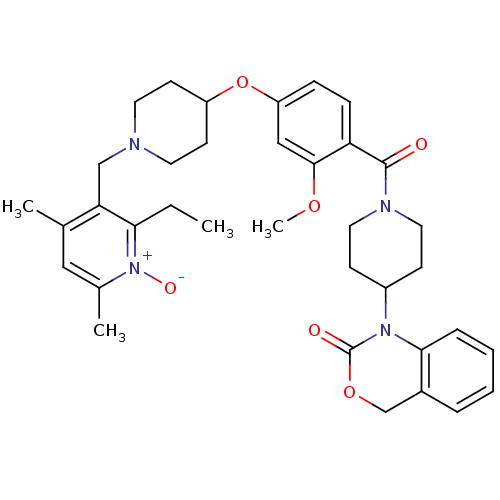 (1-(1-{4-[1-(2-Ethyl-4,6-dimethyl-1-oxy-pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012208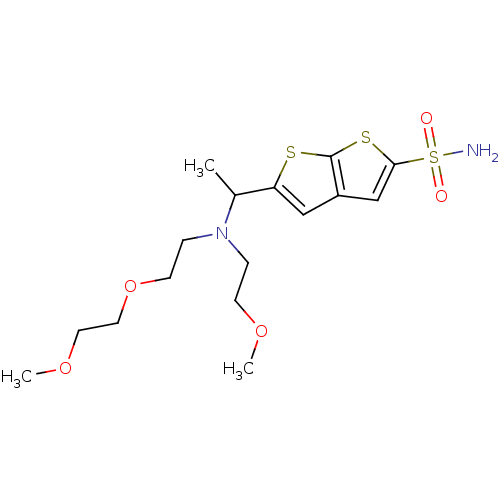 (5-{1-[[2-(2-Methoxy-ethoxy)-ethyl]-(2-methoxy-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012207 (5-{[[2-(2-Methoxy-ethoxy)-ethyl]-(2-methoxy-ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064736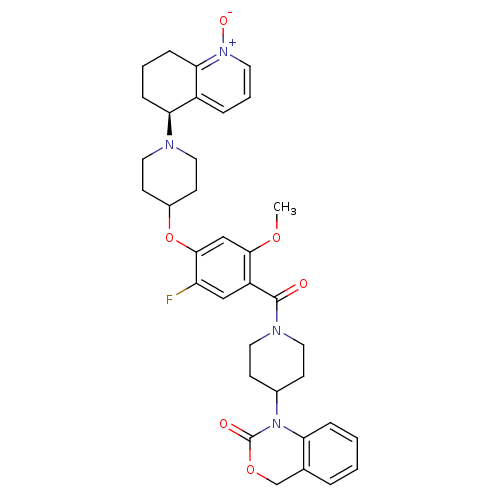 (1-(1-{5-Fluoro-2-methoxy-4-[1-((S)-1-oxy-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012225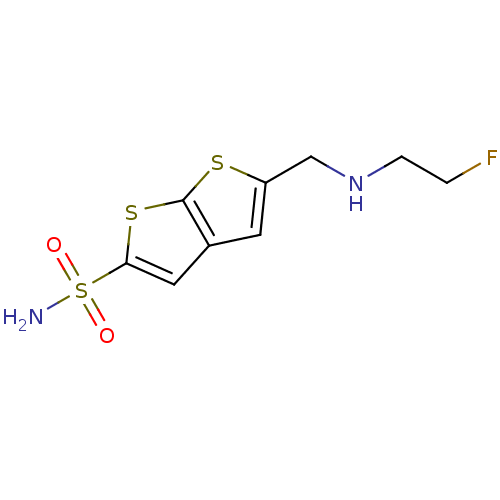 (5-[(2-Fluoro-ethylamino)-methyl]-thieno[2,3-b]thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064717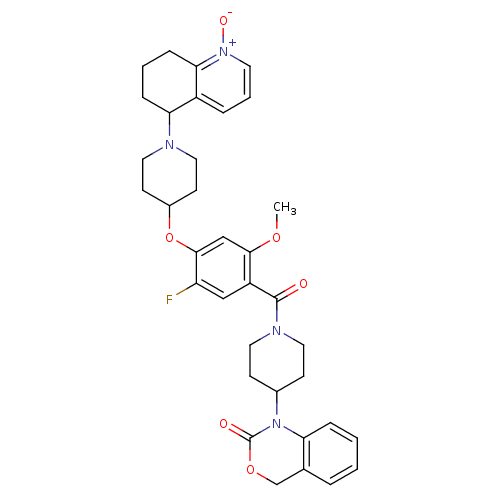 (1-(1-{5-Fluoro-2-methoxy-4-[1-(1-oxy-5,6,7,8-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012232 (5-[(2-Methylsulfanyl-ethylamino)-methyl]-thieno[2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50077032 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-(2,2,2-t...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards rat liver Vasopressin V1a receptor by using functional assay | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064737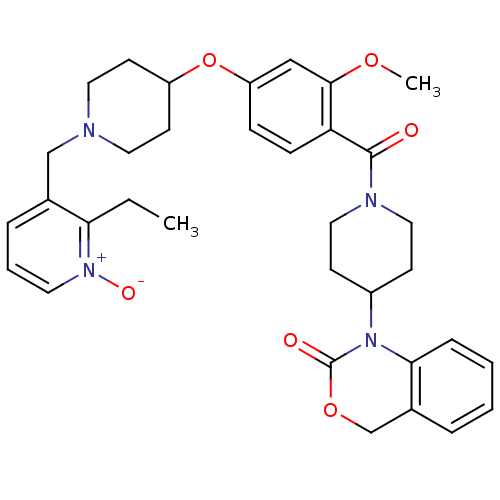 (1-(1-{4-[1-(2-Ethyl-1-oxy-pyridin-3-ylmethyl)-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50077037 (1-(1-{2-[4-(2-Morpholin-4-yl-ethoxy)-2-(2,2,2-trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat uterine oxytocin receptor (rOTr) | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003246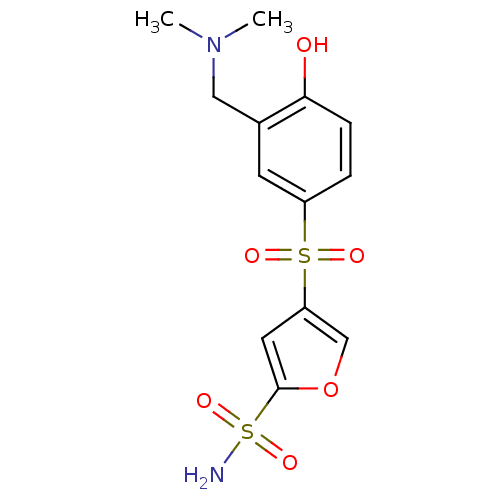 (4-(3-Dimethylaminomethyl-4-hydroxy-benzenesulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064740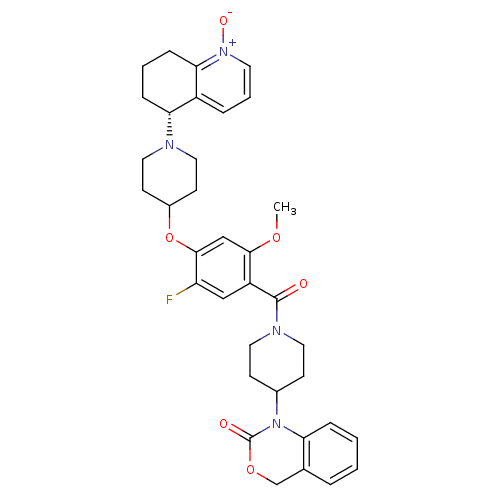 (1-(1-{5-Fluoro-2-methoxy-4-[1-((R)-1-oxy-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064739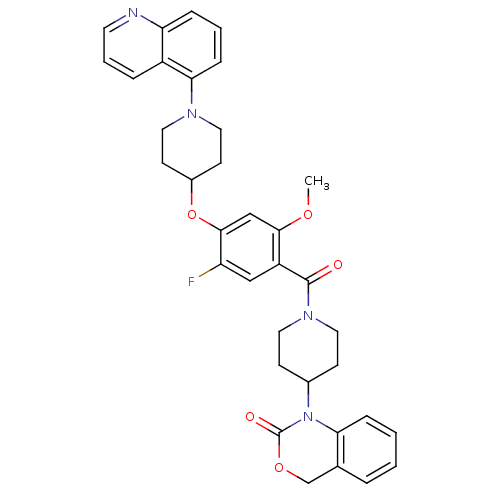 (1-{1-[5-Fluoro-2-methoxy-4-(1-quinolin-5-yl-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003246 (4-(3-Dimethylaminomethyl-4-hydroxy-benzenesulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50012217 (5-Sulfamoyl-thieno[2,3-b]thiophene-2-carboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human carbonic anhydrase II | J Med Chem 34: 1805-18 (1991) Checked by Author BindingDB Entry DOI: 10.7270/Q25D8SFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50077039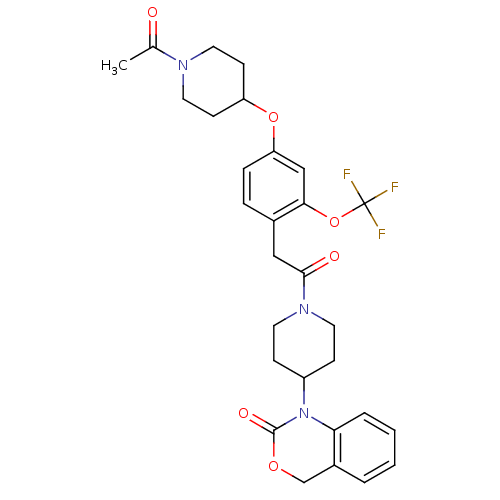 (1-(1-{2-[4-(1-Acetyl-piperidin-4-yloxy)-2-trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human oxytocin receptor | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50064730 (1-(1-{2-Methoxy-4-[1-(1-oxy-2-propyl-pyridin-3-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for cloned human oxytocin receptor (OT-R) | J Med Chem 41: 2146-63 (1998) Article DOI: 10.1021/jm9800797 BindingDB Entry DOI: 10.7270/Q2SN083Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 361 total ) | Next | Last >> |