

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM94503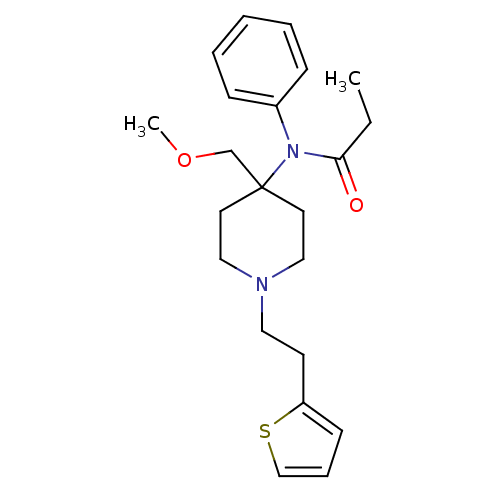 (2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013943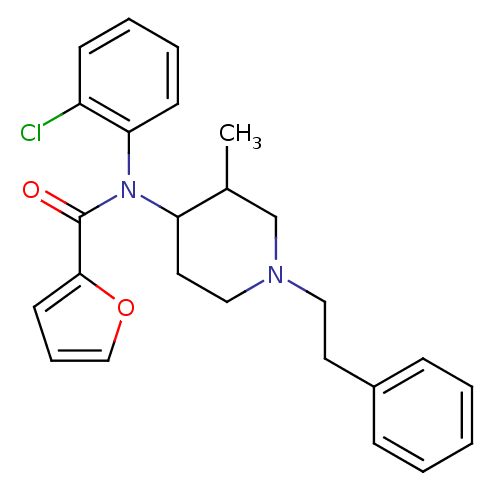 (CHEMBL327270 | Furan-2-carboxylic acid (2-chloro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013945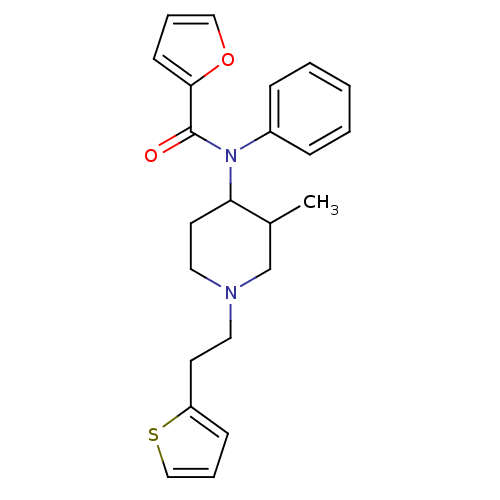 (CHEMBL431047 | Furan-2-carboxylic acid [3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934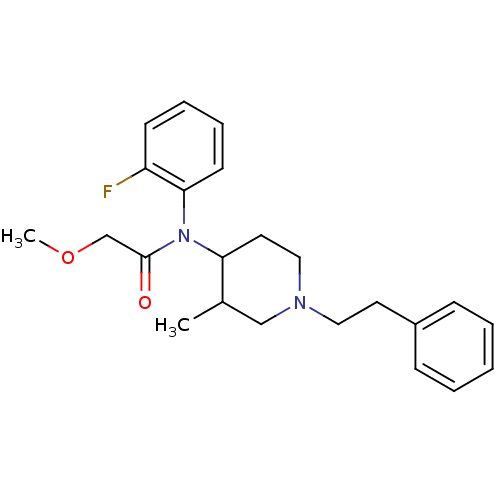 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM94503 (2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013939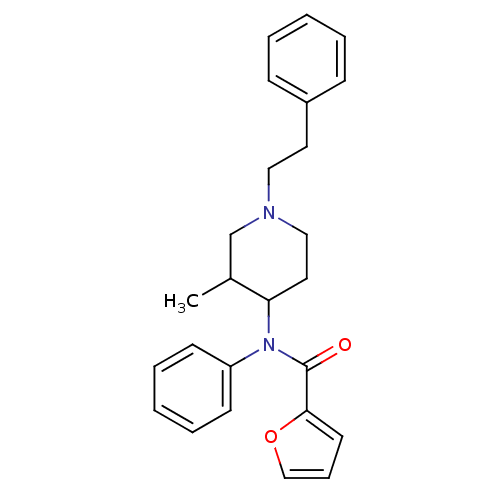 (CHEMBL95247 | Furan-2-carboxylic acid (3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013939 (CHEMBL95247 | Furan-2-carboxylic acid (3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013938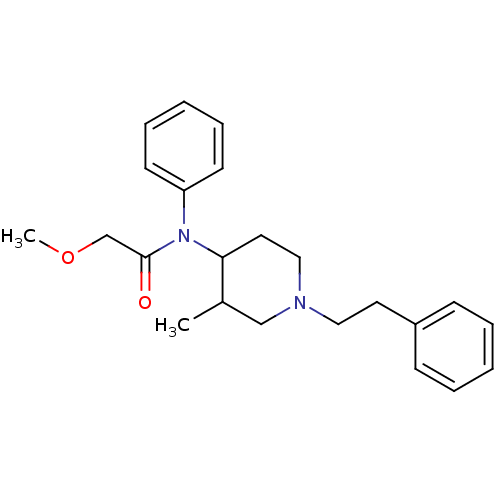 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013938 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50297307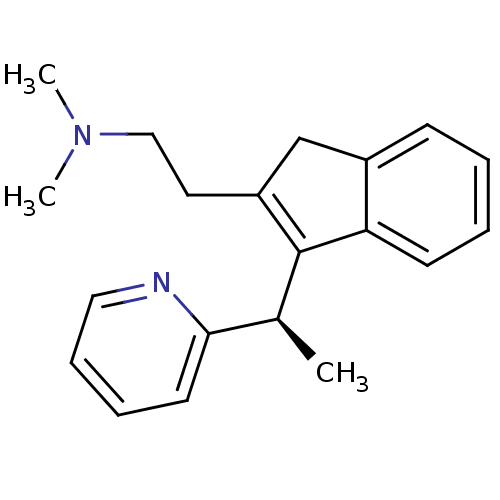 (CHEMBL564226 | R-dimethindene) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University of Mainz Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards cloned human histamine H1 receptors stably expressed in CHO-K1 cells using [3H]mepyramine | J Med Chem 46: 856-67 (2003) Article DOI: 10.1021/jm020895l BindingDB Entry DOI: 10.7270/Q2XK8J83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-dependent protein kinase catalytic subunit (Homo sapiens (Human)) | BDBM50156495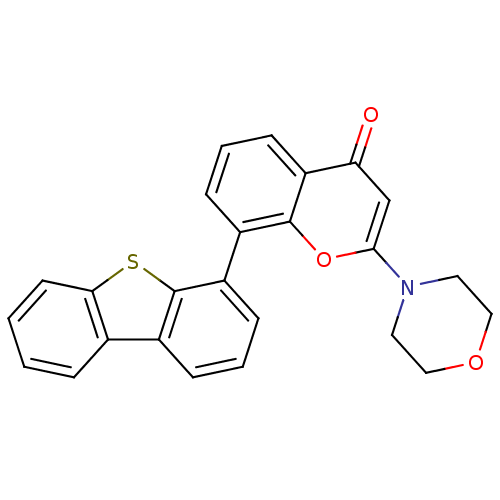 (8-(dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Natural Sciences--Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against DNA-dependent protein kinase receptor | J Med Chem 48: 7829-46 (2005) Article DOI: 10.1021/jm050444b BindingDB Entry DOI: 10.7270/Q2Z31Z60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM81452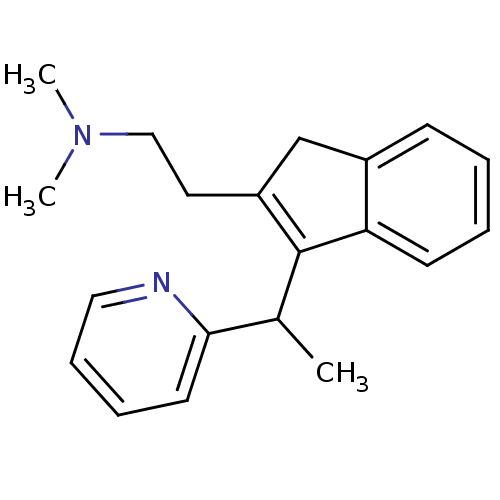 (CAS_91533 | Dimethindene | NSC_91533) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University of Mainz Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards cloned human histamine H1 receptors stably expressed in CHO-K1 cells using [3H]mepyramine | J Med Chem 46: 856-67 (2003) Article DOI: 10.1021/jm020895l BindingDB Entry DOI: 10.7270/Q2XK8J83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013949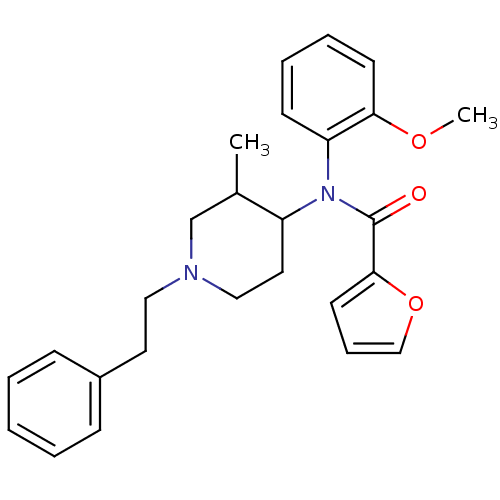 (CHEMBL95248 | Furan-2-carboxylic acid (2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50474506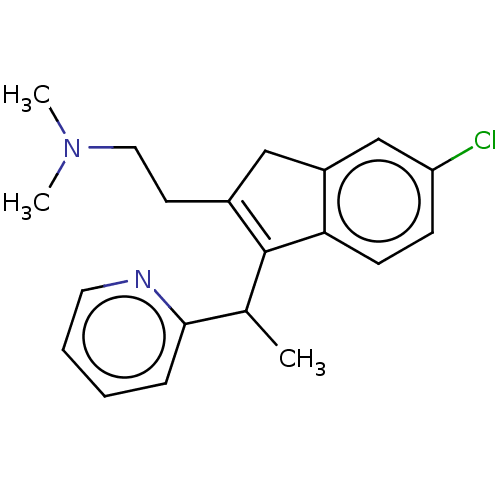 (CHEMBL167223) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University of Mainz Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards cloned human histamine H1 receptors stably expressed in CHO-K1 cells using [3H]mepyramine | J Med Chem 46: 856-67 (2003) Article DOI: 10.1021/jm020895l BindingDB Entry DOI: 10.7270/Q2XK8J83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013948 (CHEMBL95909 | N-(2-Chloro-phenyl)-2-methoxy-N-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOC Technical Center Curated by ChEMBL | Assay Description Displacement [3H]-naloxone from the Opioid receptor mu 1 isolated from rat brain membrane. | J Med Chem 32: 663-71 (1989) BindingDB Entry DOI: 10.7270/Q2N58KC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093383 (2-(4-Trifluoromethyl-phenyl)-1H-benzoimidazole-4-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093359 (2-(4-Hydroxymethyl-phenyl)-1H-benzoimidazole-4-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM22869 (6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50017412 (CHEMBL345120 | Furan-2-carboxylic acid (1-phenethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOC Technical Center Curated by ChEMBL | Assay Description Displacement [3H]-naloxone from the Opioid receptor mu 1 isolated from rat brain membrane. | J Med Chem 32: 663-71 (1989) BindingDB Entry DOI: 10.7270/Q2N58KC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093384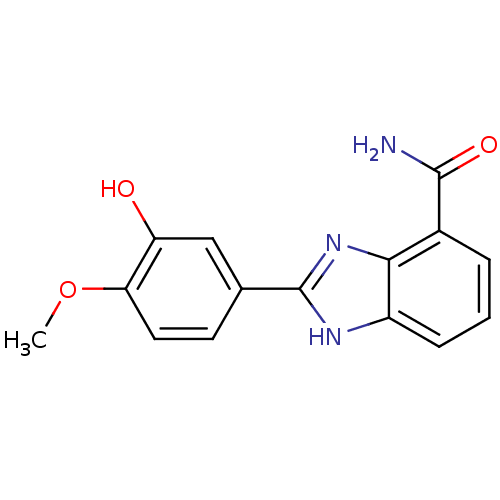 (2-(3-Hydroxy-4-methoxy-phenyl)-1H-benzoimidazole-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013947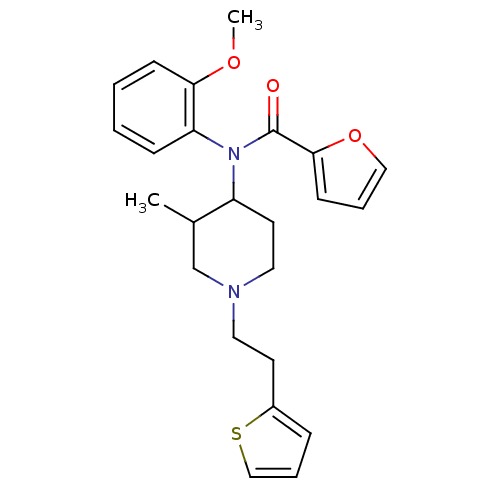 (CHEMBL95786 | Furan-2-carboxylic acid (2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013935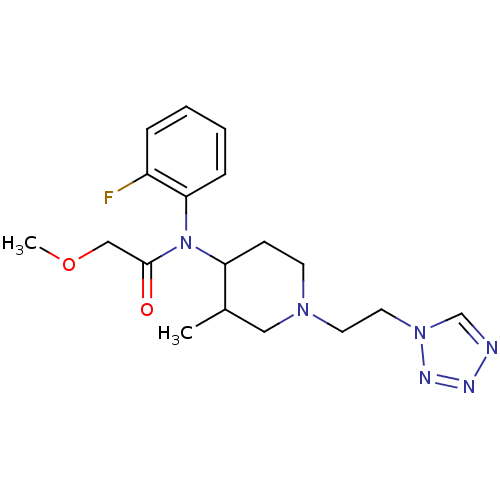 (CHEMBL95390 | N-(2-Fluoro-phenyl)-2-methoxy-N-[3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013940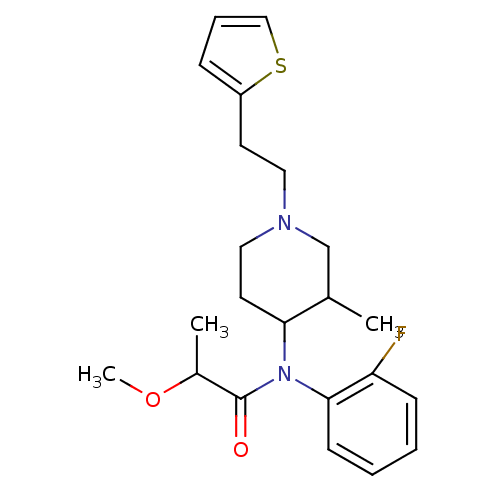 (CHEMBL319343 | N-(2-Fluoro-phenyl)-2-methoxy-N-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOC Technical Center Curated by ChEMBL | Assay Description Displacement [3H]-naloxone from the Opioid receptor mu 1 isolated from rat brain membrane. | J Med Chem 32: 663-71 (1989) BindingDB Entry DOI: 10.7270/Q2N58KC7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50017406 (CHEMBL162700 | Furan-3-carboxylic acid benzo[1,2,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOC Technical Center Curated by ChEMBL | Assay Description Displacement [3H]-naloxone from the Opioid receptor mu 1 isolated from rat brain membrane. | J Med Chem 32: 663-71 (1989) BindingDB Entry DOI: 10.7270/Q2N58KC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013942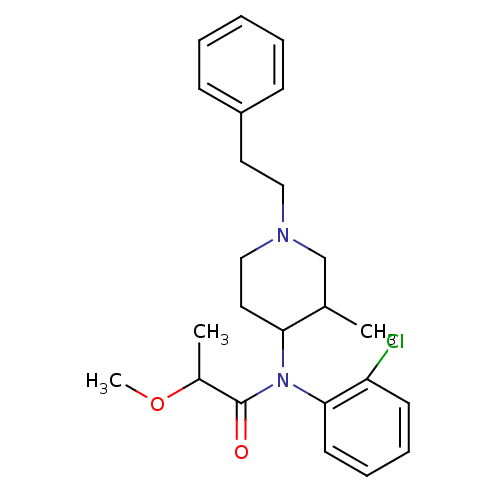 (CHEMBL318895 | N-(2-Chloro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM86496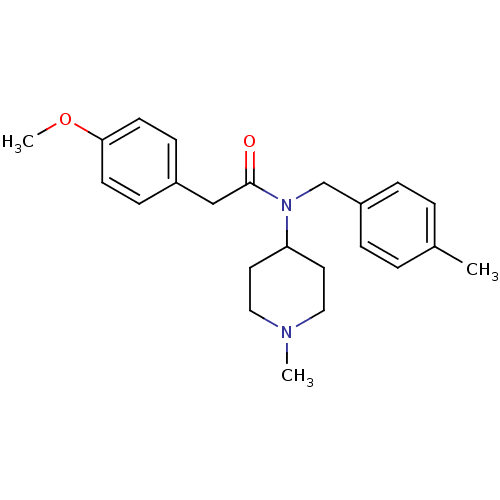 (AC-90179 | N-(4-Methylbenzyl)-N-(1-methyl-4-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals, Inc., Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 943-51 (2004) Article DOI: 10.1124/jpet.104.066688 BindingDB Entry DOI: 10.7270/Q28S4NH4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093372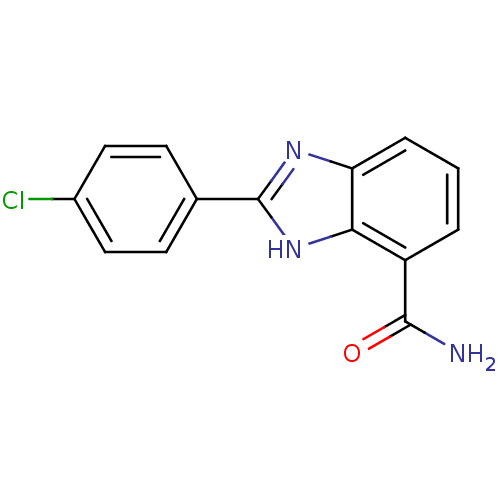 (2-(4-Chloro-phenyl)-1H-benzoimidazole-4-carboxylic...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50145214 (2-(4-((methylamino)methyl)phenyl)-1H-benzo[d]imida...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Newcastle Curated by ChEMBL | Assay Description Binding affinity towards human poly(ADP-ribose) polymerase-1 (PARP-1) | Bioorg Med Chem Lett 14: 2433-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.017 BindingDB Entry DOI: 10.7270/Q2542N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50228269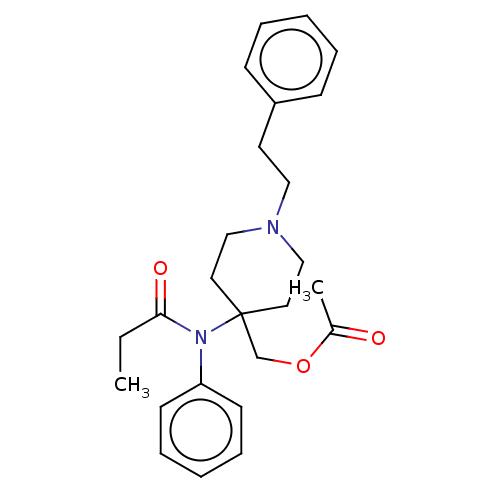 (CHEMBL178035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Antimuscarinic activity on the acetylcholine-induced inhibition of contraction of guinea pig ileum which has Muscarinic acetylcholine receptor M2 sub... | J Med Chem 32: 968-74 (1989) BindingDB Entry DOI: 10.7270/Q2XG9TBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50154756 (6-(4-Methylaminomethyl-phenyl)-3,4-dihydro-2H-[1,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D--La Jolla Laboratories Curated by ChEMBL | Assay Description Inhibition of human Poly (ADP-ribose) polymerase 1 enzyme | J Med Chem 47: 5467-81 (2004) Article DOI: 10.1021/jm030513r BindingDB Entry DOI: 10.7270/Q2PK0FM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50093379 (2-(4-Cyano-phenyl)-1H-benzoimidazole-4-carboxylic ...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University Curated by ChEMBL | Assay Description In vitro inhibition against human full length PARP protein | J Med Chem 43: 4084-97 (2000) BindingDB Entry DOI: 10.7270/Q2MP52HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122777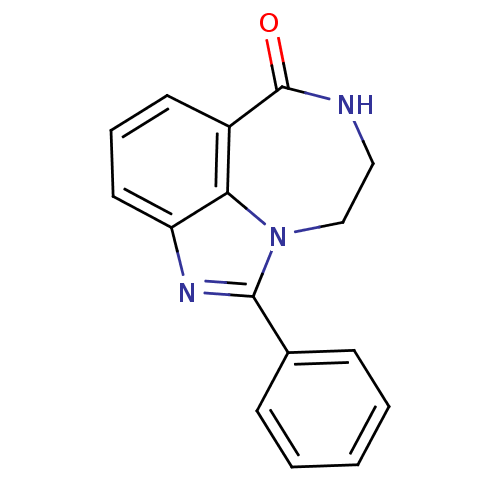 (1-Phenyl-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]azu...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50017416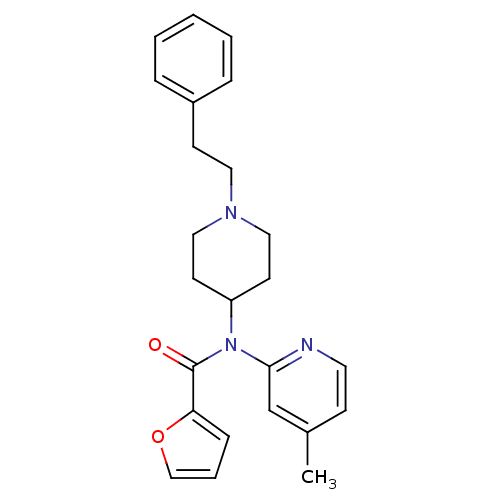 (CHEMBL347188 | Furan-2-carboxylic acid (4-methyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BOC Technical Center Curated by ChEMBL | Assay Description Displacement [3H]-naloxone from the Opioid receptor mu 1 isolated from rat brain membrane. | J Med Chem 32: 663-71 (1989) BindingDB Entry DOI: 10.7270/Q2N58KC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013936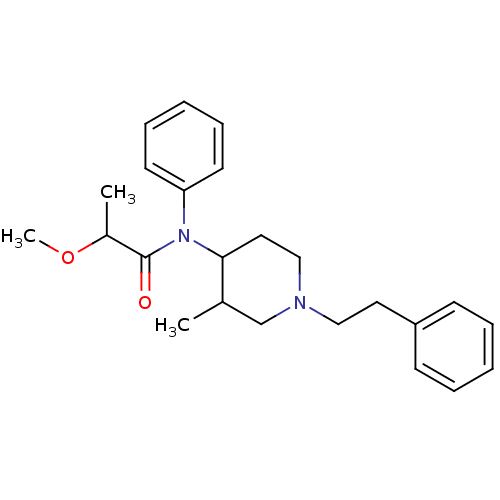 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122775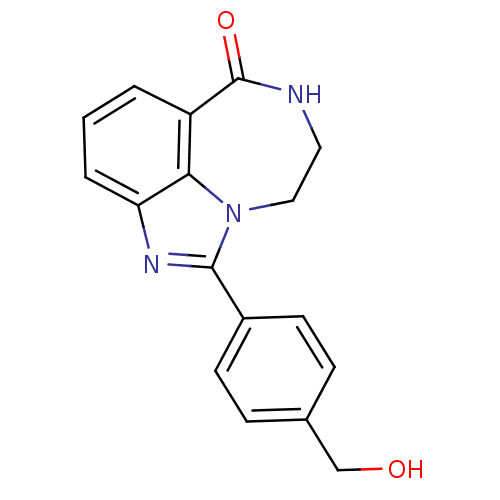 (1-(4-Hydroxymethyl-phenyl)-8,9-dihydro-7H-2,7,9a-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013936 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50145216 (2-(4-{[Bis-(2-hydroxy-ethyl)-amino]-methyl}-phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Newcastle Curated by ChEMBL | Assay Description Binding affinity towards human poly(ADP-ribose) polymerase-1 (PARP-1) | Bioorg Med Chem Lett 14: 2433-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.017 BindingDB Entry DOI: 10.7270/Q2542N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50145224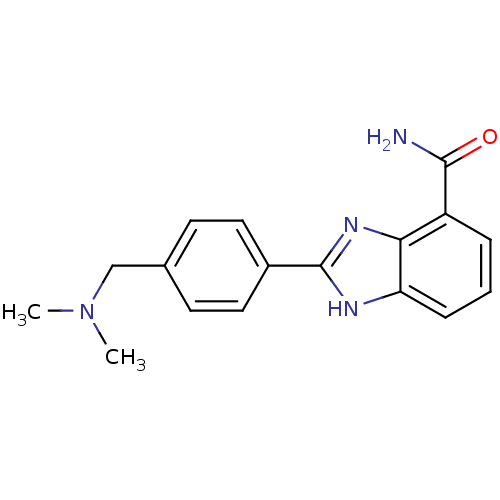 (2-(4-Dimethylaminomethyl-phenyl)-1H-benzoimidazole...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Newcastle Curated by ChEMBL | Assay Description Binding affinity towards human poly(ADP-ribose) polymerase-1 (PARP-1) | Bioorg Med Chem Lett 14: 2433-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.017 BindingDB Entry DOI: 10.7270/Q2542N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122781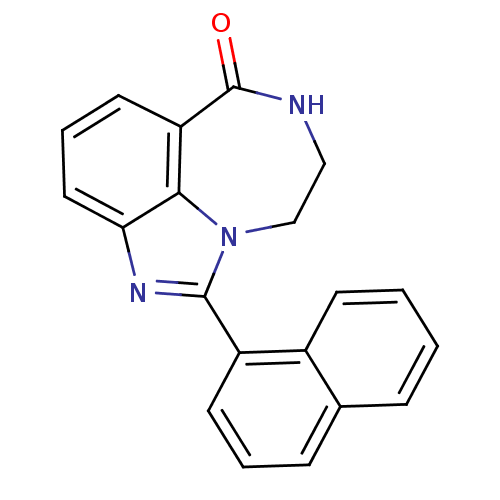 (1-Naphthalen-1-yl-8,9-dihydro-7H-2,7,9a-triaza-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120702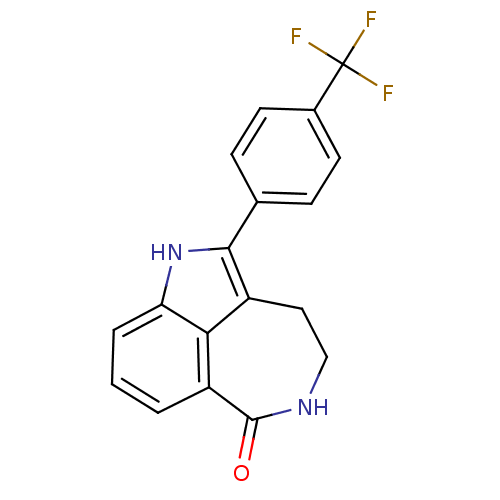 (2-(4-Trifluoromethyl-phenyl)-1,3,4,5-tetrahydro-az...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27685 (2-{4-[(dimethylamino)methyl]phenyl}-3,10-diazatric...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120707 (2-Thiophen-2-yl-1,3,4,5-tetrahydro-azepino[5,4,3-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity towards human poly(ADP-ribose) polymerase 1 (PARP-1). | J Med Chem 45: 4961-74 (2002) BindingDB Entry DOI: 10.7270/Q2T152ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3414 total ) | Next | Last >> |