

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50143784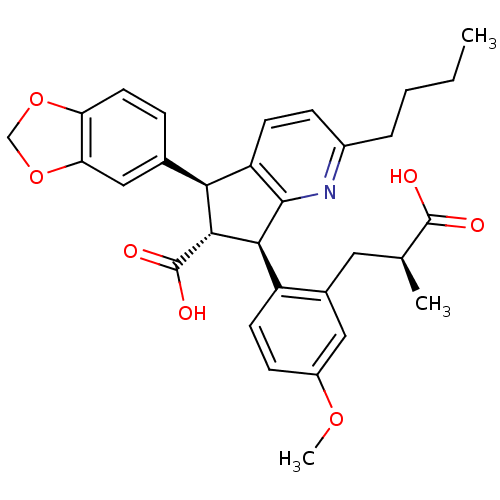 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50143784 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50061101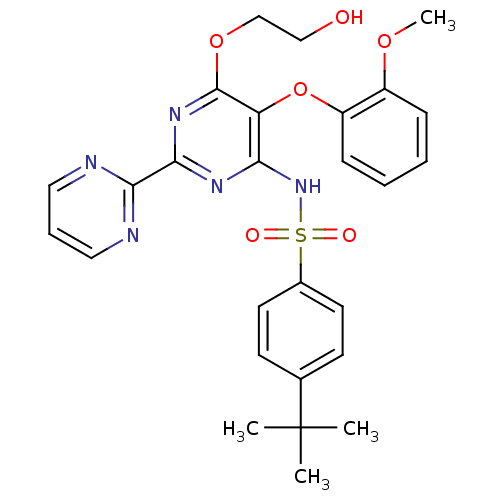 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM47085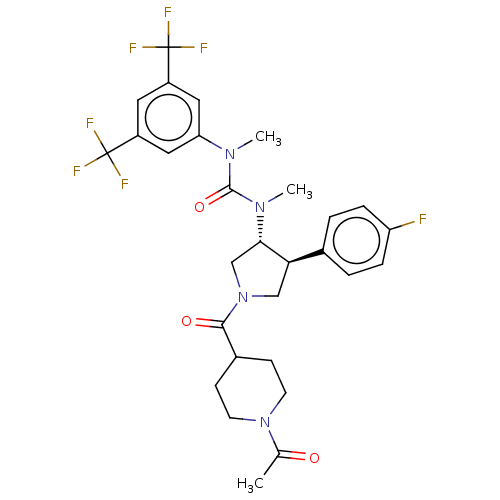 (US8592454, 71b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106978 (US8592454, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106983 (US8592454, 420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106974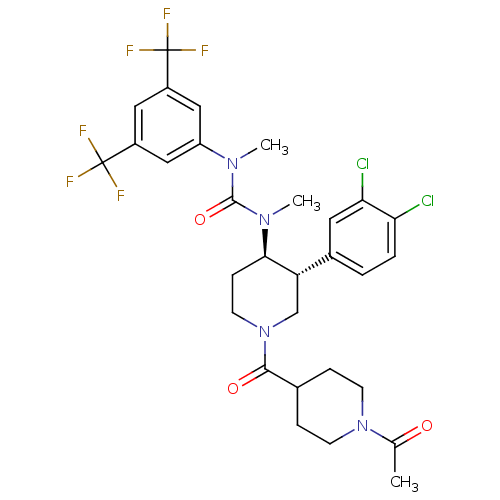 (US8592454, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106979 (US8592454, 192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106982 (US8592454, 378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106980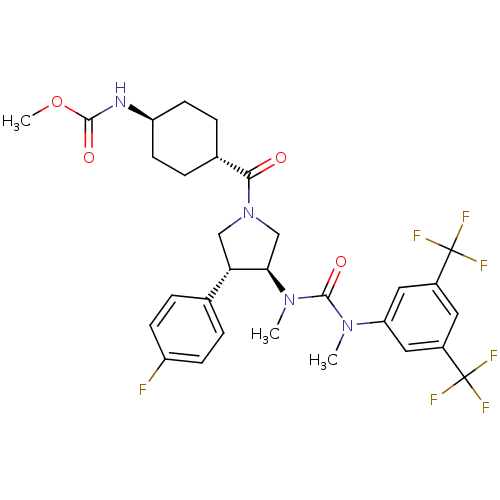 (US8592454, 233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182758 (CHEMBL3819243) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106981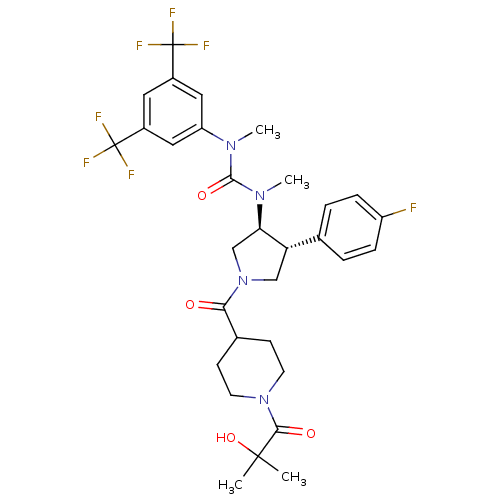 (US8592454, 308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106977 (US8592454, 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106976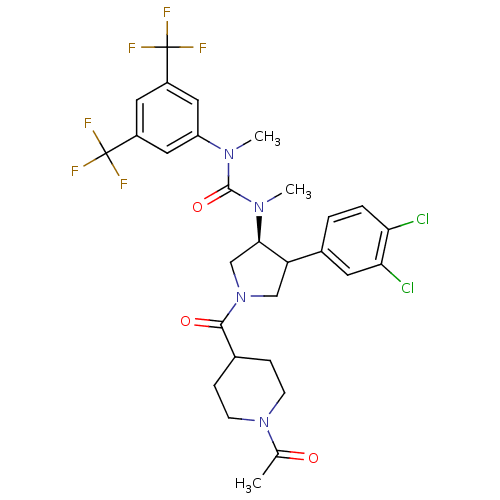 (US8592454, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182757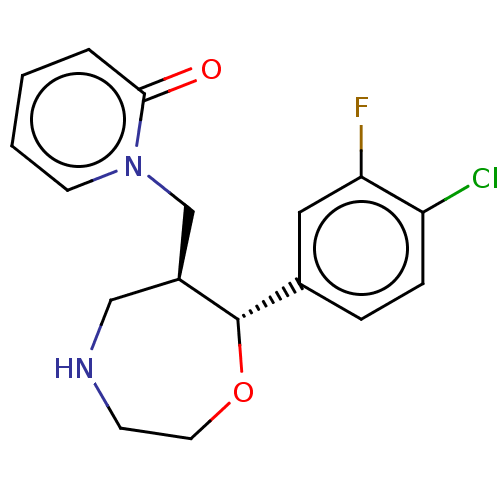 (CHEMBL3819201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182755 (CHEMBL3818117) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM97478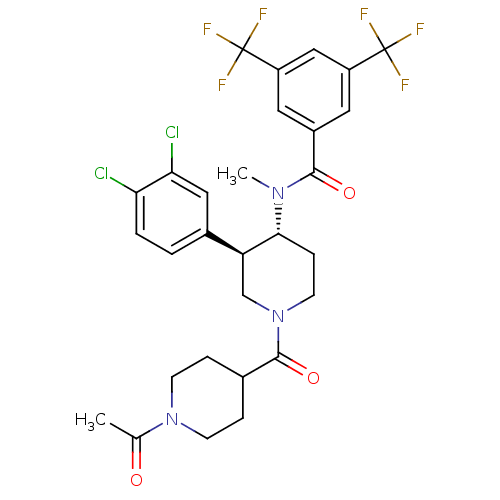 (US8470816, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182754 (CHEMBL3817915) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106973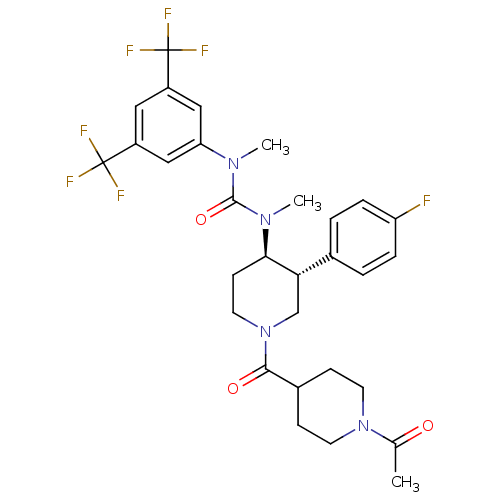 (US8592454, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182753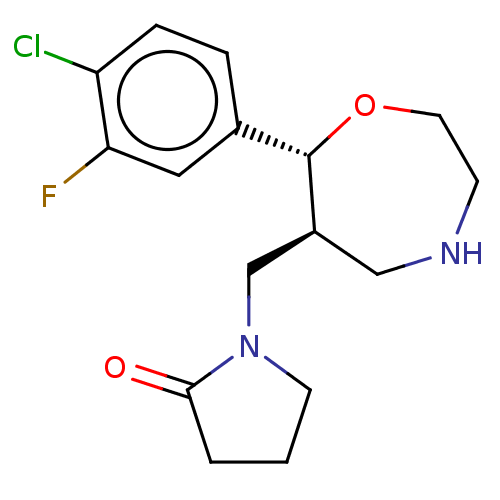 (CHEMBL3818953) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182763 (CHEMBL3818901) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97489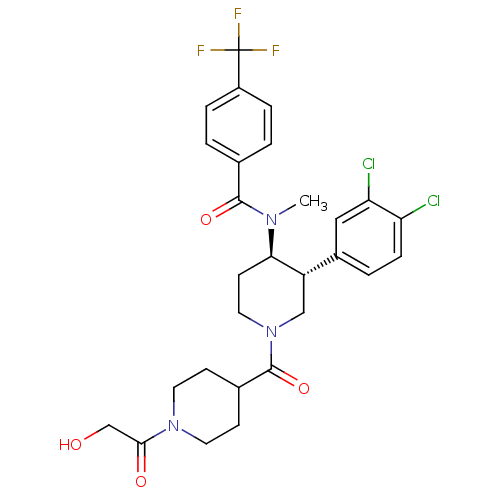 (US8470816, 632) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97486 (US8470816, 464) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97485 (US8470816, 458) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97481 (US8470816, 265) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Bioorg Med Chem Lett 5: 1989-1994 (1995) Article DOI: 10.1016/0960-894X(95)00339-U BindingDB Entry DOI: 10.7270/Q2B56K6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97490 (US8470816, 633) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97483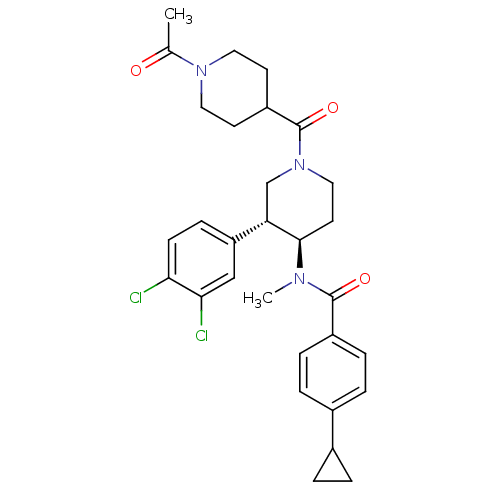 (US8470816, 292) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97479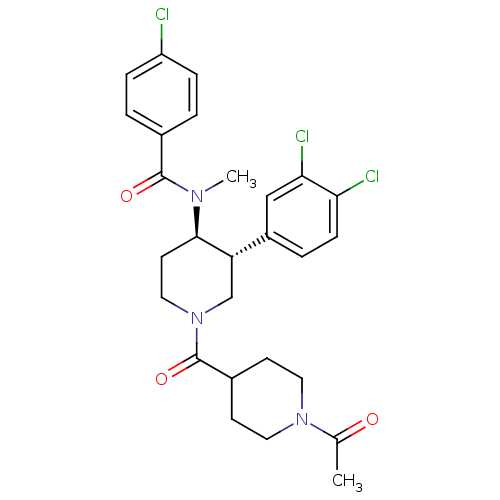 (US8470816, 251a) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97487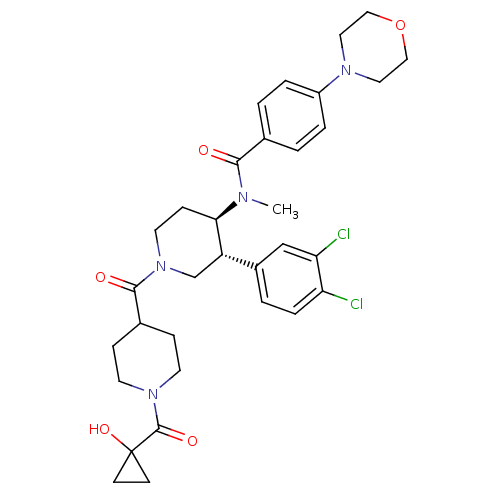 (US8470816, 623) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50098412 (CHEMBL3593400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay | Bioorg Med Chem 23: 5000-14 (2015) Article DOI: 10.1016/j.bmc.2015.05.017 BindingDB Entry DOI: 10.7270/Q22F7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50558220 (CHEMBL4791288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NET expressed in CHO-K1 cells assessed as inhibition of [3H]-norepinephrine reuptake measured after 45 mins by Microscintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.038 BindingDB Entry DOI: 10.7270/Q24B350H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97491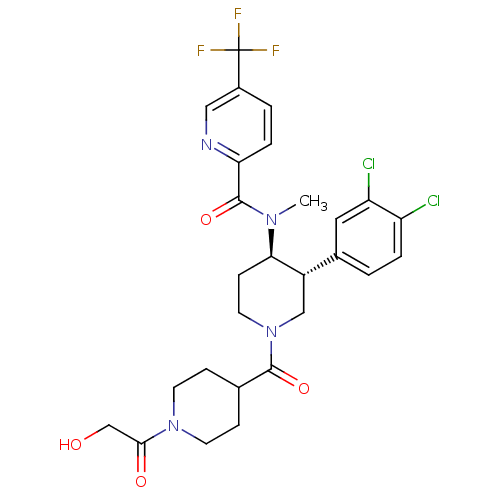 (US8470816, 635) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97482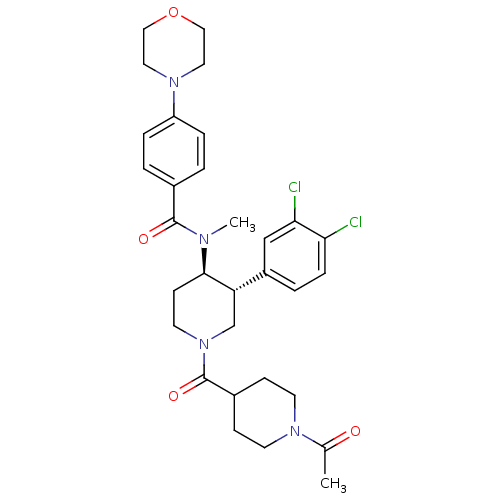 (US8470816, 268) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97472 (US8470816, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50285068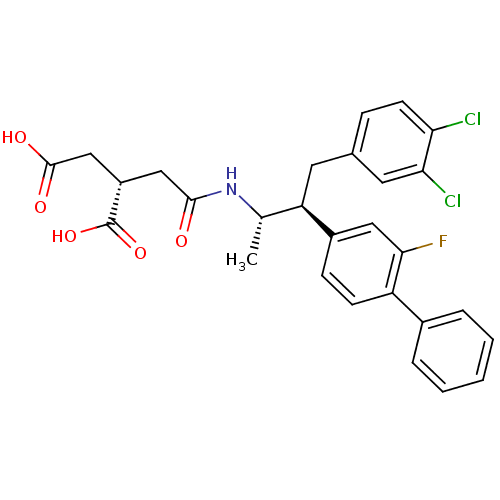 ((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Bioorg Med Chem Lett 5: 1989-1994 (1995) Article DOI: 10.1016/0960-894X(95)00339-U BindingDB Entry DOI: 10.7270/Q2B56K6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50285068 ((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against squalene synthase (SQS) obtained from HepG2 cells | Bioorg Med Chem Lett 6: 463-466 (1996) Article DOI: 10.1016/0960-894X(96)00033-9 BindingDB Entry DOI: 10.7270/Q2MG7PG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97488 (US8470816, 629) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97480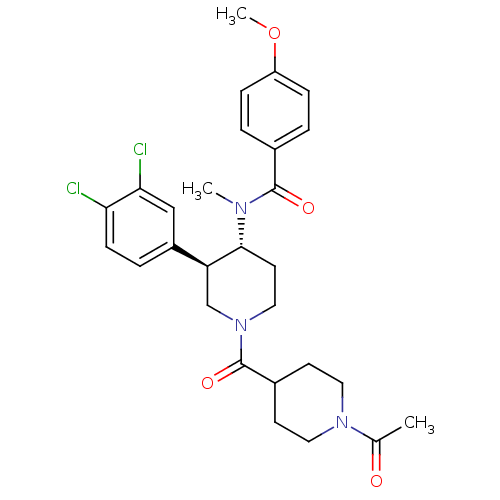 (US8470816, 252) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182762 (CHEMBL3818471) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50403481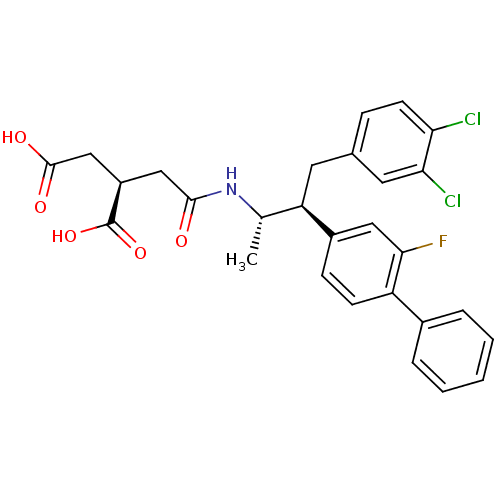 (CHEMBL2115091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Bioorg Med Chem Lett 5: 1989-1994 (1995) Article DOI: 10.1016/0960-894X(95)00339-U BindingDB Entry DOI: 10.7270/Q2B56K6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50098413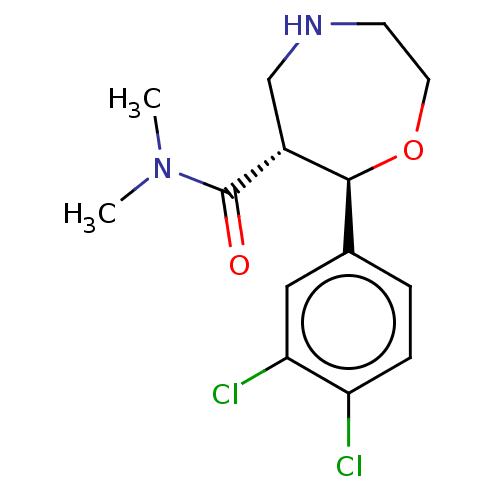 (CHEMBL3593399) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay | Bioorg Med Chem 23: 5000-14 (2015) Article DOI: 10.1016/j.bmc.2015.05.017 BindingDB Entry DOI: 10.7270/Q22F7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97478 (US8470816, 91) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97484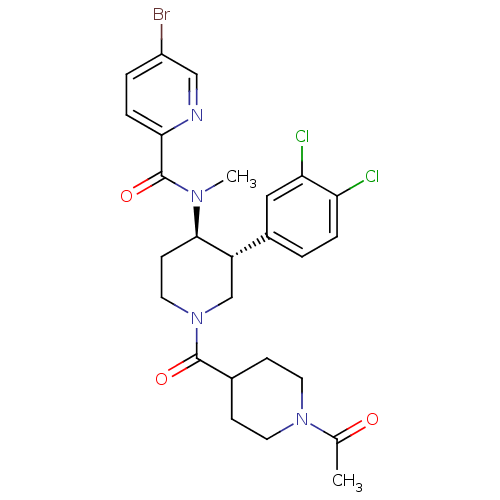 (US8470816, 318) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182761 (CHEMBL3818345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM97475 (US8470816, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligan receptor binding inhibitory activity using membrane fraction of CHO cell expressing human NK receptors. | US Patent US8470816 (2013) BindingDB Entry DOI: 10.7270/Q2NZ8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182759 (CHEMBL3819441) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50558224 (CHEMBL4753874) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NET expressed in CHO-K1 cells assessed as inhibition of [3H]-norepinephrine reuptake measured after 45 mins by Microscintillation... | Citation and Details Article DOI: 10.1016/j.bmc.2016.05.038 BindingDB Entry DOI: 10.7270/Q24B350H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50285065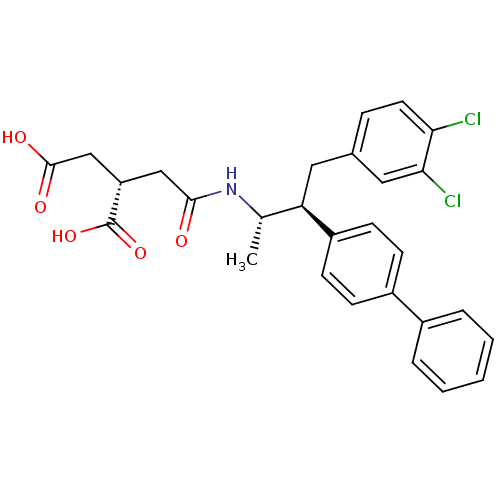 ((S)-2-{[(1S,2S)-2-Biphenyl-4-yl-3-(3,4-dichloro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Bioorg Med Chem Lett 5: 1989-1994 (1995) Article DOI: 10.1016/0960-894X(95)00339-U BindingDB Entry DOI: 10.7270/Q2B56K6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50182760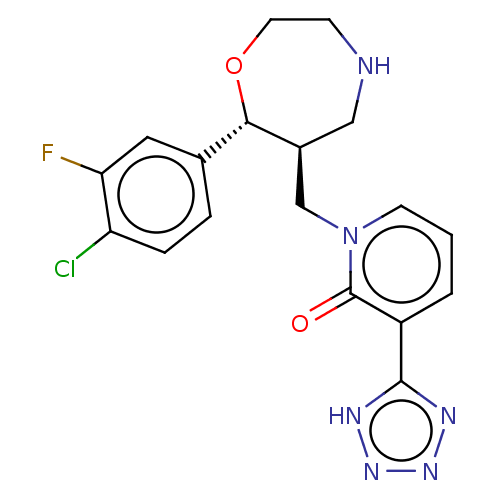 (CHEMBL3818946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human NET expressed in CHO cells assessed as [3H]-Norepinephrine reuptake incubated for 45 mins measured after 30 mins by topcount meth... | Bioorg Med Chem 24: 3716-26 (2016) Article DOI: 10.1016/j.bmc.2016.06.014 BindingDB Entry DOI: 10.7270/Q2NP26B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 240 total ) | Next | Last >> |