

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761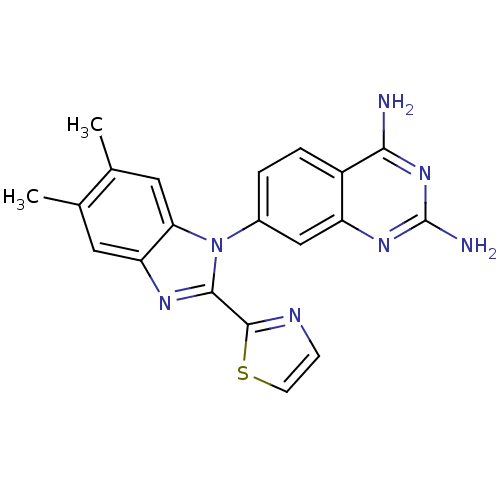 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131853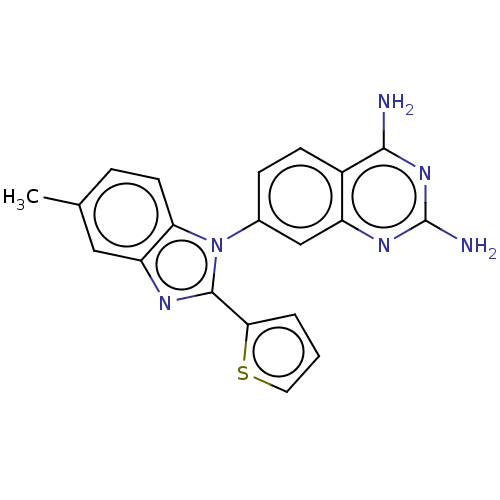 (US8835445, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757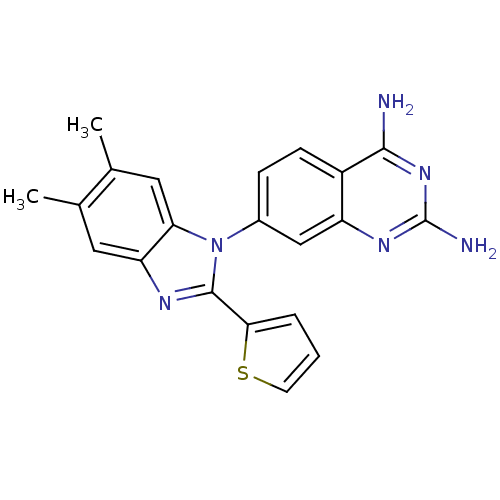 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552678 (CHEMBL4759036) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448758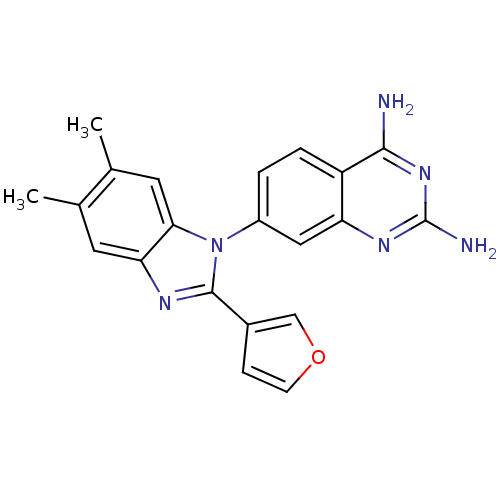 (CHEMBL3128024) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552679 (CHEMBL4762678) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552672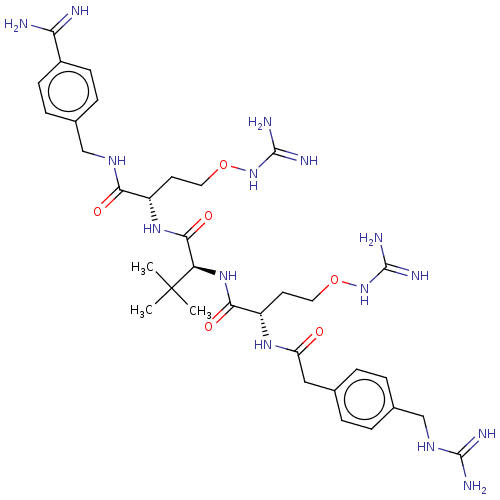 (CHEMBL4790628) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448743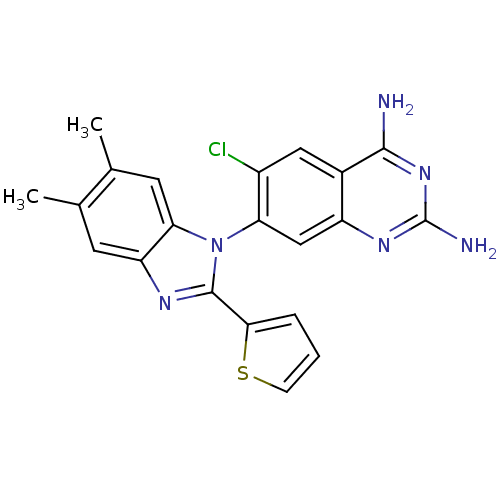 (CHEMBL3128015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744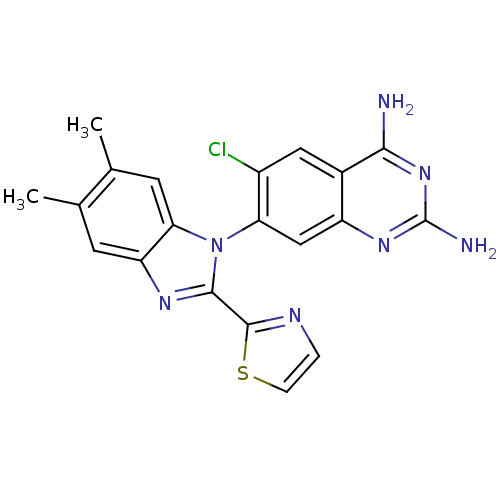 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448740 (CHEMBL3128018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131845 (US8835445, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552676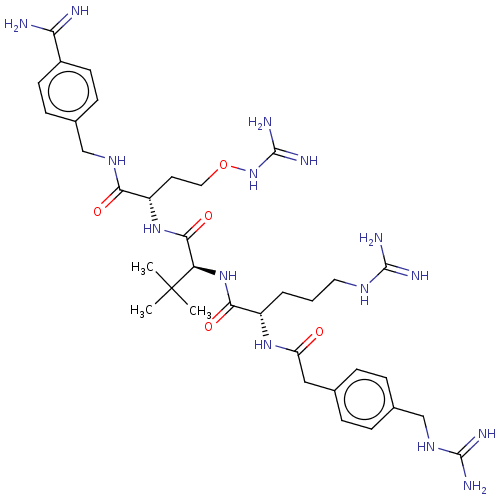 (CHEMBL4794635) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131846 (US8835445, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448741 (CHEMBL3128017) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175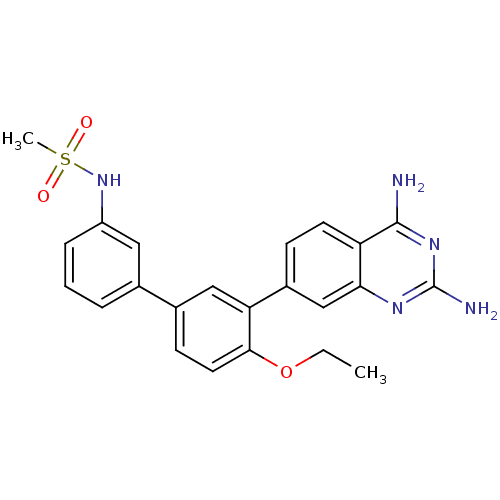 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131850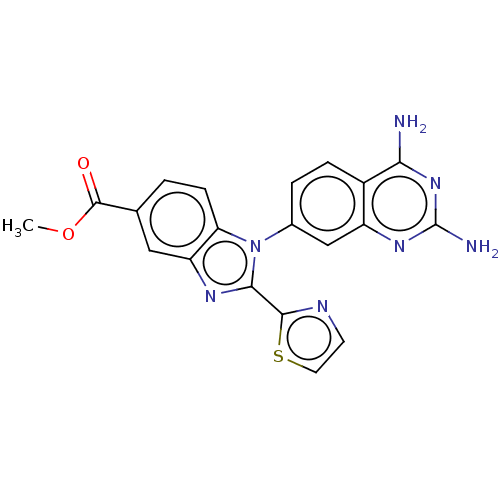 (US8835445, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448753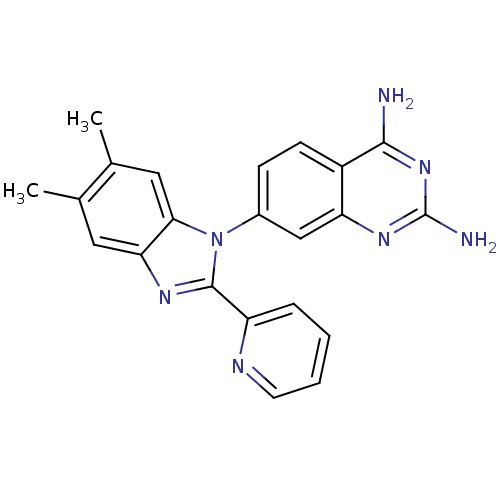 (CHEMBL3127912 | US8835445, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448753 (CHEMBL3127912 | US8835445, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448738 (CHEMBL3127909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351173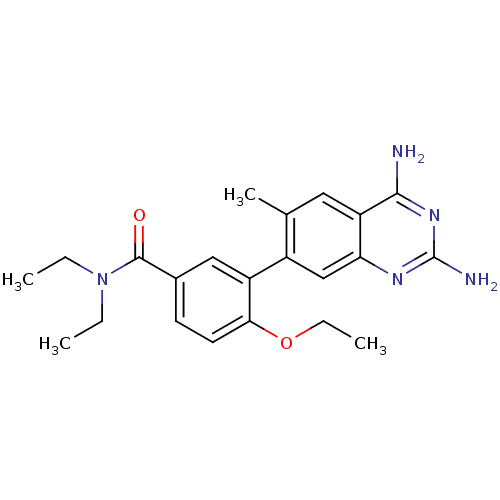 (CHEMBL1818129 | US8835445, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351173 (CHEMBL1818129 | US8835445, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448751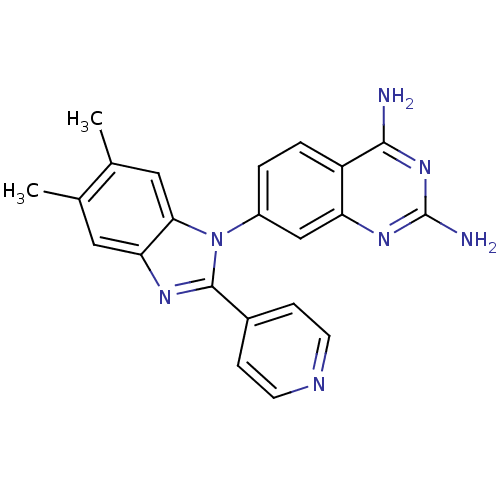 (CHEMBL3127914) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351174 (CHEMBL1818128 | US8835445, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351174 (CHEMBL1818128 | US8835445, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448745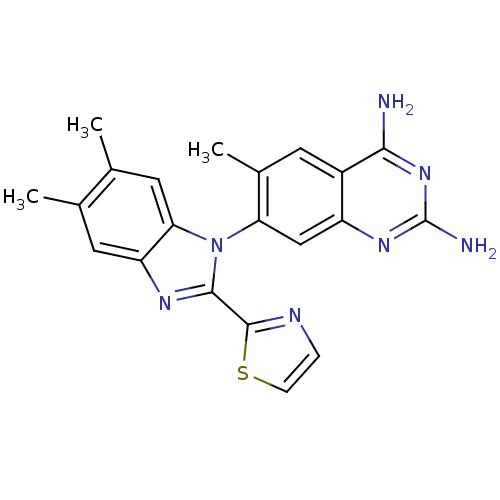 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448745 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552673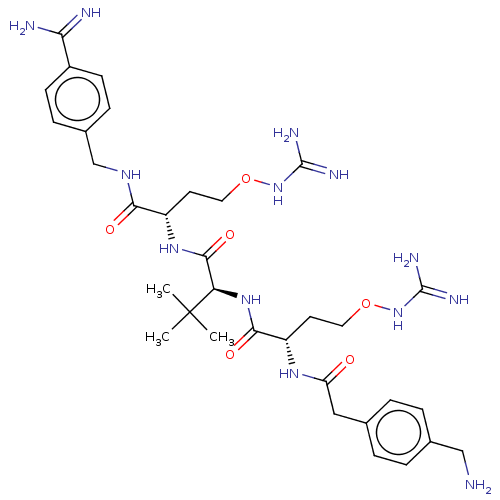 (CHEMBL4750900) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552674 (CHEMBL4742184) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131854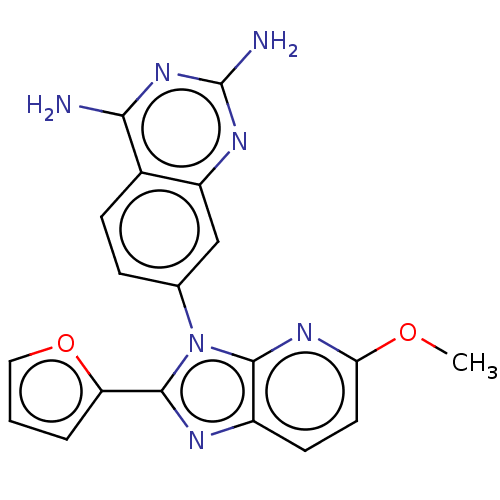 (US8835445, 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448739 (CHEMBL3128019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552677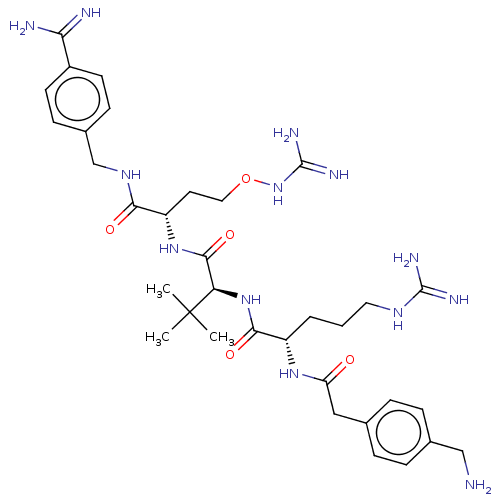 (CHEMBL4779067) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00651 BindingDB Entry DOI: 10.7270/Q2JD51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18070 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18070 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-chromen-5-yl)me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448760 (CHEMBL3128022) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM608574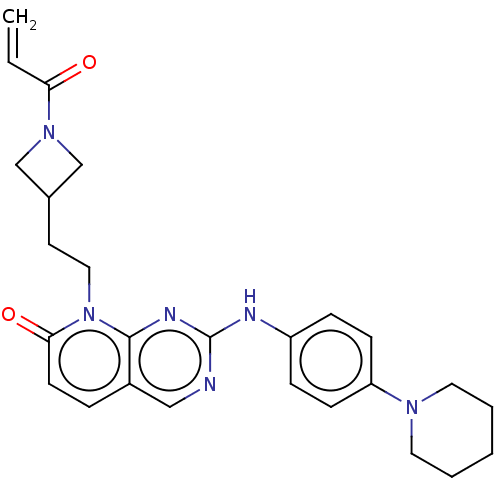 (US11697648, Example 143) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7MDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM608575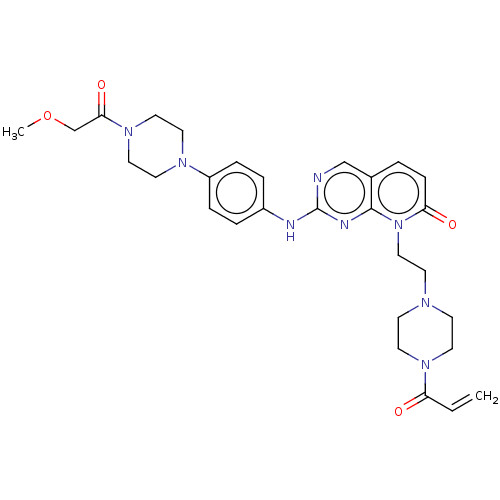 (US11697648, Example 144) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7MDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM608581 (US11697648, Example 150) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7MDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM475610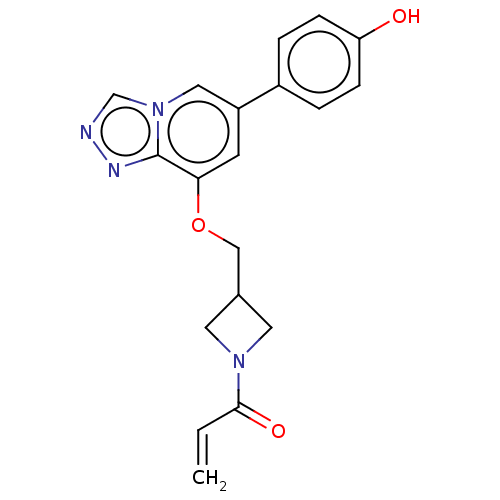 (US10851102, Example 5 | US11339160, Ex No. 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | US Patent US10851102 (2020) BindingDB Entry DOI: 10.7270/Q2N87DV3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM475613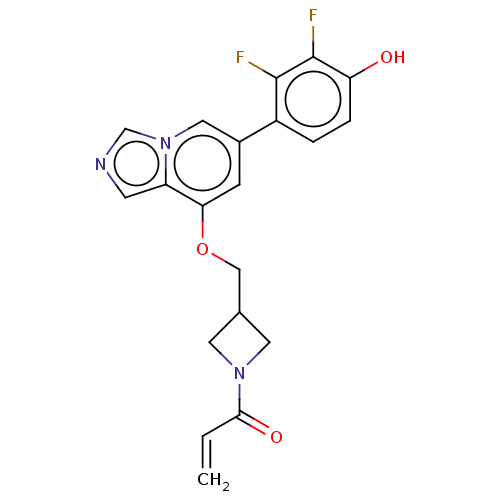 (US10851102, Example 8 | US11339160, Ex No. 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | US Patent US10851102 (2020) BindingDB Entry DOI: 10.7270/Q2N87DV3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM475615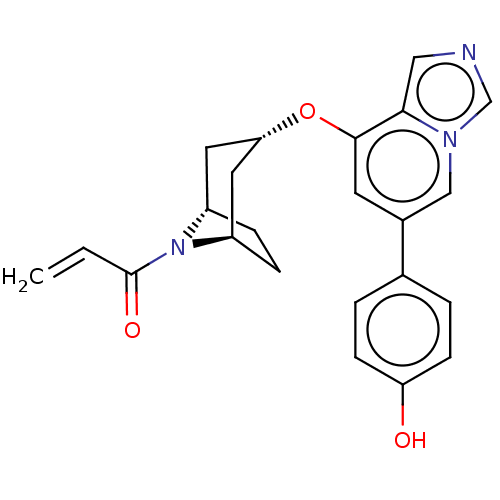 (US10851102, Example 10 | US11339160, Ex No. 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | US Patent US10851102 (2020) BindingDB Entry DOI: 10.7270/Q2N87DV3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM475619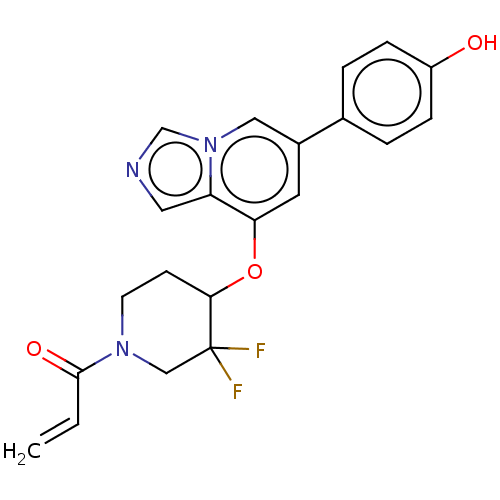 (US10851102, Example 14 | US11339160, Ex No. 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | US Patent US10851102 (2020) BindingDB Entry DOI: 10.7270/Q2N87DV3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3445 total ) | Next | Last >> |