 Found 2462 hits with Last Name = 'nie' and Initial = 't'
Found 2462 hits with Last Name = 'nie' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
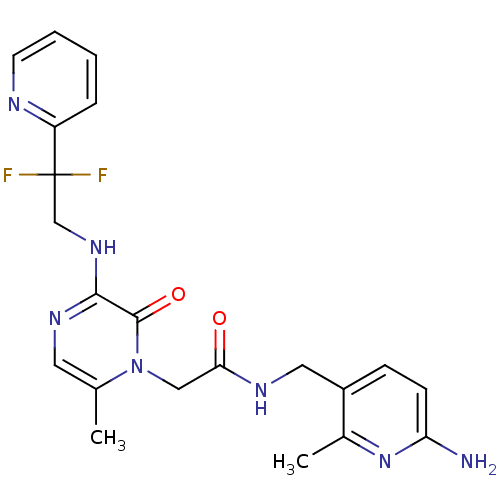
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126304
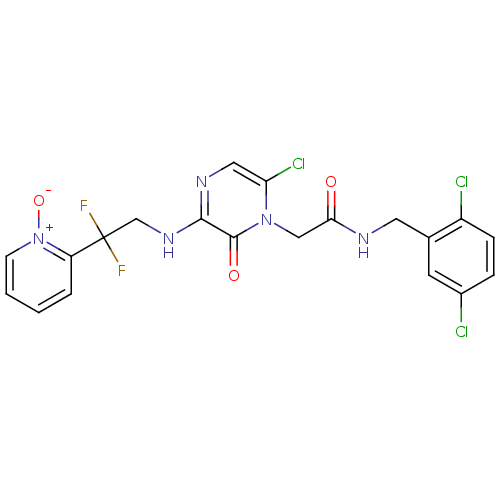
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2Cl)c1=O Show InChI InChI=1S/C20H16Cl3F2N5O3/c21-13-4-5-14(22)12(7-13)8-26-17(31)10-29-16(23)9-27-18(19(29)32)28-11-20(24,25)15-3-1-2-6-30(15)33/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50454822
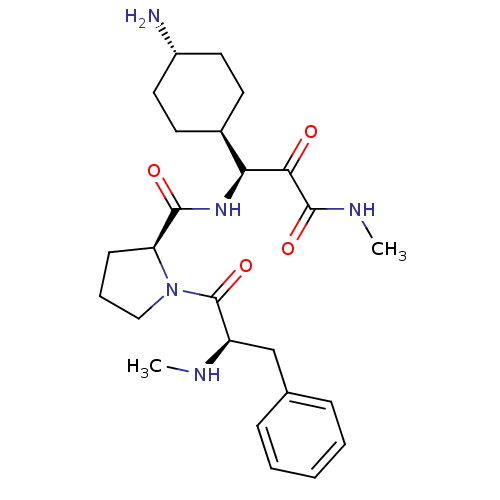
(CHEMBL2062141 | L-370518)Show SMILES [H][C@@](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)NC)(C(=O)C(=O)NC)[C@@]1([H])CC[C@H](N)CC1 |wU:1.0,wD:12.13,5.4,32.34,28.30,(9.54,-15.25,;8.45,-16.34,;7.42,-17.49,;5.92,-17.17,;5.44,-15.7,;4.89,-18.31,;5.21,-19.82,;3.87,-20.59,;2.73,-19.56,;3.35,-18.15,;2.58,-16.82,;3.35,-15.48,;1.04,-16.82,;.27,-15.48,;1.04,-14.15,;2.58,-14.15,;3.35,-12.82,;2.58,-11.48,;1.04,-11.48,;.27,-12.82,;.27,-18.15,;-1.27,-18.15,;7.98,-14.88,;6.47,-14.56,;9.01,-13.73,;10.51,-14.05,;8.53,-12.27,;9.56,-11.12,;9.96,-16.66,;8.87,-17.75,;10.99,-15.52,;12.5,-15.84,;12.97,-17.3,;14.48,-17.62,;11.94,-18.45,;10.44,-18.13,)| Show InChI InChI=1S/C25H37N5O4/c1-27-19(15-16-7-4-3-5-8-16)25(34)30-14-6-9-20(30)23(32)29-21(22(31)24(33)28-2)17-10-12-18(26)13-11-17/h3-5,7-8,17-21,27H,6,9-15,26H2,1-2H3,(H,28,33)(H,29,32)/t17-,18-,19-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123504
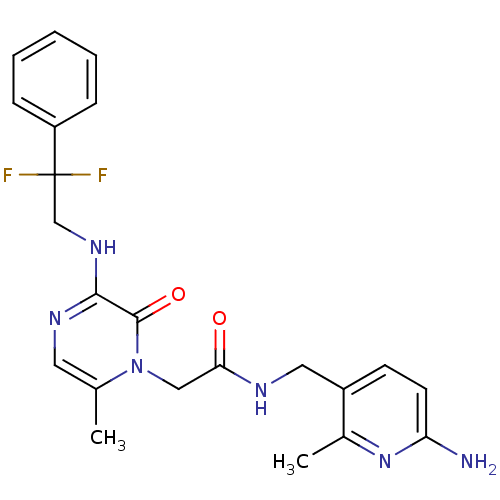
(CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24F2N6O2/c1-14-10-27-20(28-13-22(23,24)17-6-4-3-5-7-17)21(32)30(14)12-19(31)26-11-16-8-9-18(25)29-15(16)2/h3-10H,11-13H2,1-2H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82071
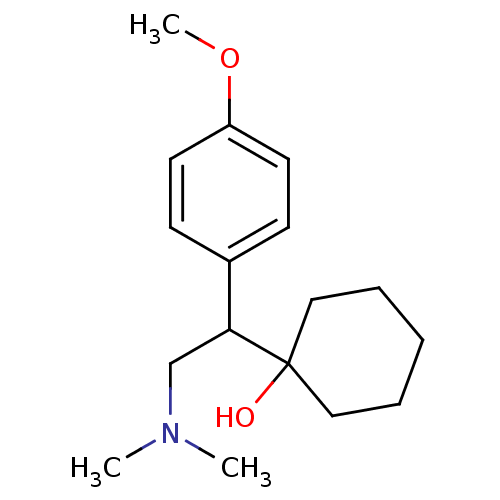
(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057828

((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...)Show SMILES N[C@H](C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.37,-6.36,;9.7,-5.59,;9.7,-4.05,;11.04,-3.28,;12.58,-3.28,;13.35,-1.94,;12.56,-.61,;11.02,-.62,;10.27,-1.96,;8.35,-3.28,;8.35,-1.74,;7.03,-.97,;5.69,-1.74,;5.69,-3.28,;7.03,-4.05,;11.05,-6.36,;11.05,-7.9,;12.36,-5.59,;12.52,-4.05,;14.03,-3.73,;14.8,-5.05,;13.77,-6.2,;14.1,-7.71,;12.96,-8.74,;15.57,-8.18,;16.72,-7.15,;18.17,-7.61,;18.09,-8.95,;19.1,-10.37,;17.98,-11.46,;19.06,-12.54,;18.09,-10.21,;17.07,-8.71,)| Show InChI InChI=1S/C27H36N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,19,22-25H,7,12-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was determined |
J Med Chem 41: 401-6 (1998)
Article DOI: 10.1021/jm9705014
BindingDB Entry DOI: 10.7270/Q2H995V2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070824
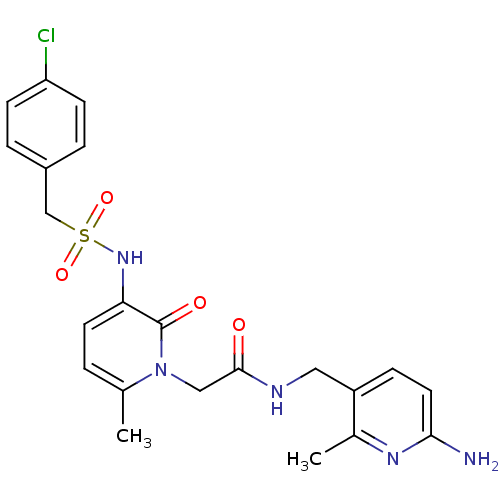
(CHEMBL47920 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccc(Cl)cc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24ClN5O4S/c1-14-3-9-19(27-33(31,32)13-16-4-7-18(23)8-5-16)22(30)28(14)12-21(29)25-11-17-6-10-20(24)26-15(17)2/h3-10,27H,11-13H2,1-2H3,(H2,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated to inhibit the thrombin enzyme |
Bioorg Med Chem Lett 8: 1719-24 (1999)
BindingDB Entry DOI: 10.7270/Q2319V13 |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126303

(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2F)c1=O Show InChI InChI=1S/C20H16Cl2F3N5O3/c21-13-4-5-14(23)12(7-13)8-26-17(31)10-29-16(22)9-27-18(19(29)32)28-11-20(24,25)15-3-1-2-6-30(15)33/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126309

(CHEMBL29744 | N-(3-Bromo-benzyl)-2-{6-chloro-3-[2,...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cccc(Br)c2)c1=O Show InChI InChI=1S/C20H17BrClF2N5O3/c21-14-5-3-4-13(8-14)9-25-17(30)11-28-16(22)10-26-18(19(28)31)27-12-20(23,24)15-6-1-2-7-29(15)32/h1-8,10H,9,11-12H2,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82071

(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM82071

(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126300
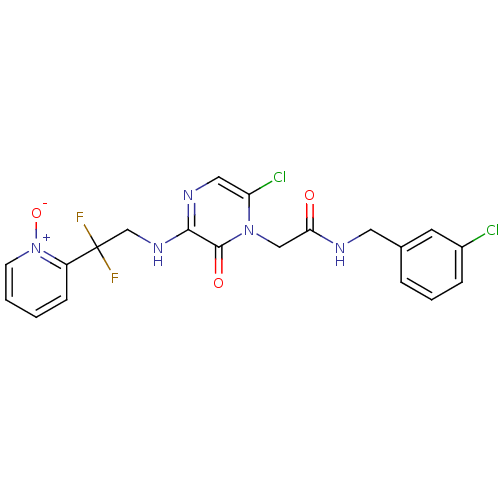
(CHEMBL25845 | N-(3-Chloro-benzyl)-2-{6-chloro-3-[2...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cccc(Cl)c2)c1=O Show InChI InChI=1S/C20H17Cl2F2N5O3/c21-14-5-3-4-13(8-14)9-25-17(30)11-28-16(22)10-26-18(19(28)31)27-12-20(23,24)15-6-1-2-7-29(15)32/h1-8,10H,9,11-12H2,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123496

(CHEMBL143138 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccn2)c1=O Show InChI InChI=1S/C21H25N7O2/c1-14-11-26-20(24-10-8-17-5-3-4-9-23-17)21(30)28(14)13-19(29)25-12-16-6-7-18(22)27-15(16)2/h3-7,9,11H,8,10,12-13H2,1-2H3,(H2,22,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130561
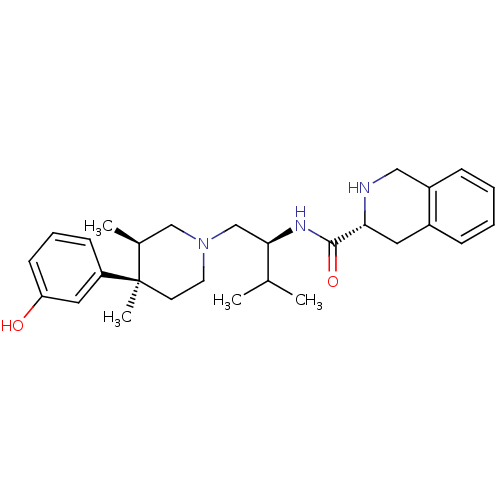
((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H39N3O2/c1-19(2)26(30-27(33)25-14-21-8-5-6-9-22(21)16-29-25)18-31-13-12-28(4,20(3)17-31)23-10-7-11-24(32)15-23/h5-11,15,19-20,25-26,29,32H,12-14,16-18H2,1-4H3,(H,30,33)/t20-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418481
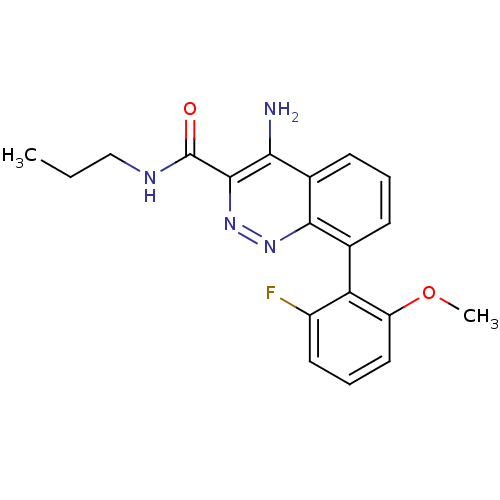
(CHEMBL1783282)Show SMILES CCCNC(=O)c1nnc2c(cccc2c1N)-c1c(F)cccc1OC |(3.52,-21.84,;2.19,-22.61,;.85,-21.84,;-.48,-22.61,;-1.81,-21.84,;-1.81,-20.3,;-3.15,-22.61,;-3.16,-24.17,;-4.5,-24.95,;-5.84,-24.18,;-7.18,-24.95,;-8.51,-24.18,;-8.51,-22.63,;-7.18,-21.86,;-5.85,-22.63,;-4.51,-21.84,;-4.52,-20.3,;-7.18,-26.49,;-5.85,-27.25,;-4.52,-26.47,;-5.85,-28.78,;-7.19,-29.56,;-8.52,-28.78,;-8.52,-27.25,;-9.85,-26.47,;-9.84,-24.93,)| Show InChI InChI=1S/C19H19FN4O2/c1-3-10-22-19(25)18-16(21)12-7-4-6-11(17(12)23-24-18)15-13(20)8-5-9-14(15)26-2/h4-9H,3,10H2,1-2H3,(H2,21,23)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50066331
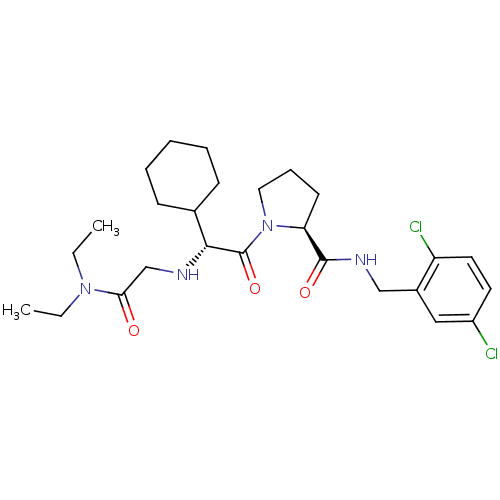
((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...)Show SMILES CCN(CC)C(=O)CN[C@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1Cl Show InChI InChI=1S/C26H38Cl2N4O3/c1-3-31(4-2)23(33)17-29-24(18-9-6-5-7-10-18)26(35)32-14-8-11-22(32)25(34)30-16-19-15-20(27)12-13-21(19)28/h12-13,15,18,22,24,29H,3-11,14,16-17H2,1-2H3,(H,30,34)/t22-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated on serine protease thrombin. |
J Med Chem 41: 3210-9 (1998)
Article DOI: 10.1021/jm9801713
BindingDB Entry DOI: 10.7270/Q24Q7T4G |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069189
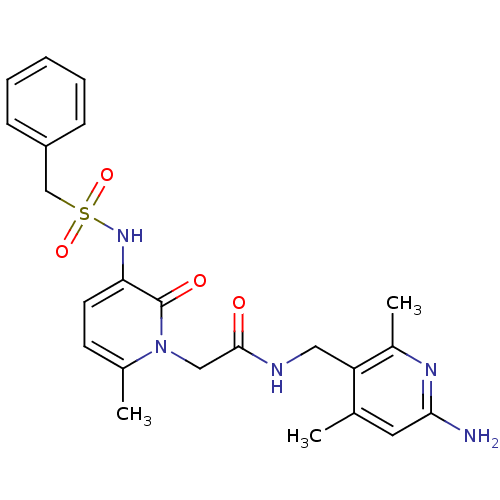
(CHEMBL353431 | N-(6-Amino-2,4-dimethyl-pyridin-3-y...)Show SMILES Cc1cc(N)nc(C)c1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C23H27N5O4S/c1-15-11-21(24)26-17(3)19(15)12-25-22(29)13-28-16(2)9-10-20(23(28)30)27-33(31,32)14-18-7-5-4-6-8-18/h4-11,27H,12-14H2,1-3H3,(H2,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was evaluated |
Bioorg Med Chem Lett 8: 817-22 (1999)
BindingDB Entry DOI: 10.7270/Q2JH3K9Z |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067795

(CHEMBL138855 | N-(6-Amino-2,4-dimethyl-pyridin-3-y...)Show SMILES Cc1cc(N)nc(C)c1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C23H28N6O2/c1-15-11-20(24)28-17(3)19(15)13-26-21(30)14-29-16(2)12-27-22(23(29)31)25-10-9-18-7-5-4-6-8-18/h4-8,11-12H,9-10,13-14H2,1-3H3,(H2,24,28)(H,25,27)(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against thrombin (IIa) was determined |
J Med Chem 41: 4466-74 (1998)
Article DOI: 10.1021/jm980368v
BindingDB Entry DOI: 10.7270/Q2KK99X0 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418483
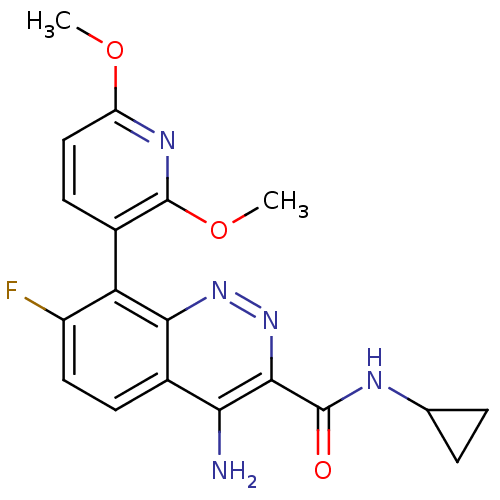
(CHEMBL1783284)Show SMILES COc1ccc(c(OC)n1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(-7.37,-43.23,;-6.03,-42.46,;-6.03,-40.92,;-4.69,-40.15,;-4.69,-38.61,;-6.02,-37.85,;-7.36,-38.61,;-8.69,-37.83,;-10.03,-38.6,;-7.36,-40.15,;-6.02,-36.31,;-7.35,-35.54,;-8.69,-36.31,;-7.35,-34,;-6.02,-33.23,;-4.69,-33.99,;-3.35,-33.2,;-3.36,-31.66,;-1.99,-33.98,;-2,-35.54,;-3.34,-36.31,;-4.68,-35.54,;-.65,-33.21,;-.65,-31.67,;.68,-33.98,;2.01,-33.21,;3.55,-33.2,;2.78,-31.87,)| Show InChI InChI=1S/C19H18FN5O3/c1-27-13-8-6-10(19(23-13)28-2)14-12(20)7-5-11-15(21)17(25-24-16(11)14)18(26)22-9-3-4-9/h5-9H,3-4H2,1-2H3,(H2,21,24)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123486
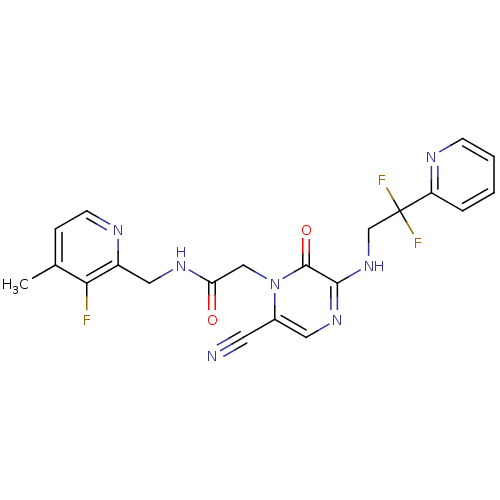
(2-[6-Cyano-3-(2,2-difluoro-2-pyridin-2-yl-ethylami...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(cnc(NCC(F)(F)c3ccccn3)c2=O)C#N)c1F Show InChI InChI=1S/C21H18F3N7O2/c1-13-5-7-26-15(18(13)22)10-28-17(32)11-31-14(8-25)9-29-19(20(31)33)30-12-21(23,24)16-4-2-3-6-27-16/h2-7,9H,10-12H2,1H3,(H,28,32)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418482
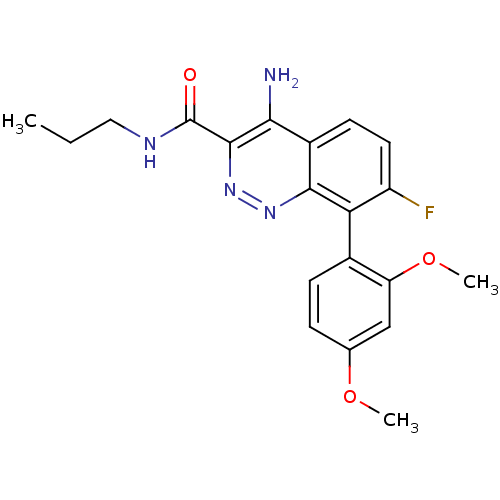
(CHEMBL1783283)Show SMILES CCCNC(=O)c1nnc2c(c(F)ccc2c1N)-c1ccc(OC)cc1OC |(30.61,-19.8,;29.28,-20.57,;27.94,-19.8,;26.61,-20.57,;25.28,-19.8,;25.28,-18.26,;23.94,-20.57,;23.93,-22.13,;22.59,-22.9,;21.25,-22.14,;19.91,-22.9,;18.58,-22.13,;17.24,-22.9,;18.58,-20.59,;19.91,-19.82,;21.24,-20.58,;22.58,-19.79,;22.57,-18.25,;19.91,-24.44,;21.24,-25.2,;21.24,-26.74,;19.9,-27.51,;19.9,-29.05,;18.57,-29.82,;18.57,-26.74,;18.57,-25.2,;17.24,-24.42,;15.9,-25.19,)| Show InChI InChI=1S/C20H21FN4O3/c1-4-9-23-20(26)19-17(22)13-7-8-14(21)16(18(13)24-25-19)12-6-5-11(27-2)10-15(12)28-3/h5-8,10H,4,9H2,1-3H3,(H2,22,24)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50066338
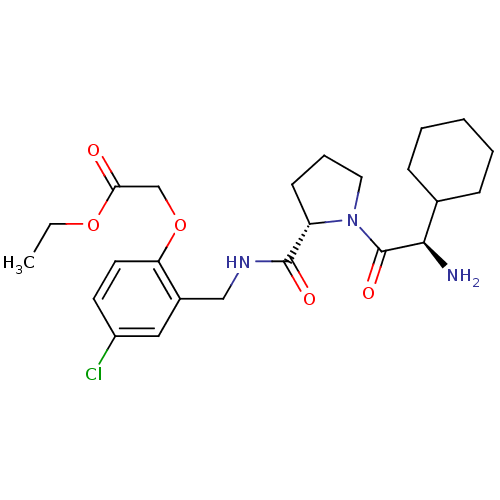
(CHEMBL327115 | [2-({[(S)-1-((R)-2-Amino-2-cyclohex...)Show SMILES CCOC(=O)COc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@H](N)C1CCCCC1 Show InChI InChI=1S/C24H34ClN3O5/c1-2-32-21(29)15-33-20-11-10-18(25)13-17(20)14-27-23(30)19-9-6-12-28(19)24(31)22(26)16-7-4-3-5-8-16/h10-11,13,16,19,22H,2-9,12,14-15,26H2,1H3,(H,27,30)/t19-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated on serine protease thrombin. |
J Med Chem 41: 3210-9 (1998)
Article DOI: 10.1021/jm9801713
BindingDB Entry DOI: 10.7270/Q24Q7T4G |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50066332

((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...)Show SMILES N[C@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1OCC(N)=O Show InChI InChI=1S/C22H31ClN4O4/c23-16-8-9-18(31-13-19(24)28)15(11-16)12-26-21(29)17-7-4-10-27(17)22(30)20(25)14-5-2-1-3-6-14/h8-9,11,14,17,20H,1-7,10,12-13,25H2,(H2,24,28)(H,26,29)/t17-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated on serine protease thrombin. |
J Med Chem 41: 3210-9 (1998)
Article DOI: 10.1021/jm9801713
BindingDB Entry DOI: 10.7270/Q24Q7T4G |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327257
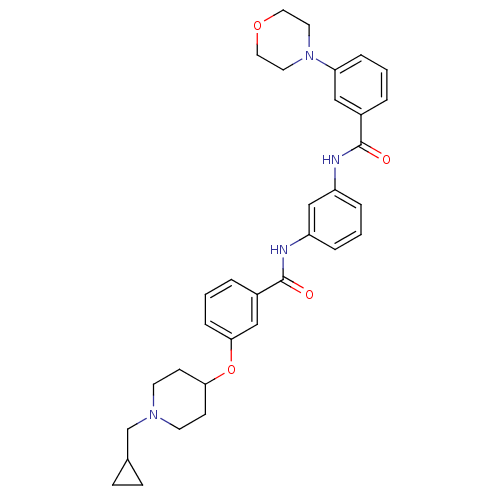
(3-(1-(cyclopropylmethyl)piperidin-4-yloxy)-N-(3-(3...)Show SMILES O=C(Nc1cccc(NC(=O)c2cccc(c2)N2CCOCC2)c1)c1cccc(OC2CCN(CC3CC3)CC2)c1 Show InChI InChI=1S/C33H38N4O4/c38-32(25-4-1-8-29(20-25)37-16-18-40-19-17-37)34-27-6-3-7-28(22-27)35-33(39)26-5-2-9-31(21-26)41-30-12-14-36(15-13-30)23-24-10-11-24/h1-9,20-22,24,30H,10-19,23H2,(H,34,38)(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067796
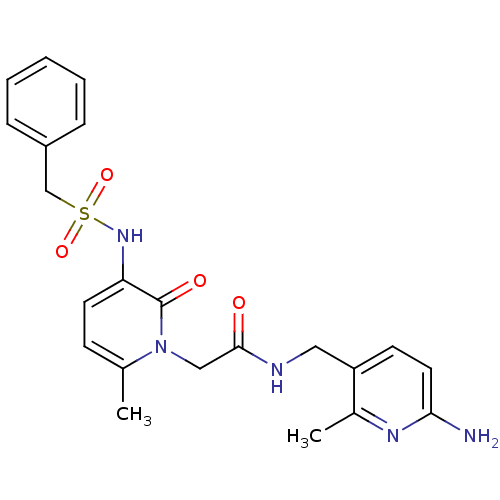
(CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C22H25N5O4S/c1-15-8-10-19(26-32(30,31)14-17-6-4-3-5-7-17)22(29)27(15)13-21(28)24-12-18-9-11-20(23)25-16(18)2/h3-11,26H,12-14H2,1-2H3,(H2,23,25)(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was evaluated |
Bioorg Med Chem Lett 8: 817-22 (1999)
BindingDB Entry DOI: 10.7270/Q2JH3K9Z |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067796

(CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C22H25N5O4S/c1-15-8-10-19(26-32(30,31)14-17-6-4-3-5-7-17)22(29)27(15)13-21(28)24-12-18-9-11-20(23)25-16(18)2/h3-11,26H,12-14H2,1-2H3,(H2,23,25)(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated to inhibit the thrombin enzyme |
Bioorg Med Chem Lett 8: 1719-24 (1999)
BindingDB Entry DOI: 10.7270/Q2319V13 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50069190

(CHEMBL287614 | N-(1-Carbamimidoyl-piperidin-4-ylme...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCC1CCN(CC1)C(N)=N Show InChI InChI=1S/C22H30N6O4S/c1-16-7-8-19(26-33(31,32)15-18-5-3-2-4-6-18)21(30)28(16)14-20(29)25-13-17-9-11-27(12-10-17)22(23)24/h2-8,17,26H,9-15H2,1H3,(H3,23,24)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin was evaluated |
Bioorg Med Chem Lett 8: 817-22 (1999)
BindingDB Entry DOI: 10.7270/Q2JH3K9Z |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067796

(CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C22H25N5O4S/c1-15-8-10-19(26-32(30,31)14-17-6-4-3-5-7-17)22(29)27(15)13-21(28)24-12-18-9-11-20(23)25-16(18)2/h3-11,26H,12-14H2,1-2H3,(H2,23,25)(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against thrombin (IIa) was determined |
J Med Chem 41: 4466-74 (1998)
Article DOI: 10.1021/jm980368v
BindingDB Entry DOI: 10.7270/Q2KK99X0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067798

(CHEMBL336438 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES C[C@H](Cc1ccccc1)Nc1ncc(C)n(CC(=O)NCc2ccc(N)nc2C)c1=O Show InChI InChI=1S/C23H28N6O2/c1-15(11-18-7-5-4-6-8-18)27-22-23(31)29(16(2)12-26-22)14-21(30)25-13-19-9-10-20(24)28-17(19)3/h4-10,12,15H,11,13-14H2,1-3H3,(H2,24,28)(H,25,30)(H,26,27)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against thrombin (IIa) was determined |
J Med Chem 41: 4466-74 (1998)
Article DOI: 10.1021/jm980368v
BindingDB Entry DOI: 10.7270/Q2KK99X0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123479
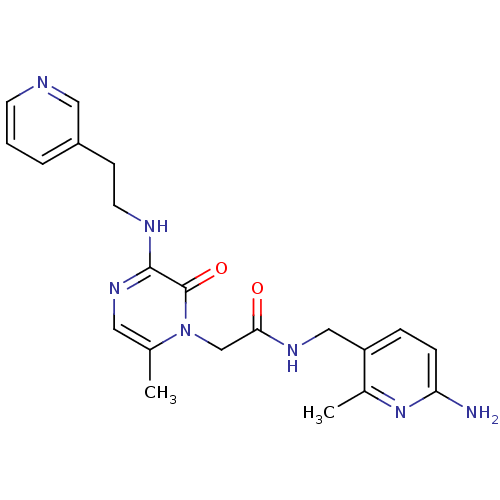
(CHEMBL143008 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2cccnc2)c1=O Show InChI InChI=1S/C21H25N7O2/c1-14-10-26-20(24-9-7-16-4-3-8-23-11-16)21(30)28(14)13-19(29)25-12-17-5-6-18(22)27-15(17)2/h3-6,8,10-11H,7,9,12-13H2,1-2H3,(H2,22,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418488

(CHEMBL1783285)Show SMILES COc1cc(c(OC)nn1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(23.34,-39.57,;22.01,-40.34,;20.67,-39.57,;20.67,-38.03,;19.34,-37.28,;18,-38.03,;16.67,-37.26,;15.34,-38.02,;18,-39.57,;19.33,-40.35,;19.34,-35.74,;18.01,-34.97,;16.67,-35.73,;18.01,-33.42,;19.34,-32.65,;20.67,-33.41,;22.01,-32.63,;22,-31.09,;23.38,-33.4,;23.37,-34.96,;22.02,-35.74,;20.68,-34.97,;24.71,-32.63,;24.71,-31.09,;26.04,-33.4,;27.38,-32.63,;28.91,-32.63,;28.14,-31.29,)| Show InChI InChI=1S/C18H17FN6O3/c1-27-12-7-10(18(28-2)25-22-12)13-11(19)6-5-9-14(20)16(24-23-15(9)13)17(26)21-8-3-4-8/h5-8H,3-4H2,1-2H3,(H2,20,23)(H,21,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50066333
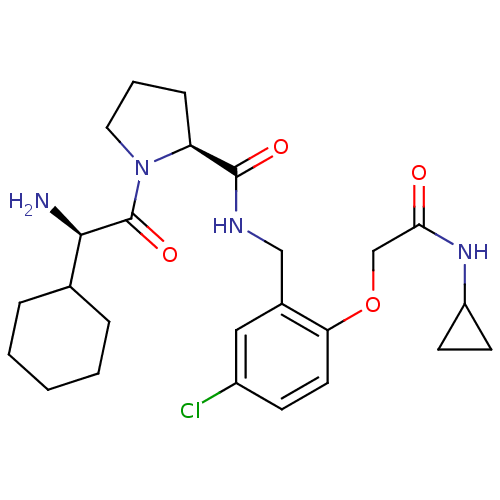
((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...)Show SMILES N[C@H](C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCc1cc(Cl)ccc1OCC(=O)NC1CC1 Show InChI InChI=1S/C25H35ClN4O4/c26-18-8-11-21(34-15-22(31)29-19-9-10-19)17(13-18)14-28-24(32)20-7-4-12-30(20)25(33)23(27)16-5-2-1-3-6-16/h8,11,13,16,19-20,23H,1-7,9-10,12,14-15,27H2,(H,28,32)(H,29,31)/t20-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated on serine protease thrombin. |
J Med Chem 41: 3210-9 (1998)
Article DOI: 10.1021/jm9801713
BindingDB Entry DOI: 10.7270/Q24Q7T4G |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM82071

(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50066334
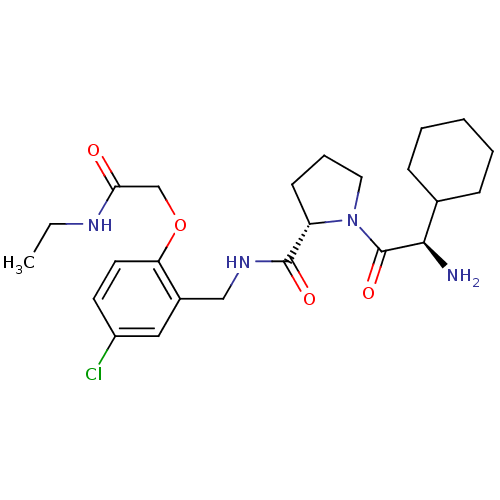
((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...)Show SMILES CCNC(=O)COc1ccc(Cl)cc1CNC(=O)[C@@H]1CCCN1C(=O)[C@H](N)C1CCCCC1 Show InChI InChI=1S/C24H35ClN4O4/c1-2-27-21(30)15-33-20-11-10-18(25)13-17(20)14-28-23(31)19-9-6-12-29(19)24(32)22(26)16-7-4-3-5-8-16/h10-11,13,16,19,22H,2-9,12,14-15,26H2,1H3,(H,27,30)(H,28,31)/t19-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated on serine protease thrombin. |
J Med Chem 41: 3210-9 (1998)
Article DOI: 10.1021/jm9801713
BindingDB Entry DOI: 10.7270/Q24Q7T4G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Norepinephrine transporter
(RAT) | BDBM82071

(CAS_93413-69-5 | CAS_99300-78-4 | NSC_62923 | VENL...)Show InChI InChI=1S/C17H27NO2/c1-18(2)13-16(17(19)11-5-4-6-12-17)14-7-9-15(20-3)10-8-14/h7-10,16,19H,4-6,11-13H2,1-3H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126295

(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES Cc1cccc(CNC(=O)Cn2c(Cl)cnc(NCC(F)(F)c3cccc[n+]3[O-])c2=O)c1 Show InChI InChI=1S/C21H20ClF2N5O3/c1-14-5-4-6-15(9-14)10-25-18(30)12-28-17(22)11-26-19(20(28)31)27-13-21(23,24)16-7-2-3-8-29(16)32/h2-9,11H,10,12-13H2,1H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067797
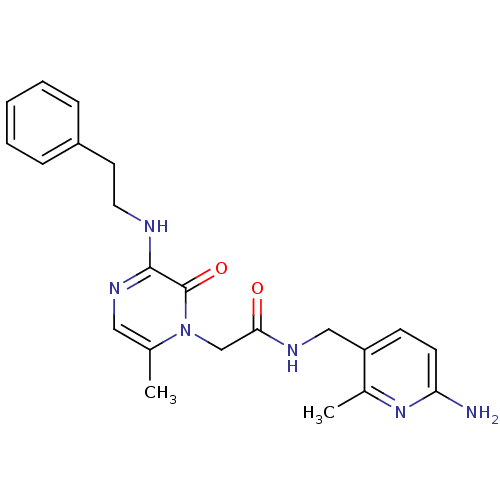
(CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C22H26N6O2/c1-15-12-26-21(24-11-10-17-6-4-3-5-7-17)22(30)28(15)14-20(29)25-13-18-8-9-19(23)27-16(18)2/h3-9,12H,10-11,13-14H2,1-2H3,(H2,23,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against thrombin (IIa) was determined |
J Med Chem 41: 4466-74 (1998)
Article DOI: 10.1021/jm980368v
BindingDB Entry DOI: 10.7270/Q2KK99X0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50067797

(CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C22H26N6O2/c1-15-12-26-21(24-11-10-17-6-4-3-5-7-17)22(30)28(15)14-20(29)25-13-18-8-9-19(23)27-16(18)2/h3-9,12H,10-11,13-14H2,1-2H3,(H2,23,27)(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126294
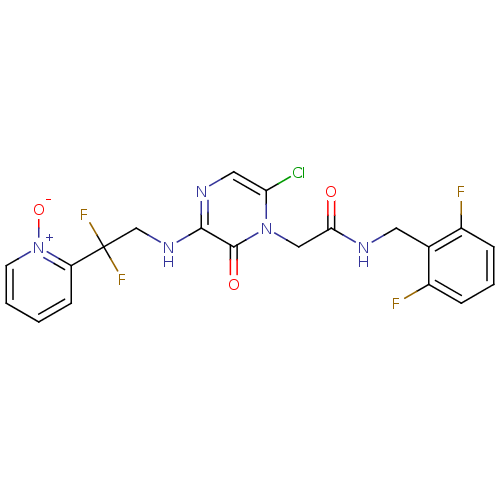
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2c(F)cccc2F)c1=O Show InChI InChI=1S/C20H16ClF4N5O3/c21-16-9-27-18(28-11-20(24,25)15-6-1-2-7-30(15)33)19(32)29(16)10-17(31)26-8-12-13(22)4-3-5-14(12)23/h1-7,9H,8,10-11H2,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070823

(CHEMBL45480 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...)Show SMILES CCCc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C24H29N5O4S/c1-3-7-20-11-12-21(28-34(32,33)16-18-8-5-4-6-9-18)24(31)29(20)15-23(30)26-14-19-10-13-22(25)27-17(19)2/h4-6,8-13,28H,3,7,14-16H2,1-2H3,(H2,25,27)(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated to inhibit the thrombin enzyme |
Bioorg Med Chem Lett 8: 1719-24 (1999)
BindingDB Entry DOI: 10.7270/Q2319V13 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123497
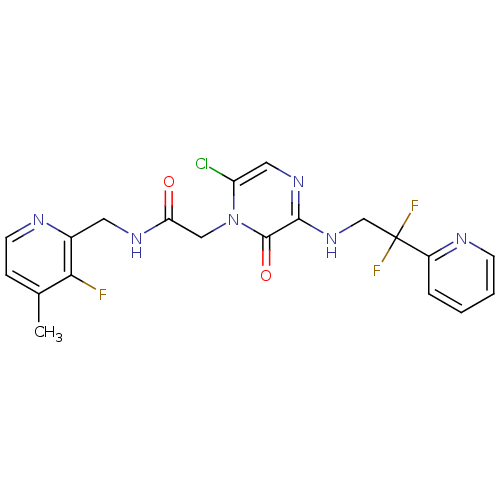
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(Cl)cnc(NCC(F)(F)c3ccccn3)c2=O)c1F Show InChI InChI=1S/C20H18ClF3N6O2/c1-12-5-7-25-13(17(12)22)8-27-16(31)10-30-15(21)9-28-18(19(30)32)29-11-20(23,24)14-4-2-3-6-26-14/h2-7,9H,8,10-11H2,1H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418445

(CHEMBL1783245)Show InChI InChI=1S/C16H22N4O/c1-4-8-18-16(21)15-13(17)12-7-5-6-11(9-10(2)3)14(12)19-20-15/h5-7,10H,4,8-9H2,1-3H3,(H2,17,19)(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418486

(CHEMBL1783276)Show SMILES COc1ccc(OC)c(c1)-c1c(F)ccc2c(N)c3c(cn(C4CCC4)c3=O)[nH]c12 |(-3.15,-48.69,;-3.15,-47.15,;-4.49,-46.38,;-5.83,-47.16,;-7.16,-46.39,;-7.16,-44.85,;-8.49,-44.07,;-9.83,-44.83,;-5.82,-44.09,;-4.49,-44.85,;-5.82,-42.55,;-7.15,-41.78,;-8.49,-42.55,;-7.15,-40.23,;-5.82,-39.46,;-4.49,-40.23,;-3.15,-39.44,;-3.16,-37.9,;-1.79,-40.22,;-1.8,-41.78,;-.31,-42.27,;.61,-41.01,;2.15,-41.02,;3.23,-42.12,;4.32,-41.04,;3.24,-39.94,;-.3,-39.74,;.19,-38.28,;-3.14,-42.55,;-4.48,-41.78,)| Show InChI InChI=1S/C23H22FN3O3/c1-29-13-6-9-18(30-2)15(10-13)19-16(24)8-7-14-21(25)20-17(26-22(14)19)11-27(23(20)28)12-4-3-5-12/h6-12,26H,3-5,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123503
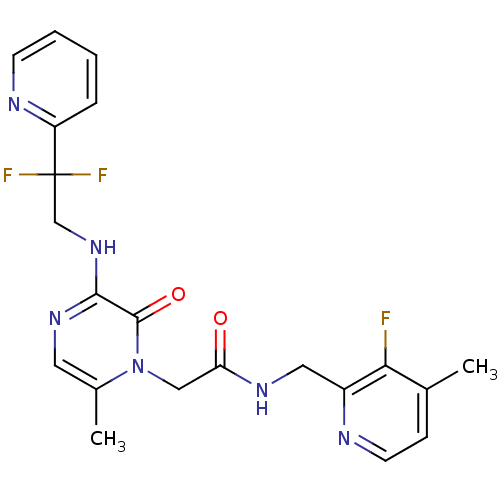
(2-[3-(2,2-Difluoro-2-pyridin-2-yl-ethylamino)-6-me...)Show SMILES Cc1ccnc(CNC(=O)Cn2c(C)cnc(NCC(F)(F)c3ccccn3)c2=O)c1F Show InChI InChI=1S/C21H21F3N6O2/c1-13-6-8-25-15(18(13)22)10-27-17(31)11-30-14(2)9-28-19(20(30)32)29-12-21(23,24)16-5-3-4-7-26-16/h3-9H,10-12H2,1-2H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123500

(CHEMBL143139 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1nc(N)ccc1CNC(=O)Cn1c(C)cnc(NCC(C)(C)c2ccccc2)c1=O Show InChI InChI=1S/C24H30N6O2/c1-16-12-27-22(28-15-24(3,4)19-8-6-5-7-9-19)23(32)30(16)14-21(31)26-13-18-10-11-20(25)29-17(18)2/h5-12H,13-15H2,1-4H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123493
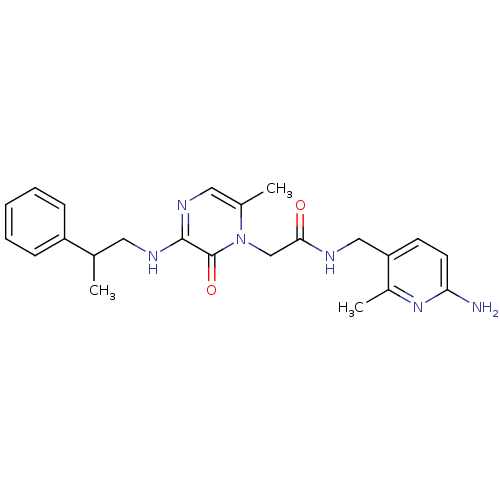
(CHEMBL142566 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES CC(CNc1ncc(C)n(CC(=O)NCc2ccc(N)nc2C)c1=O)c1ccccc1 Show InChI InChI=1S/C23H28N6O2/c1-15(18-7-5-4-6-8-18)11-26-22-23(31)29(16(2)12-27-22)14-21(30)25-13-19-9-10-20(24)28-17(19)3/h4-10,12,15H,11,13-14H2,1-3H3,(H2,24,28)(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126306

(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2c(F)ccc(F)c2F)c1=O Show InChI InChI=1S/C20H15ClF5N5O3/c21-15-8-28-18(29-10-20(25,26)14-3-1-2-6-31(14)34)19(33)30(15)9-16(32)27-7-11-12(22)4-5-13(23)17(11)24/h1-6,8H,7,9-10H2,(H,27,32)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
Bioorg Med Chem Lett 13: 1353-7 (2003)
BindingDB Entry DOI: 10.7270/Q2833RC9 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-2/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50418472
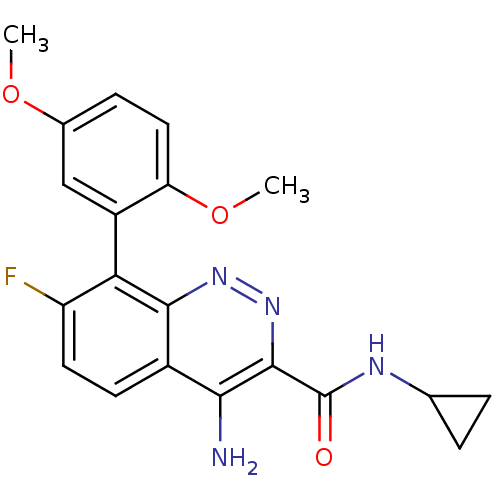
(CHEMBL1783271)Show SMILES COc1ccc(OC)c(c1)-c1c(F)ccc2c(N)c(nnc12)C(=O)NC1CC1 |(23.64,-5.48,;23.63,-3.94,;22.3,-3.17,;20.96,-3.95,;19.63,-3.17,;19.63,-1.63,;18.3,-.86,;16.96,-1.62,;20.97,-.88,;22.29,-1.63,;20.97,.66,;19.63,1.43,;18.3,.67,;19.64,2.98,;20.97,3.75,;22.3,2.99,;23.64,3.77,;23.63,5.31,;24.99,3,;24.99,1.44,;23.65,.66,;22.31,1.43,;26.33,3.78,;26.32,5.32,;27.66,3.01,;28.99,3.78,;30.53,3.78,;29.75,5.12,)| Show InChI InChI=1S/C20H19FN4O3/c1-27-11-5-8-15(28-2)13(9-11)16-14(21)7-6-12-17(22)19(25-24-18(12)16)20(26)23-10-3-4-10/h5-10H,3-4H2,1-2H3,(H2,22,24)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]flunitrazepam from benzodiazepine binding site GABAA alpha2beta3gamma2 receptor expressed in Sf9 cells after 1 hr |
Bioorg Med Chem 19: 2927-38 (2011)
Article DOI: 10.1016/j.bmc.2011.03.035
BindingDB Entry DOI: 10.7270/Q29S1S98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data