 Found 2331 hits with Last Name = 'sim' and Initial = 't'
Found 2331 hits with Last Name = 'sim' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
GTPase KRas
(Homo sapiens (Human)) | BDBM50501345

(CHEMBL3958857)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H21ClN6O12P2/c15-3-7(22)17-1-2-30-34(26,27)33-35(28,29)31-4-6-9(23)10(24)13(32-6)21-5-18-8-11(21)19-14(16)20-12(8)25/h5-6,9-10,13,23-24H,1-4H2,(H,17,22)(H,26,27)(H,28,29)(H3,16,19,20,25)/t6-,9-,10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50156071
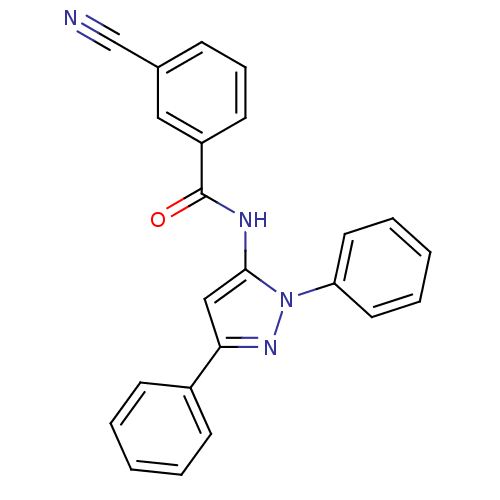
(3-Cyano-N-(2,5-diphenyl-2H-pyrazol-3-yl)-benzamide...)Show SMILES O=C(Nc1cc(nn1-c1ccccc1)-c1ccccc1)c1cccc(c1)C#N Show InChI InChI=1S/C23H16N4O/c24-16-17-8-7-11-19(14-17)23(28)25-22-15-21(18-9-3-1-4-10-18)26-27(22)20-12-5-2-6-13-20/h1-15H,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEP from human mGluR5 |
Bioorg Med Chem Lett 20: 7381-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.036
BindingDB Entry DOI: 10.7270/Q27S7P13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501343

(CHEMBL3922435)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OCCNC(=O)C=C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H22N6O12P2/c1-2-8(22)17-3-4-30-34(26,27)33-35(28,29)31-5-7-10(23)11(24)14(32-7)21-6-18-9-12(21)19-15(16)20-13(9)25/h2,6-7,10-11,14,23-24H,1,3-5H2,(H,17,22)(H,26,27)(H,28,29)(H3,16,19,20,25)/t7-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501342

(CHEMBL3904433)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OCCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H25ClN6O11P2/c17-4-9(24)19-2-1-3-32-35(28,29)7-36(30,31)33-5-8-11(25)12(26)15(34-8)23-6-20-10-13(23)21-16(18)22-14(10)27/h6,8,11-12,15,25-26H,1-5,7H2,(H,19,24)(H,28,29)(H,30,31)(H3,18,21,22,27)/t8-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501347
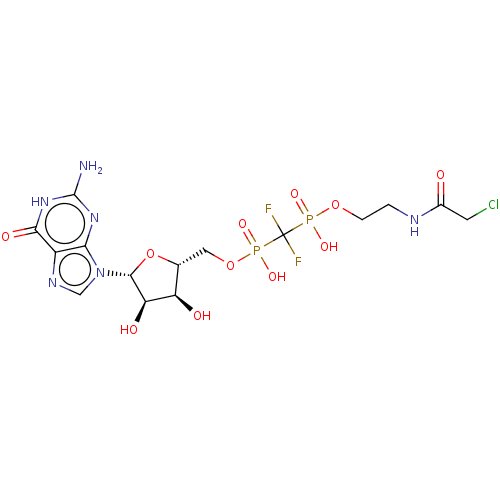
(CHEMBL3935647)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)C(F)(F)P(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H21ClF2N6O11P2/c16-3-7(25)20-1-2-33-36(29,30)15(17,18)37(31,32)34-4-6-9(26)10(27)13(35-6)24-5-21-8-11(24)22-14(19)23-12(8)28/h5-6,9-10,13,26-27H,1-4H2,(H,20,25)(H,29,30)(H,31,32)(H3,19,22,23,28)/t6-,9-,10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501344

(CHEMBL3932440)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)C(F)(F)P(O)(=O)OCCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H23ClF2N6O11P2/c17-4-8(26)21-2-1-3-34-37(30,31)16(18,19)38(32,33)35-5-7-10(27)11(28)14(36-7)25-6-22-9-12(25)23-15(20)24-13(9)29/h6-7,10-11,14,27-28H,1-5H2,(H,21,26)(H,30,31)(H,32,33)(H3,20,23,24,29)/t7-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119291
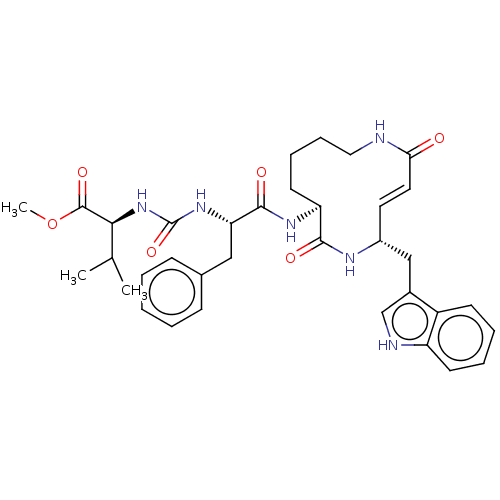
(CHEMBL3613827)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C |r,t:29| Show InChI InChI=1S/C35H44N6O6/c1-22(2)31(34(45)47-3)41-35(46)40-29(19-23-11-5-4-6-12-23)33(44)39-28-15-9-10-18-36-30(42)17-16-25(38-32(28)43)20-24-21-37-27-14-8-7-13-26(24)27/h4-8,11-14,16-17,21-22,25,28-29,31,37H,9-10,15,18-20H2,1-3H3,(H,36,42)(H,38,43)(H,39,44)(H2,40,41,46)/b17-16+/t25-,28+,29+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119290
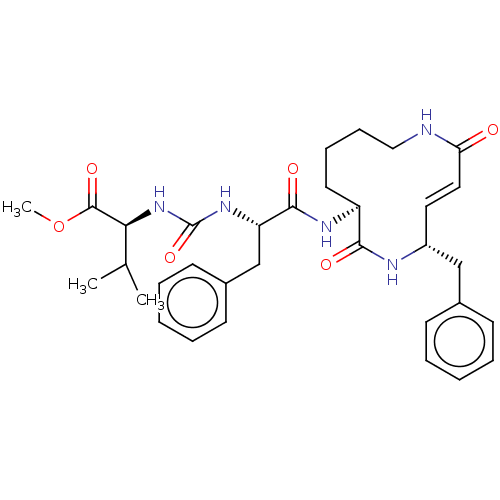
(CHEMBL3613826)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2)NC1=O)C(C)C |r,t:29| Show InChI InChI=1S/C33H43N5O6/c1-22(2)29(32(42)44-3)38-33(43)37-27(21-24-14-8-5-9-15-24)31(41)36-26-16-10-11-19-34-28(39)18-17-25(35-30(26)40)20-23-12-6-4-7-13-23/h4-9,12-15,17-18,22,25-27,29H,10-11,16,19-21H2,1-3H3,(H,34,39)(H,35,40)(H,36,41)(H2,37,38,43)/b18-17+/t25-,26+,27+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119297

(CHEMBL3613817)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(F)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42FN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-11-6-7-14-31-23(36)13-12-21(32-26(22)37)16-19-9-8-10-20(30)15-19/h8-10,12-13,15,17-18,21-22,24-25H,6-7,11,14,16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b13-12+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119294
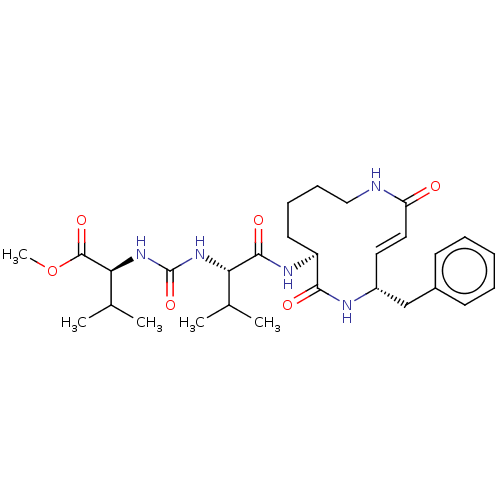
(CHEMBL3613814)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H43N5O6/c1-18(2)24(33-29(39)34-25(19(3)4)28(38)40-5)27(37)32-22-13-9-10-16-30-23(35)15-14-21(31-26(22)36)17-20-11-7-6-8-12-20/h6-8,11-12,14-15,18-19,21-22,24-25H,9-10,13,16-17H2,1-5H3,(H,30,35)(H,31,36)(H,32,37)(H2,33,34,39)/b15-14+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 743 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119296
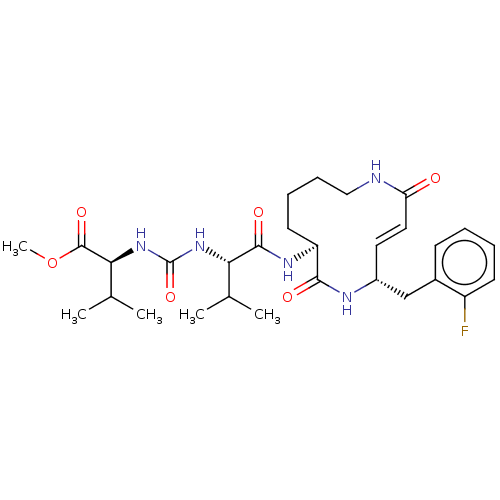
(CHEMBL3613816)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2F)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42FN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-12-8-9-15-31-23(36)14-13-20(32-26(22)37)16-19-10-6-7-11-21(19)30/h6-7,10-11,13-14,17-18,20,22,24-25H,8-9,12,15-16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t20-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 906 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501341

(CHEMBL3897666)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OC2CCN(C2)C(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H25ClN6O11P2/c18-3-10(25)23-2-1-8(4-23)35-37(31,32)7-36(29,30)33-5-9-12(26)13(27)16(34-9)24-6-20-11-14(24)21-17(19)22-15(11)28/h6,8-9,12-13,16,26-27H,1-5,7H2,(H,29,30)(H,31,32)(H3,19,21,22,28)/t8?,9-,12-,13-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119289

(CHEMBL3613825)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@@H](NC1=O)C(C)C)C(C)C |r,t:29| Show InChI InChI=1S/C29H43N5O6/c1-18(2)21-14-15-24(35)30-16-10-9-13-22(26(36)31-21)32-27(37)23(17-20-11-7-6-8-12-20)33-29(39)34-25(19(3)4)28(38)40-5/h6-8,11-12,14-15,18-19,21-23,25H,9-10,13,16-17H2,1-5H3,(H,30,35)(H,31,36)(H,32,37)(H2,33,34,39)/b15-14+/t21-,22+,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119299

(CHEMBL3613818)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cc(F)cc(F)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H41F2N5O6/c1-16(2)24(35-29(41)36-25(17(3)4)28(40)42-5)27(39)34-22-8-6-7-11-32-23(37)10-9-21(33-26(22)38)14-18-12-19(30)15-20(31)13-18/h9-10,12-13,15-17,21-22,24-25H,6-8,11,14H2,1-5H3,(H,32,37)(H,33,38)(H,34,39)(H2,35,36,41)/b10-9+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 952 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119301
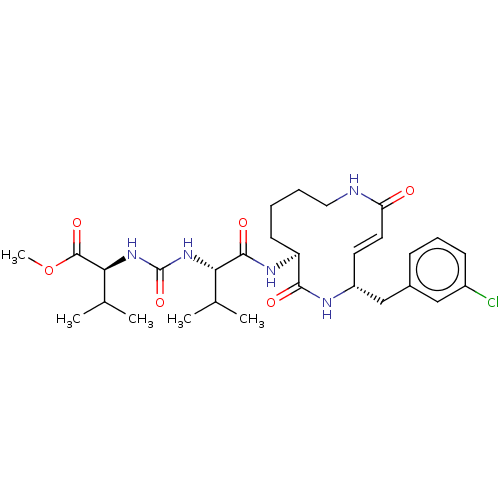
(CHEMBL3613820)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(Cl)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42ClN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-11-6-7-14-31-23(36)13-12-21(32-26(22)37)16-19-9-8-10-20(30)15-19/h8-10,12-13,15,17-18,21-22,24-25H,6-7,11,14,16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b13-12+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 963 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119304

(CHEMBL3613823)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccc(C)cc2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C30H45N5O6/c1-18(2)25(34-30(40)35-26(19(3)4)29(39)41-6)28(38)33-23-9-7-8-16-31-24(36)15-14-22(32-27(23)37)17-21-12-10-20(5)11-13-21/h10-15,18-19,22-23,25-26H,7-9,16-17H2,1-6H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b15-14+/t22-,23+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119302
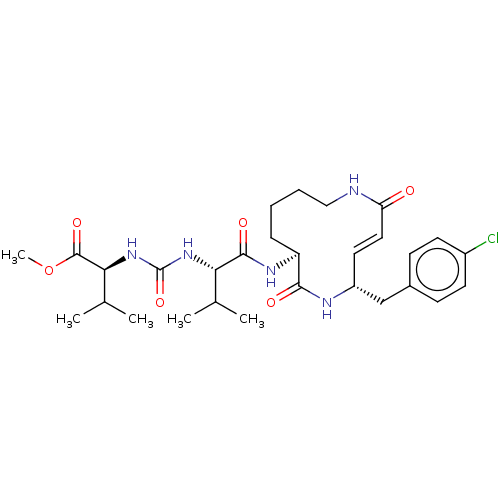
(CHEMBL3613821)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccc(Cl)cc2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42ClN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-8-6-7-15-31-23(36)14-13-21(32-26(22)37)16-19-9-11-20(30)12-10-19/h9-14,17-18,21-22,24-25H,6-8,15-16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501332

(CHEMBL3982443)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CS(=O)(=O)NCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H23ClN7O10PS/c16-3-8(24)18-1-2-20-35(30,31)6-34(28,29)32-4-7-10(25)11(26)14(33-7)23-5-19-9-12(23)21-15(17)22-13(9)27/h5,7,10-11,14,20,25-26H,1-4,6H2,(H,18,24)(H,28,29)(H3,17,21,22,27)/t7-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119288

(CHEMBL3613824)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2c[nH]c3ccccc23)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C31H44N6O6/c1-18(2)26(36-31(42)37-27(19(3)4)30(41)43-5)29(40)35-24-12-8-9-15-32-25(38)14-13-21(34-28(24)39)16-20-17-33-23-11-7-6-10-22(20)23/h6-7,10-11,13-14,17-19,21,24,26-27,33H,8-9,12,15-16H2,1-5H3,(H,32,38)(H,34,39)(H,35,40)(H2,36,37,42)/b14-13+/t21-,24+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119300

(CHEMBL3613819)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2ccccc2Cl)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C29H42ClN5O6/c1-17(2)24(34-29(40)35-25(18(3)4)28(39)41-5)27(38)33-22-12-8-9-15-31-23(36)14-13-20(32-26(22)37)16-19-10-6-7-11-21(19)30/h6-7,10-11,13-14,17-18,20,22,24-25H,8-9,12,15-16H2,1-5H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t20-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119292
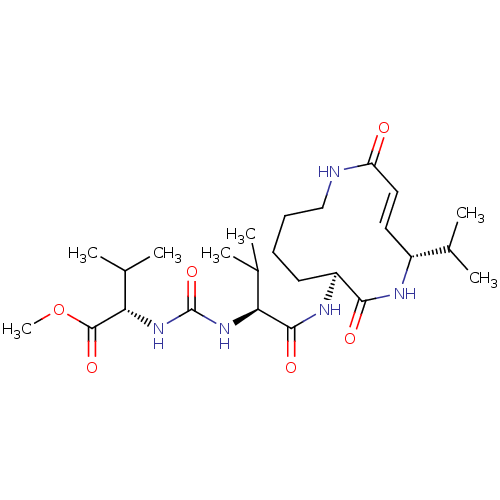
(CHEMBL3613812)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@@H](NC1=O)C(C)C)C(C)C |r,t:24| Show InChI InChI=1S/C25H43N5O6/c1-14(2)17-11-12-19(31)26-13-9-8-10-18(22(32)27-17)28-23(33)20(15(3)4)29-25(35)30-21(16(5)6)24(34)36-7/h11-12,14-18,20-21H,8-10,13H2,1-7H3,(H,26,31)(H,27,32)(H,28,33)(H2,29,30,35)/b12-11+/t17-,18+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501335

(CHEMBL3940601)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COC(=O)CP(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H22ClN6O10P/c17-3-8(24)19-1-2-32-34(29,30)5-9(25)31-4-7-11(26)12(27)15(33-7)23-6-20-10-13(23)21-16(18)22-14(10)28/h6-7,11-12,15,26-27H,1-5H2,(H,19,24)(H,29,30)(H3,18,21,22,28)/t7-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119303

(CHEMBL3613822)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(C)c2)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C30H45N5O6/c1-18(2)25(34-30(40)35-26(19(3)4)29(39)41-6)28(38)33-23-12-7-8-15-31-24(36)14-13-22(32-27(23)37)17-21-11-9-10-20(5)16-21/h9-11,13-14,16,18-19,22-23,25-26H,7-8,12,15,17H2,1-6H3,(H,31,36)(H,32,37)(H,33,38)(H2,34,35,40)/b14-13+/t22-,23+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50331975
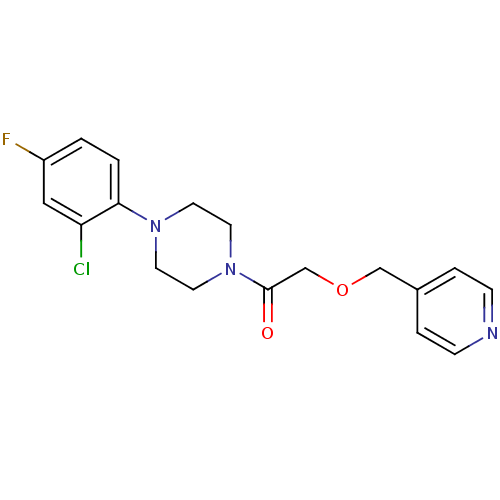
(1-(4-(2-chloro-4-fluorophenyl)piperazin-1-yl)-2-(p...)Show InChI InChI=1S/C18H19ClFN3O2/c19-16-11-15(20)1-2-17(16)22-7-9-23(10-8-22)18(24)13-25-12-14-3-5-21-6-4-14/h1-6,11H,7-10,12-13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEP from human mGluR5 |
Bioorg Med Chem Lett 20: 7381-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.036
BindingDB Entry DOI: 10.7270/Q27S7P13 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501339

(CHEMBL3961855)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H23ClN6O11P2/c16-3-8(23)18-1-2-31-34(27,28)6-35(29,30)32-4-7-10(24)11(25)14(33-7)22-5-19-9-12(22)20-15(17)21-13(9)26/h5,7,10-11,14,24-25H,1-4,6H2,(H,18,23)(H,27,28)(H,29,30)(H3,17,20,21,26)/t7-,10-,11-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119293

(CHEMBL3613813)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](CC(C)C)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C26H45N5O6/c1-15(2)14-18-11-12-20(32)27-13-9-8-10-19(23(33)28-18)29-24(34)21(16(3)4)30-26(36)31-22(17(5)6)25(35)37-7/h11-12,15-19,21-22H,8-10,13-14H2,1-7H3,(H,27,32)(H,28,33)(H,29,34)(H2,30,31,36)/b12-11+/t18-,19+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501350

(CHEMBL3974440)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)OC2CCCN(C2)C(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H27ClN6O11P2/c19-4-11(26)24-3-1-2-9(5-24)36-38(32,33)8-37(30,31)34-6-10-13(27)14(28)17(35-10)25-7-21-12-15(25)22-18(20)23-16(12)29/h7,9-10,13-14,17,27-28H,1-6,8H2,(H,30,31)(H,32,33)(H3,20,22,23,29)/t9?,10-,13-,14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501334

(CHEMBL3953729)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H20ClN7O10S/c16-3-7(24)18-1-2-31-15(28)22-34(29,30)32-4-6-9(25)10(26)13(33-6)23-5-19-8-11(23)20-14(17)21-12(8)27/h5-6,9-10,13,25-26H,1-4H2,(H,18,24)(H,22,28)(H3,17,20,21,27)/t6-,9-,10-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501333

(CHEMBL3968831)Show SMILES CC(C)(CNC(=O)CCl)COP(O)(=O)CP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c1nc(N)[nH]c2=O |r| Show InChI InChI=1S/C18H29ClN6O11P2/c1-18(2,5-21-10(26)3-19)6-35-38(32,33)8-37(30,31)34-4-9-12(27)13(28)16(36-9)25-7-22-11-14(25)23-17(20)24-15(11)29/h7,9,12-13,16,27-28H,3-6,8H2,1-2H3,(H,21,26)(H,30,31)(H,32,33)(H3,20,23,24,29)/t9-,12-,13-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50119295
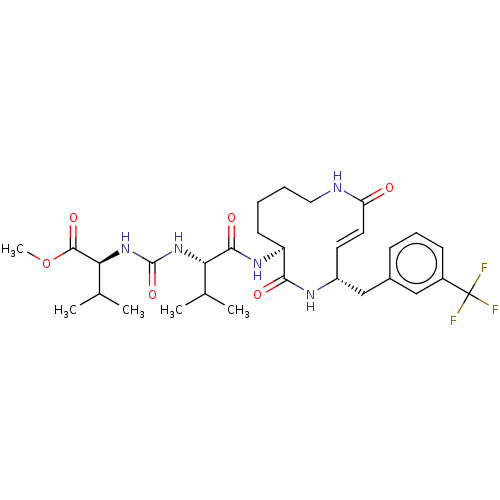
(CHEMBL3613815)Show SMILES COC(=O)[C@@H](NC(=O)N[C@@H](C(C)C)C(=O)N[C@H]1CCCCNC(=O)\C=C\[C@H](Cc2cccc(c2)C(F)(F)F)NC1=O)C(C)C |r,t:24| Show InChI InChI=1S/C30H42F3N5O6/c1-17(2)24(37-29(43)38-25(18(3)4)28(42)44-5)27(41)36-22-11-6-7-14-34-23(39)13-12-21(35-26(22)40)16-19-9-8-10-20(15-19)30(31,32)33/h8-10,12-13,15,17-18,21-22,24-25H,6-7,11,14,16H2,1-5H3,(H,34,39)(H,35,40)(H,36,41)(H2,37,38,43)/b13-12+/t21-,22+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of human 20S proteasome beta5 subunit using Suc-LLVY-AMC as substrate by fluorescence assay |
Bioorg Med Chem 23: 6218-22 (2015)
Article DOI: 10.1016/j.bmc.2015.07.041
BindingDB Entry DOI: 10.7270/Q25B0499 |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501337

(CHEMBL3941415)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)C(F)P(O)(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H22ClFN6O11P2/c16-3-7(24)19-1-2-32-35(28,29)14(17)36(30,31)33-4-6-9(25)10(26)13(34-6)23-5-20-8-11(23)21-15(18)22-12(8)27/h5-6,9-10,13-14,25-26H,1-4H2,(H,19,24)(H,28,29)(H,30,31)(H3,18,21,22,27)/t6-,9-,10-,13-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501338

(CHEMBL3911460)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COC(=O)CS(=O)(=O)NCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H22ClN7O9S/c17-3-8(25)19-1-2-21-34(30,31)5-9(26)32-4-7-11(27)12(28)15(33-7)24-6-20-10-13(24)22-16(18)23-14(10)29/h6-7,11-12,15,21,27-28H,1-5H2,(H,19,25)(H3,18,22,23,29)/t7-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501348

(CHEMBL3913450)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CC(=O)OCCNC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H22ClN6O10P/c17-3-8(24)19-1-2-31-9(25)5-34(29,30)32-4-7-11(26)12(27)15(33-7)23-6-20-10-13(23)21-16(18)22-14(10)28/h6-7,11-12,15,26-27H,1-5H2,(H,19,24)(H,29,30)(H3,18,21,22,28)/t7-,11-,12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501340

(CHEMBL3936077)Show SMILES COC(=O)\C=C\CCCOP(O)(=O)CP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c1nc(N)[nH]c2=O |r| Show InChI InChI=1S/C18H27N5O12P2/c1-32-11(24)5-3-2-4-6-33-36(28,29)9-37(30,31)34-7-10-13(25)14(26)17(35-10)23-8-20-12-15(23)21-18(19)22-16(12)27/h3,5,8,10,13-14,17,25-26H,2,4,6-7,9H2,1H3,(H,28,29)(H,30,31)(H3,19,21,22,27)/b5-3+/t10-,13-,14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501349

(CHEMBL3981450)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CNc2c(NCCCNC(=O)CCl)c(=O)c2=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H23ClN8O7/c20-4-8(29)22-2-1-3-23-9-10(14(32)13(9)31)24-5-7-12(30)15(33)18(35-7)28-6-25-11-16(28)26-19(21)27-17(11)34/h6-7,12,15,18,23-24,30,33H,1-5H2,(H,22,29)(H3,21,26,27,34)/t7-,12-,15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501336

(CHEMBL3965944)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)O[C@@H]2CC[C@H](C2)NC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H27ClN6O11P2/c19-4-11(26)22-8-1-2-9(3-8)36-38(32,33)7-37(30,31)34-5-10-13(27)14(28)17(35-10)25-6-21-12-15(25)23-18(20)24-16(12)29/h6,8-10,13-14,17,27-28H,1-5,7H2,(H,22,26)(H,30,31)(H,32,33)(H3,20,23,24,29)/t8-,9-,10-,13-,14-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50501346

(CHEMBL3945018)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(=O)CP(O)(=O)O[C@@H]2CC[C@@H](C2)NC(=O)CCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H27ClN6O11P2/c19-4-11(26)22-8-1-2-9(3-8)36-38(32,33)7-37(30,31)34-5-10-13(27)14(28)17(35-10)25-6-21-12-15(25)23-18(20)24-16(12)29/h6,8-10,13-14,17,27-28H,1-5,7H2,(H,22,26)(H,30,31)(H,32,33)(H3,20,23,24,29)/t8-,9+,10+,13+,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GMP-stabilized FLAG-tagged KRAS G12C mutant (unknown origin) using desthiobiotin-GTP probe by alphascreen assay |
ACS Med Chem Lett 8: 61-66 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00373
BindingDB Entry DOI: 10.7270/Q24F1TRH |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50420189
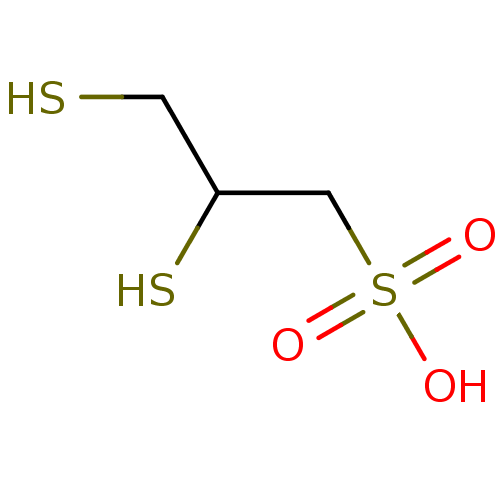
(CHEMBL1624767)Show InChI InChI=1S/C3H8O3S3/c4-9(5,6)2-3(8)1-7/h3,7-8H,1-2H2,(H,4,5,6) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 62: 921-6 (2002)
BindingDB Entry DOI: 10.7270/Q2Z320X6 |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Rattus norvegicus) | BDBM50420190
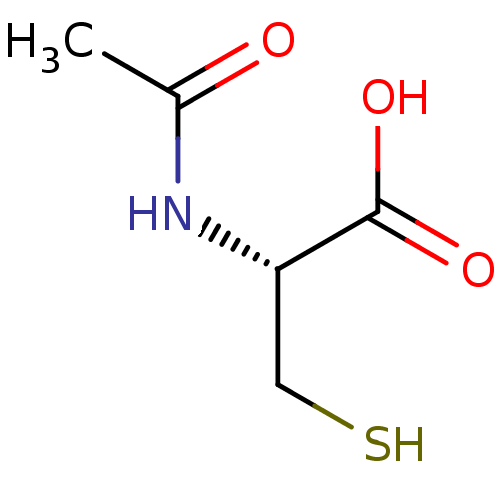
(5052 | ACETYLCYSTEINE)Show InChI InChI=1S/C5H9NO3S/c1-3(7)6-4(2-10)5(8)9/h4,10H,2H2,1H3,(H,6,7)(H,8,9)/t4-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester School of Medicine
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of PAH uptake in Xenopus laevis oocytes |
Mol Pharmacol 62: 921-6 (2002)
BindingDB Entry DOI: 10.7270/Q2Z320X6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in E275R |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D220V |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in wild type |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110686

(14-Ethylidene-2-isopropyl-5-(6-methoxy-3,5-dimethy...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@@H](NC(=O)[C@@H](C)[C@@H](CC(=O)C(=CC)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O)C(C)C |w:32.33| Show InChI InChI=1S/C41H58N4O10/c1-10-32-33(46)22-29(40(51)52)26(6)37(48)44-36(23(2)3)39(50)42-30(27(7)38(49)43-31(41(53)54)18-19-35(47)45(32)8)17-16-24(4)20-25(5)34(55-9)21-28-14-12-11-13-15-28/h10-17,20,23,25-27,29-31,34,36H,18-19,21-22H2,1-9H3,(H,42,50)(H,43,49)(H,44,48)(H,51,52)(H,53,54)/b17-16+,24-20+,32-10?/t25-,26-,27-,29+,30-,31+,34-,36-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D220V |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50328584
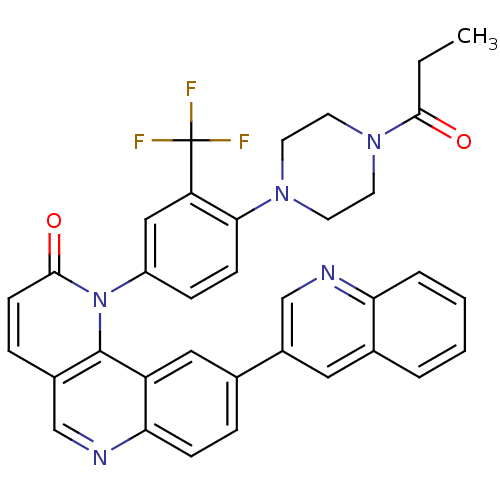
(1-(4-(4-Propionylpiperazin-1-yl)-3-(trifluoromethy...)Show SMILES CCC(=O)N1CCN(CC1)c1ccc(cc1C(F)(F)F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1 Show InChI InChI=1S/C35H28F3N5O2/c1-2-32(44)42-15-13-41(14-16-42)31-11-9-26(19-28(31)35(36,37)38)43-33(45)12-8-24-20-40-30-10-7-22(18-27(30)34(24)43)25-17-23-5-3-4-6-29(23)39-21-25/h3-12,17-21H,2,13-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR complex 1 after 30 mins by FRET assay |
J Med Chem 53: 7146-55 (2010)
Article DOI: 10.1021/jm101144f
BindingDB Entry DOI: 10.7270/Q2M32W0R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B
(Gallus gallus) | BDBM50110685

(25-Isobutyl-15-(6-methoxy-3,5-dimethyl-7-phenyl-he...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](CC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C47H68N6O12/c1-25(2)21-37-38(54)24-34(46(61)62)28(5)41(56)48-30(7)43(58)50-35(18-17-26(3)22-27(4)39(65-11)23-33-15-13-12-14-16-33)29(6)42(57)51-36(47(63)64)19-20-40(55)53(10)32(9)45(60)49-31(8)44(59)52-37/h12-18,22,25,27-31,34-37,39H,9,19-21,23-24H2,1-8,10-11H3,(H,48,56)(H,49,60)(H,50,58)(H,51,57)(H,52,59)(H,61,62)(H,63,64)/b18-17+,26-22+/t27-,28-,29-,30-,31+,34+,35-,36+,37-,39-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of microcystin analogues to catalytic subunits of Serine/threonine protein phosphatase 2A (PP2Ac) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Cyclin-Y
(Homo sapiens) | BDBM50535590
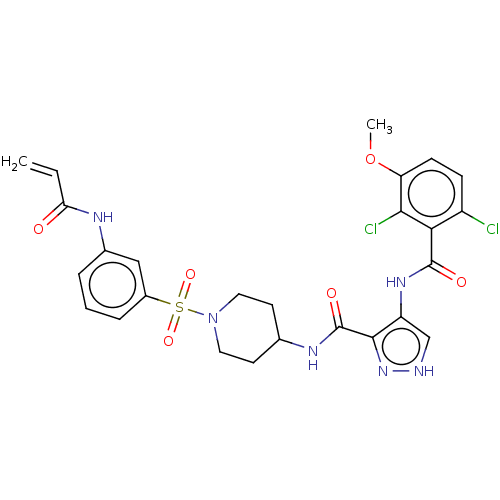
(CHEMBL4563703)Show SMILES OC(=O)C(F)(F)F.COc1ccc(Cl)c(C(=O)Nc2c[nH]nc2C(=O)NC2CCN(CC2)S(=O)(=O)c2cccc(NC(=O)C=C)c2)c1Cl Show InChI InChI=1S/C26H26Cl2N6O6S/c1-3-21(35)30-16-5-4-6-17(13-16)41(38,39)34-11-9-15(10-12-34)31-26(37)24-19(14-29-33-24)32-25(36)22-18(27)7-8-20(40-2)23(22)28/h3-8,13-15H,1,9-12H2,2H3,(H,29,33)(H,30,35)(H,31,37)(H,32,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay |
Bioorg Med Chem Lett 29: 1985-1993 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.024
BindingDB Entry DOI: 10.7270/Q29P355R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50399540

(FORETINIB | US10464902, Foretinib | US10882853, Co...)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)Nc4ccc(F)cc4)cc3F)ccnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C34H34F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3-9,12,19-21H,2,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of c-Met kinase (unknown origin) |
Eur J Med Chem 90: 195-208 (2015)
Article DOI: 10.1016/j.ejmech.2014.11.023
BindingDB Entry DOI: 10.7270/Q2M61MX6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data