 Found 634 hits with Last Name = 'yamashita' and Initial = 't'
Found 634 hits with Last Name = 'yamashita' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50065257

((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...)Show SMILES C[C@@H]1N[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C7H15NO4/c1-3-5(10)7(12)6(11)4(2-9)8-3/h3-12H,2H2,1H3/t3-,4+,5+,6+,7+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically |
J Nat Prod 65: 198-202 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1KN6 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50242272
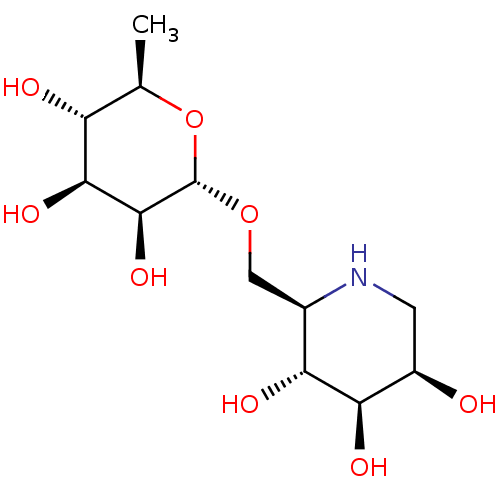
(6-O-alpha-rhamnopyranosyl-DMJ | CHEMBL469655)Show SMILES C[C@H]1O[C@H](OC[C@H]2NC[C@@H](O)[C@@H](O)[C@@H]2O)[C@@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C12H23NO8/c1-4-7(15)10(18)11(19)12(21-4)20-3-5-8(16)9(17)6(14)2-13-5/h4-19H,2-3H2,1H3/t4-,5-,6-,7-,8-,9-,10+,11+,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically |
J Nat Prod 65: 198-202 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1KN6 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50242268
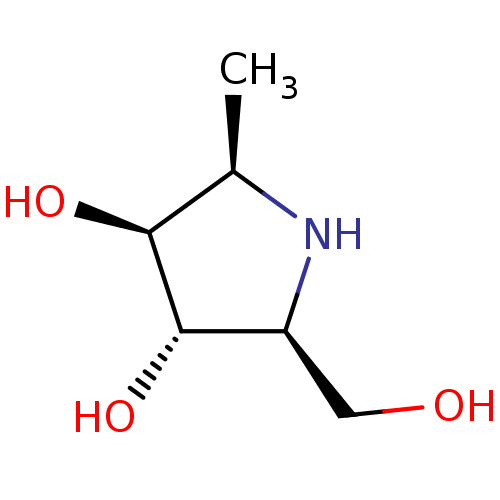
(2,5-Imino-1,2,5-trideoxy-L-glucitol | CHEMBL502230)Show InChI InChI=1S/C6H13NO3/c1-3-5(9)6(10)4(2-8)7-3/h3-10H,2H2,1H3/t3-,4+,5+,6+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically |
J Nat Prod 65: 198-202 (2002)
BindingDB Entry DOI: 10.7270/Q2ZW1KN6 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35714

(CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...)Show SMILES CN(C)C1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3C)cc2)c2ccccc12 Show InChI InChI=1S/C27H29N3O2/c1-19-9-4-5-10-22(19)26(31)28-21-16-14-20(15-17-21)27(32)30-18-8-13-24(29(2)3)23-11-6-7-12-25(23)30/h4-7,9-12,14-17,24H,8,13,18H2,1-3H3,(H,28,31) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383116
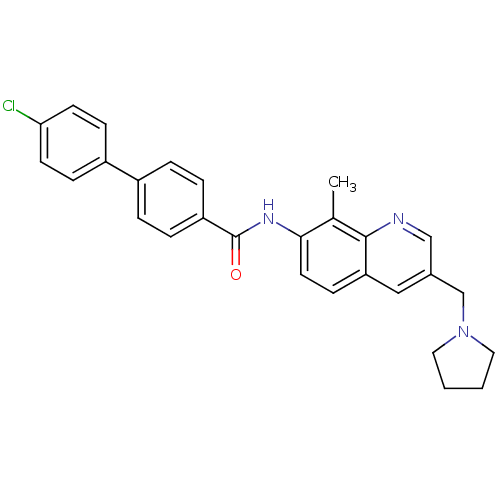
(CHEMBL2031736)Show SMILES Cc1c(NC(=O)c2ccc(cc2)-c2ccc(Cl)cc2)ccc2cc(CN3CCCC3)cnc12 Show InChI InChI=1S/C28H26ClN3O/c1-19-26(13-10-24-16-20(17-30-27(19)24)18-32-14-2-3-15-32)31-28(33)23-6-4-21(5-7-23)22-8-11-25(29)12-9-22/h4-13,16-17H,2-3,14-15,18H2,1H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50254020
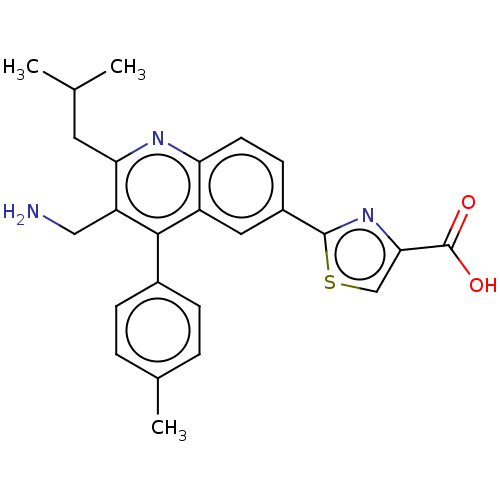
(CHEMBL4068446)Show SMILES Cl.Cl.CC(C)Cc1nc2ccc(cc2c(-c2ccc(C)cc2)c1CN)-c1nc(cs1)C(O)=O Show InChI InChI=1S/C25H25N3O2S/c1-14(2)10-21-19(12-26)23(16-6-4-15(3)5-7-16)18-11-17(8-9-20(18)27-21)24-28-22(13-31-24)25(29)30/h4-9,11,13-14H,10,12,26H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins |
Bioorg Med Chem Lett 27: 3565-3571 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.048
BindingDB Entry DOI: 10.7270/Q2PK0JKX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50351324
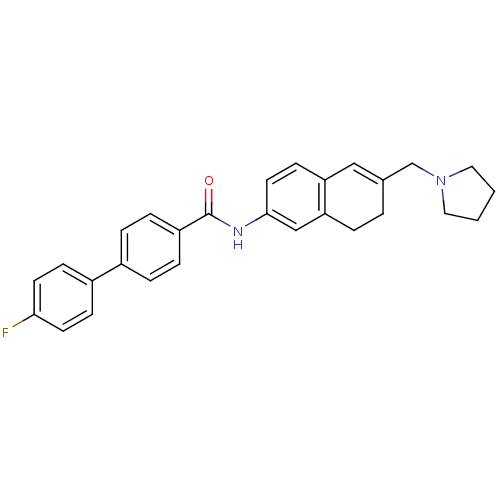
(CHEMBL1818901)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C28H27FN2O/c29-26-12-9-22(10-13-26)21-5-7-23(8-6-21)28(32)30-27-14-11-24-17-20(3-4-25(24)18-27)19-31-15-1-2-16-31/h5-14,17-18H,1-4,15-16,19H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human 5HT2C receptor expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383114
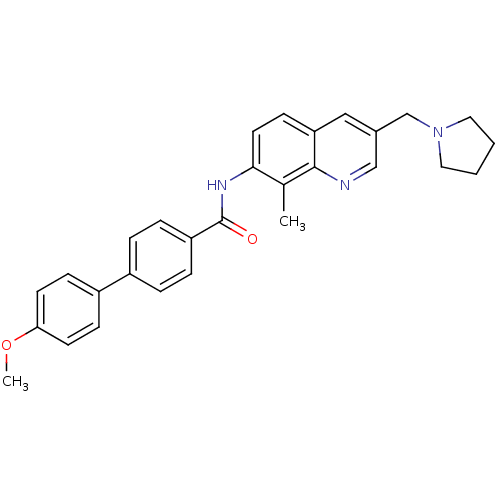
(CHEMBL2031734)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)cnc2c1C Show InChI InChI=1S/C29H29N3O2/c1-20-27(14-11-25-17-21(18-30-28(20)25)19-32-15-3-4-16-32)31-29(33)24-7-5-22(6-8-24)23-9-12-26(34-2)13-10-23/h5-14,17-18H,3-4,15-16,19H2,1-2H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383112
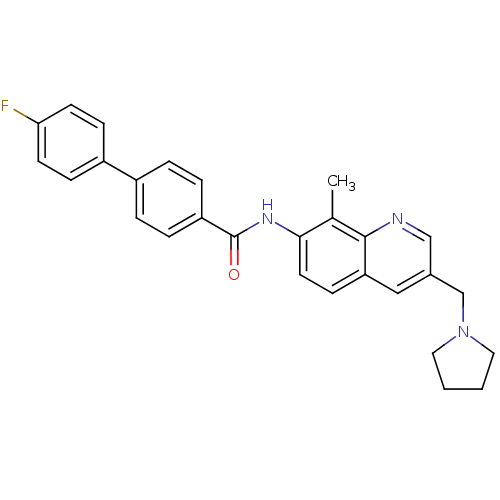
(CHEMBL2029372)Show SMILES Cc1c(NC(=O)c2ccc(cc2)-c2ccc(F)cc2)ccc2cc(CN3CCCC3)cnc12 Show InChI InChI=1S/C28H26FN3O/c1-19-26(13-10-24-16-20(17-30-27(19)24)18-32-14-2-3-15-32)31-28(33)23-6-4-21(5-7-23)22-8-11-25(29)12-9-22/h4-13,16-17H,2-3,14-15,18H2,1H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383096
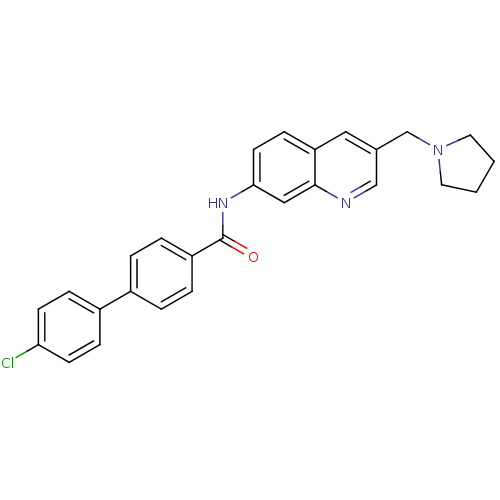
(CHEMBL2031716)Show SMILES Clc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)cnc2c1 Show InChI InChI=1S/C27H24ClN3O/c28-24-10-7-21(8-11-24)20-3-5-22(6-4-20)27(32)30-25-12-9-23-15-19(17-29-26(23)16-25)18-31-13-1-2-14-31/h3-12,15-17H,1-2,13-14,18H2,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383091
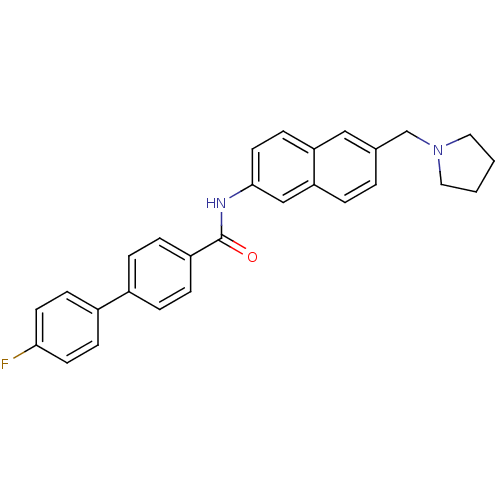
(CHEMBL2031573)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)ccc2c1 Show InChI InChI=1S/C28H25FN2O/c29-26-12-9-22(10-13-26)21-5-7-23(8-6-21)28(32)30-27-14-11-24-17-20(3-4-25(24)18-27)19-31-15-1-2-16-31/h3-14,17-18H,1-2,15-16,19H2,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383092
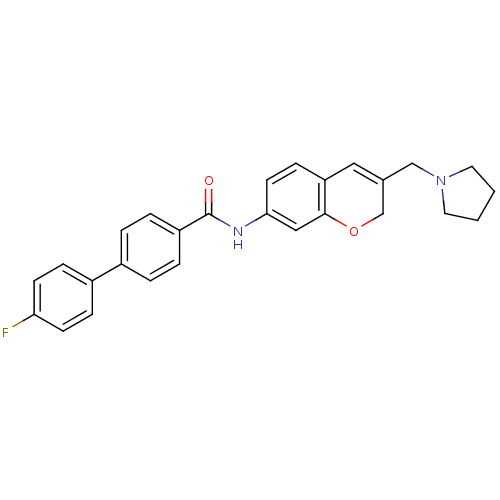
(CHEMBL2031574)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)COc2c1 |t:22| Show InChI InChI=1S/C27H25FN2O2/c28-24-10-7-21(8-11-24)20-3-5-22(6-4-20)27(31)29-25-12-9-23-15-19(18-32-26(23)16-25)17-30-13-1-2-14-30/h3-12,15-16H,1-2,13-14,17-18H2,(H,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383107

(CHEMBL2031727)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)cnc2c1F Show InChI InChI=1S/C27H23F2N3O/c28-23-10-7-20(8-11-23)19-3-5-21(6-4-19)27(33)31-24-12-9-22-15-18(16-30-26(22)25(24)29)17-32-13-1-2-14-32/h3-12,15-16H,1-2,13-14,17H2,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383115
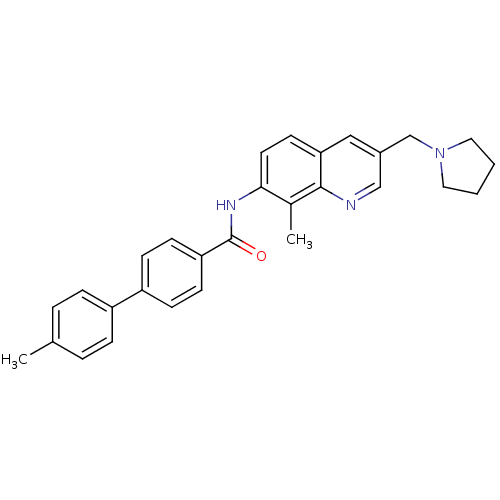
(CHEMBL2031735)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)cnc2c1C Show InChI InChI=1S/C29H29N3O/c1-20-5-7-23(8-6-20)24-9-11-25(12-10-24)29(33)31-27-14-13-26-17-22(18-30-28(26)21(27)2)19-32-15-3-4-16-32/h5-14,17-18H,3-4,15-16,19H2,1-2H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50333177
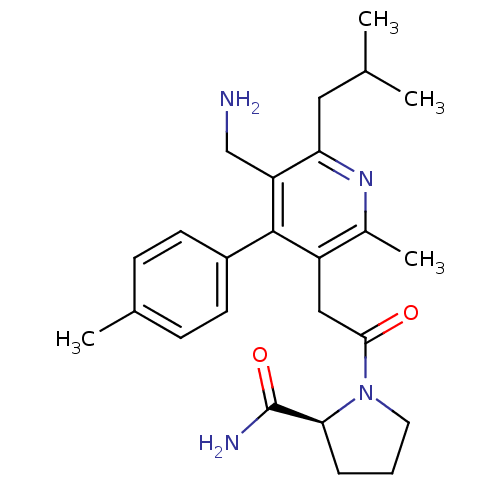
(1-{[5-(Aminomethyl)-2-methyl-4-(4-methylphenyl)-6-...)Show SMILES CC(C)Cc1nc(C)c(CC(=O)N2CCC[C@H]2C(N)=O)c(-c2ccc(C)cc2)c1CN |r| Show InChI InChI=1S/C25H34N4O2/c1-15(2)12-21-20(14-26)24(18-9-7-16(3)8-10-18)19(17(4)28-21)13-23(30)29-11-5-6-22(29)25(27)31/h7-10,15,22H,5-6,11-14,26H2,1-4H3,(H2,27,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry |
Bioorg Med Chem 19: 172-85 (2011)
Article DOI: 10.1016/j.bmc.2010.11.038
BindingDB Entry DOI: 10.7270/Q2TB174K |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50336806

(1-(3-(aminomethyl)-2-isobutyl-4-p-tolylquinolin-6-...)Show SMILES CC(C)Cc1nc2ccc(cc2c(-c2ccc(C)cc2)c1CN)N1CC(=O)NCC1=O Show InChI InChI=1S/C25H28N4O2/c1-15(2)10-22-20(12-26)25(17-6-4-16(3)5-7-17)19-11-18(8-9-21(19)28-22)29-14-23(30)27-13-24(29)31/h4-9,11,15H,10,12-14,26H2,1-3H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco2 cells after 60 mins |
J Med Chem 54: 831-50 (2012)
Article DOI: 10.1021/jm101236h
BindingDB Entry DOI: 10.7270/Q2QR4Z4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50333175
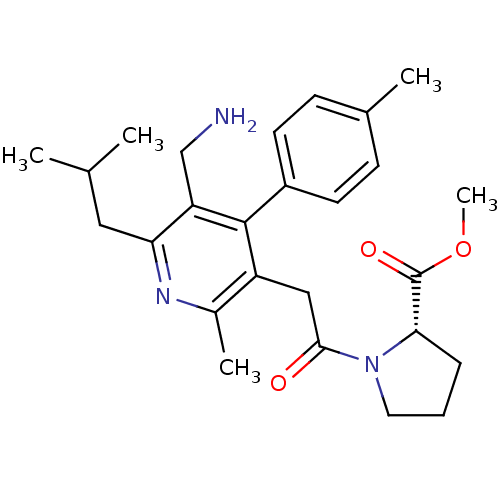
(CHEMBL1644848 | Methyl 1-{[5-(aminomethyl)-2-methy...)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)Cc1c(C)nc(CC(C)C)c(CN)c1-c1ccc(C)cc1 |r| Show InChI InChI=1S/C26H35N3O3/c1-16(2)13-22-21(15-27)25(19-10-8-17(3)9-11-19)20(18(4)28-22)14-24(30)29-12-6-7-23(29)26(31)32-5/h8-11,16,23H,6-7,12-15,27H2,1-5H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry |
Bioorg Med Chem 19: 172-85 (2011)
Article DOI: 10.1016/j.bmc.2010.11.038
BindingDB Entry DOI: 10.7270/Q2TB174K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383090
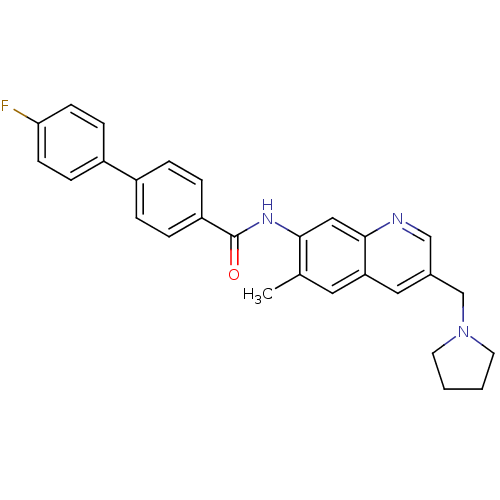
(CHEMBL2031730)Show SMILES Cc1cc2cc(CN3CCCC3)cnc2cc1NC(=O)c1ccc(cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C28H26FN3O/c1-19-14-24-15-20(18-32-12-2-3-13-32)17-30-27(24)16-26(19)31-28(33)23-6-4-21(5-7-23)22-8-10-25(29)11-9-22/h4-11,14-17H,2-3,12-13,18H2,1H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383111

(CHEMBL2031732)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1cc2ncc(CN3CCCC3)cc2cc1Cl Show InChI InChI=1S/C27H23ClFN3O/c28-24-14-22-13-18(17-32-11-1-2-12-32)16-30-25(22)15-26(24)31-27(33)21-5-3-19(4-6-21)20-7-9-23(29)10-8-20/h3-10,13-16H,1-2,11-12,17H2,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50351312

(CHEMBL1818800)Show SMILES Clc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C28H27ClN2O/c29-26-12-9-22(10-13-26)21-5-7-23(8-6-21)28(32)30-27-14-11-24-17-20(3-4-25(24)18-27)19-31-15-1-2-16-31/h5-14,17-18H,1-4,15-16,19H2,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383110

(CHEMBL2031731)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1cc2ncc(CN3CCCC3)cc2cc1F Show InChI InChI=1S/C27H23F2N3O/c28-23-9-7-20(8-10-23)19-3-5-21(6-4-19)27(33)31-26-15-25-22(14-24(26)29)13-18(16-30-25)17-32-11-1-2-12-32/h3-10,13-16H,1-2,11-12,17H2,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50351322
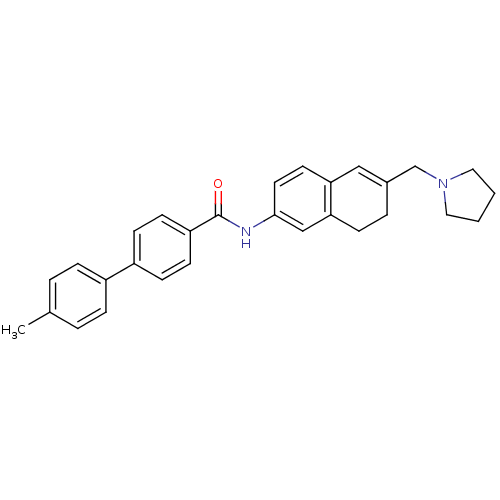
(CHEMBL1818810)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C29H30N2O/c1-21-4-7-23(8-5-21)24-10-12-25(13-11-24)29(32)30-28-15-14-26-18-22(6-9-27(26)19-28)20-31-16-2-3-17-31/h4-5,7-8,10-15,18-19H,2-3,6,9,16-17,20H2,1H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50383112

(CHEMBL2029372)Show SMILES Cc1c(NC(=O)c2ccc(cc2)-c2ccc(F)cc2)ccc2cc(CN3CCCC3)cnc12 Show InChI InChI=1S/C28H26FN3O/c1-19-26(13-10-24-16-20(17-30-27(19)24)18-32-14-2-3-15-32)31-28(33)23-6-4-21(5-7-23)22-8-11-25(29)12-9-22/h4-13,16-17H,2-3,14-15,18H2,1H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from rat MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383113
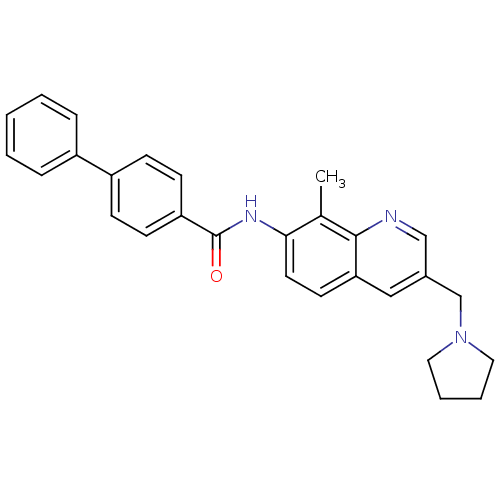
(CHEMBL2031733)Show SMILES Cc1c(NC(=O)c2ccc(cc2)-c2ccccc2)ccc2cc(CN3CCCC3)cnc12 Show InChI InChI=1S/C28H27N3O/c1-20-26(14-13-25-17-21(18-29-27(20)25)19-31-15-5-6-16-31)30-28(32)24-11-9-23(10-12-24)22-7-3-2-4-8-22/h2-4,7-14,17-18H,5-6,15-16,19H2,1H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50333167
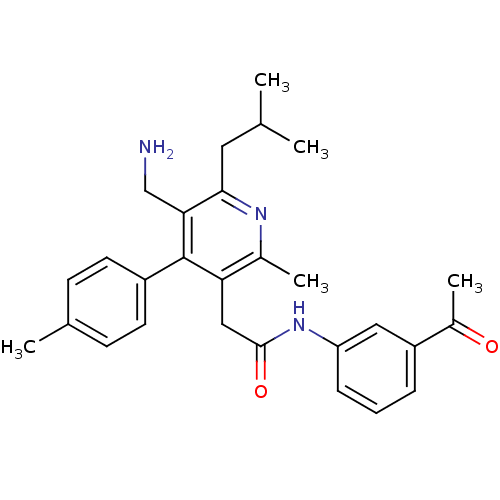
(3-({[5-(Aminomethyl)-2-methyl-4-(4-methylphenyl)-6...)Show SMILES CC(C)Cc1nc(C)c(CC(=O)Nc2cccc(c2)C(C)=O)c(-c2ccc(C)cc2)c1CN Show InChI InChI=1S/C28H33N3O2/c1-17(2)13-26-25(16-29)28(21-11-9-18(3)10-12-21)24(19(4)30-26)15-27(33)31-23-8-6-7-22(14-23)20(5)32/h6-12,14,17H,13,15-16,29H2,1-5H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry |
Bioorg Med Chem 19: 172-85 (2011)
Article DOI: 10.1016/j.bmc.2010.11.038
BindingDB Entry DOI: 10.7270/Q2TB174K |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50351308
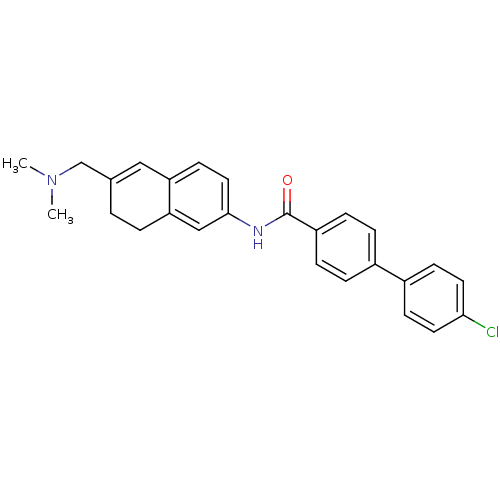
(CHEMBL1818795)Show SMILES CN(C)CC1=Cc2ccc(NC(=O)c3ccc(cc3)-c3ccc(Cl)cc3)cc2CC1 |t:4| Show InChI InChI=1S/C26H25ClN2O/c1-29(2)17-18-3-4-23-16-25(14-11-22(23)15-18)28-26(30)21-7-5-19(6-8-21)20-9-12-24(27)13-10-20/h5-16H,3-4,17H2,1-2H3,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50351313

(CHEMBL1818801)Show SMILES Clc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C29H29ClN2O/c30-27-13-10-23(11-14-27)22-6-8-24(9-7-22)29(33)31-28-15-12-25-18-21(4-5-26(25)19-28)20-32-16-2-1-3-17-32/h6-15,18-19H,1-5,16-17,20H2,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50351324

(CHEMBL1818901)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C28H27FN2O/c29-26-12-9-22(10-13-26)21-5-7-23(8-6-21)28(32)30-27-14-11-24-17-20(3-4-25(24)18-27)19-31-15-1-2-16-31/h5-14,17-18H,1-4,15-16,19H2,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50351324

(CHEMBL1818901)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C28H27FN2O/c29-26-12-9-22(10-13-26)21-5-7-23(8-6-21)28(32)30-27-14-11-24-17-20(3-4-25(24)18-27)19-31-15-1-2-16-31/h5-14,17-18H,1-4,15-16,19H2,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50253979

(CHEMBL4068477)Show SMILES Cl.Cl.CC(C)Cc1ncc(-c2nc(cs2)C(N)=O)c(-c2ccc(C)cc2)c1CN Show InChI InChI=1S/C21H24N4OS/c1-12(2)8-17-15(9-22)19(14-6-4-13(3)5-7-14)16(10-24-17)21-25-18(11-27-21)20(23)26/h4-7,10-12H,8-9,22H2,1-3H3,(H2,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins |
Bioorg Med Chem Lett 27: 3565-3571 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.048
BindingDB Entry DOI: 10.7270/Q2PK0JKX |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50268236

(CHEMBL4101718)Show SMILES CC(C)Oc1cc(-c2ccc(F)cc2)c(cc1CN1CC2(C1)CC(=NO2)N1CCC(C)(CC1)C(O)=O)C1CC1 |c:26| Show InChI InChI=1S/C31H38FN3O4/c1-20(2)38-27-15-26(22-6-8-24(32)9-7-22)25(21-4-5-21)14-23(27)17-34-18-31(19-34)16-28(33-39-31)35-12-10-30(3,11-13-35)29(36)37/h6-9,14-15,20-21H,4-5,10-13,16-19H2,1-3H3,(H,36,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Division, Takeda Pharmaceutical Co., Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: takeshi.yamasaki@takeda.com.
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR5 expressed in CHO cells assessed as inhibition of SST14-induced forskolin-stimulated intracellular cAMP level incub... |
Bioorg Med Chem 25: 4153-4162 (2017)
Article DOI: 10.1016/j.bmc.2017.06.003
BindingDB Entry DOI: 10.7270/Q28G8P5G |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50351312

(CHEMBL1818800)Show SMILES Clc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C28H27ClN2O/c29-26-12-9-22(10-13-26)21-5-7-23(8-6-21)28(32)30-27-14-11-24-17-20(3-4-25(24)18-27)19-31-15-1-2-16-31/h5-14,17-18H,1-4,15-16,19H2,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
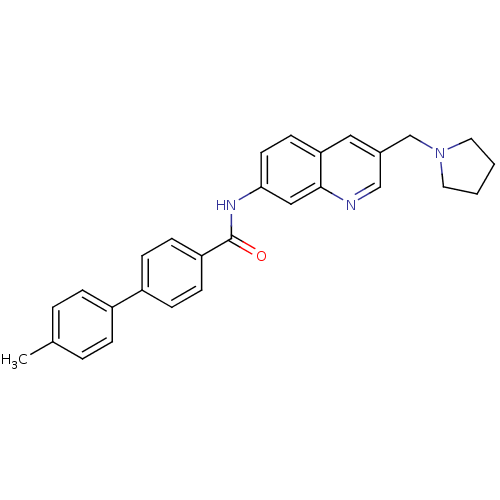
(Homo sapiens (Human)) | BDBM50383088
(CHEMBL2031715)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)cnc2c1 Show InChI InChI=1S/C28H27N3O/c1-20-4-6-22(7-5-20)23-8-10-24(11-9-23)28(32)30-26-13-12-25-16-21(18-29-27(25)17-26)19-31-14-2-3-15-31/h4-13,16-18H,2-3,14-15,19H2,1H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from human MCHR1 expressed in CHO cells after 1 hr by scintillation counting |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
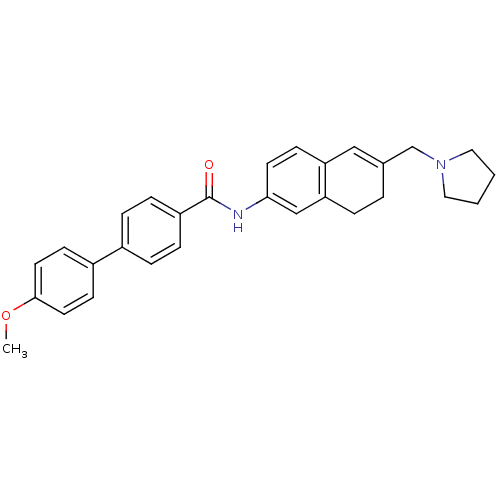
(Homo sapiens (Human)) | BDBM50351323
(CHEMBL1818811)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:23| Show InChI InChI=1S/C29H30N2O2/c1-33-28-14-11-23(12-15-28)22-6-8-24(9-7-22)29(32)30-27-13-10-25-18-21(4-5-26(25)19-27)20-31-16-2-3-17-31/h6-15,18-19H,2-5,16-17,20H2,1H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from human MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383107

(CHEMBL2031727)Show SMILES Fc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2cc(CN3CCCC3)cnc2c1F Show InChI InChI=1S/C27H23F2N3O/c28-23-10-7-20(8-11-23)19-3-5-21(6-4-19)27(33)31-24-12-9-22-15-18(16-30-26(22)25(24)29)17-32-13-1-2-14-32/h3-12,15-16H,1-2,13-14,17H2,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Antagonist activity at human MCHR1 expressed in CHO cells assessed as inhibition of MCH-induced [3H]arachidonic acid release after 16 hrs by liquid s... |
J Med Chem 55: 2353-66 (2012)
Article DOI: 10.1021/jm201596h
BindingDB Entry DOI: 10.7270/Q2GH9JZQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50351322

(CHEMBL1818810)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccc2C=C(CN3CCCC3)CCc2c1 |t:22| Show InChI InChI=1S/C29H30N2O/c1-21-4-7-23(8-5-21)24-10-12-25(13-11-24)29(32)30-28-15-14-26-18-22(6-9-27(26)19-28)20-31-16-2-3-17-31/h4-5,7-8,10-15,18-19H,2-3,6,9,16-17,20H2,1H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from rat MCHR1 expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 19: 5539-52 (2011)
Article DOI: 10.1016/j.bmc.2011.07.038
BindingDB Entry DOI: 10.7270/Q26H4HT4 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50336818
(5-(Aminomethyl)-2-benzyl-6-(2,2-dimethylpropyl)-4-...)Show SMILES Cc1ccc(cc1)-c1c(CN)c(CC(C)(C)C)nc(Cc2ccccc2)c1C(O)=O Show InChI InChI=1S/C26H30N2O2/c1-17-10-12-19(13-11-17)23-20(16-27)22(15-26(2,3)4)28-21(24(23)25(29)30)14-18-8-6-5-7-9-18/h5-13H,14-16,27H2,1-4H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco2 cells after 60 mins |
J Med Chem 54: 831-50 (2012)
Article DOI: 10.1021/jm101236h
BindingDB Entry DOI: 10.7270/Q2QR4Z4M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data