 Found 42520 hits with Last Name = 'he' and Initial = 'w'
Found 42520 hits with Last Name = 'he' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655

(A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Binding affinity to wild type HIV1 protease |
J Med Chem 55: 1424-44 (2012)
Article DOI: 10.1021/jm2010332
BindingDB Entry DOI: 10.7270/Q2D79F84 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM209097
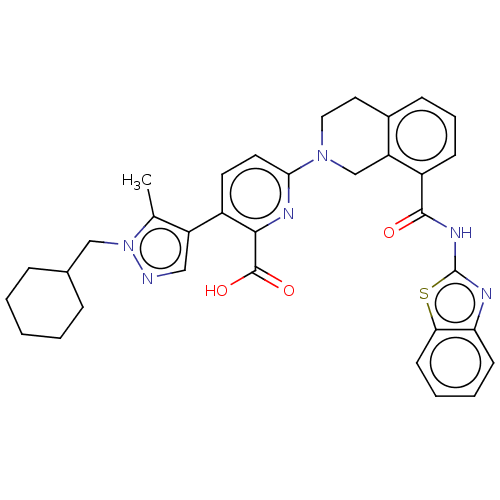
(US9266877, 43)Show SMILES Cc1c(cnn1CC1CCCCC1)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C34H34N6O3S/c1-21-26(18-35-40(21)19-22-8-3-2-4-9-22)24-14-15-30(37-31(24)33(42)43)39-17-16-23-10-7-11-25(27(23)20-39)32(41)38-34-36-28-12-5-6-13-29(28)44-34/h5-7,10-15,18,22H,2-4,8-9,16-17,19-20H2,1H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054156
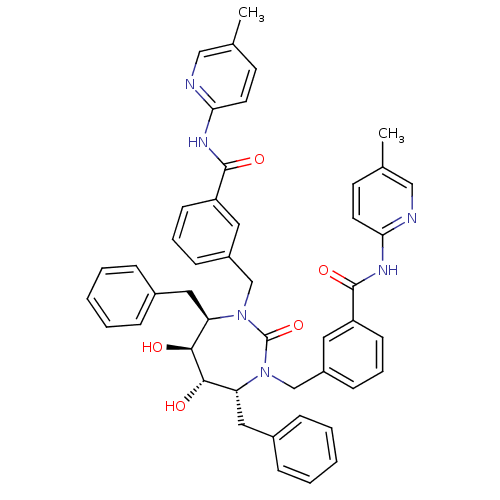
((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...)Show SMILES Cc1ccc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4ccc(C)cn4)C3=O)c2)nc1 Show InChI InChI=1S/C47H46N6O5/c1-31-19-21-41(48-27-31)50-45(56)37-17-9-15-35(23-37)29-52-39(25-33-11-5-3-6-12-33)43(54)44(55)40(26-34-13-7-4-8-14-34)53(47(52)58)30-36-16-10-18-38(24-36)46(57)51-42-22-20-32(2)28-49-42/h3-24,27-28,39-40,43-44,54-55H,25-26,29-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162797
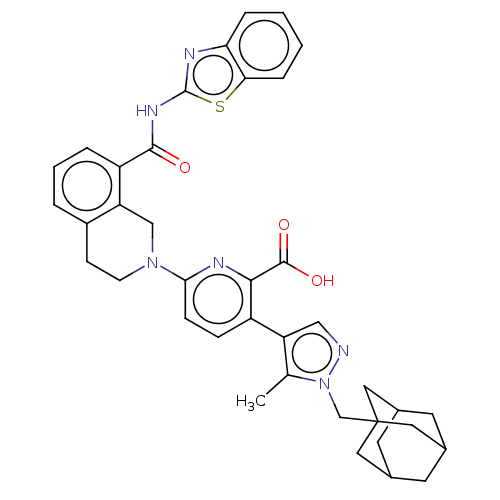
(CHEMBL3793424)Show SMILES Cc1c(cnn1CC12CC3CC(CC(C3)C1)C2)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 |TLB:14:9:16:12.13.15,14:13:16:10.9.8,THB:12:11:8:14.13.15,12:13:10.11.16:8| Show InChI InChI=1S/C38H38N6O3S/c1-22-29(19-39-44(22)21-38-16-23-13-24(17-38)15-25(14-23)18-38)27-9-10-33(41-34(27)36(46)47)43-12-11-26-5-4-6-28(30(26)20-43)35(45)42-37-40-31-7-2-3-8-32(31)48-37/h2-10,19,23-25H,11-18,20-21H2,1H3,(H,46,47)(H,40,42,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754
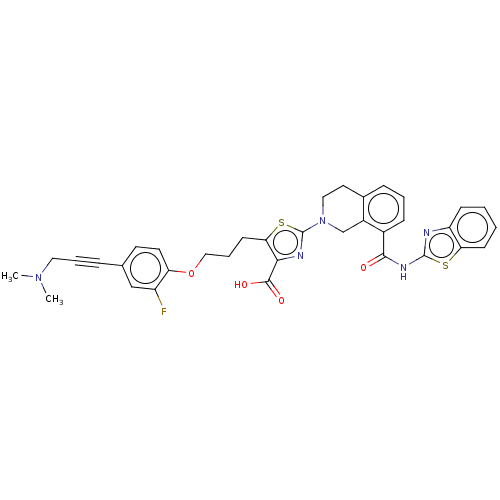
(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030752
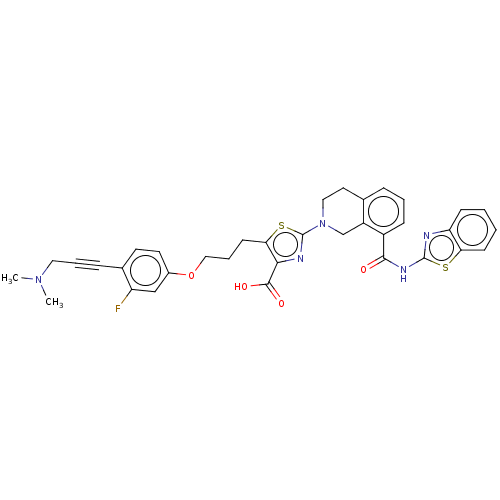
(CHEMBL3342333)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-9-23-14-15-24(20-27(23)36)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-22-8-5-10-25(26(22)21-41)32(42)39-34-37-28-11-3-4-12-29(28)46-34/h3-5,8,10-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759
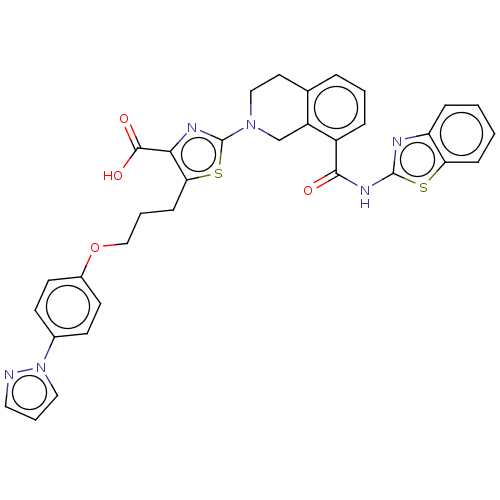
(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758
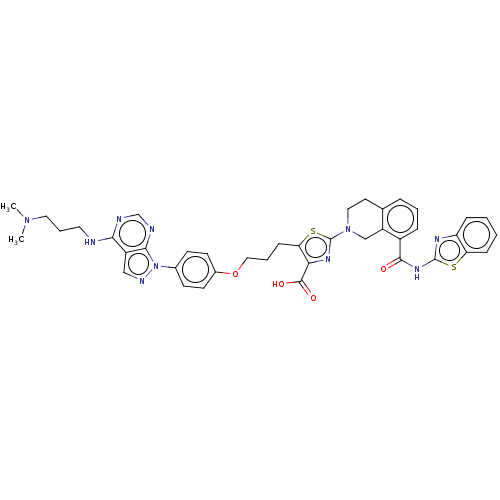
(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757
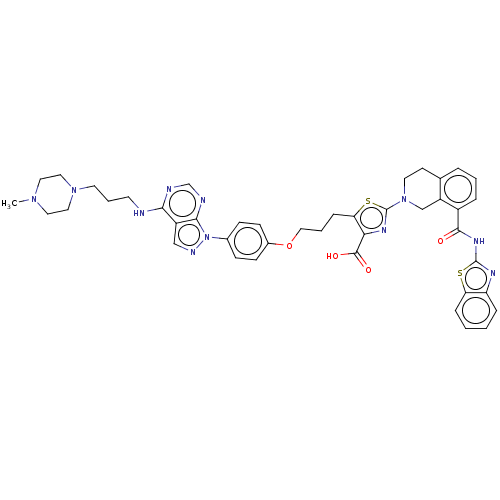
(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054159
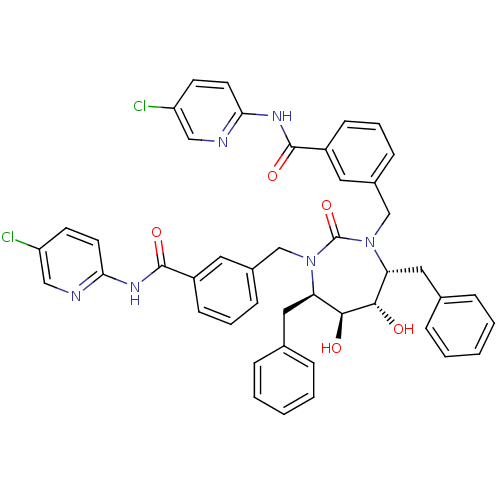
(5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ccc(Cl)cn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ccc(Cl)cn2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C45H40Cl2N6O5/c46-35-17-19-39(48-25-35)50-43(56)33-15-7-13-31(21-33)27-52-37(23-29-9-3-1-4-10-29)41(54)42(55)38(24-30-11-5-2-6-12-30)53(45(52)58)28-32-14-8-16-34(22-32)44(57)51-40-20-18-36(47)26-49-40/h1-22,25-26,37-38,41-42,54-55H,23-24,27-28H2,(H,48,50,56)(H,49,51,57)/t37-,38-,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50561528
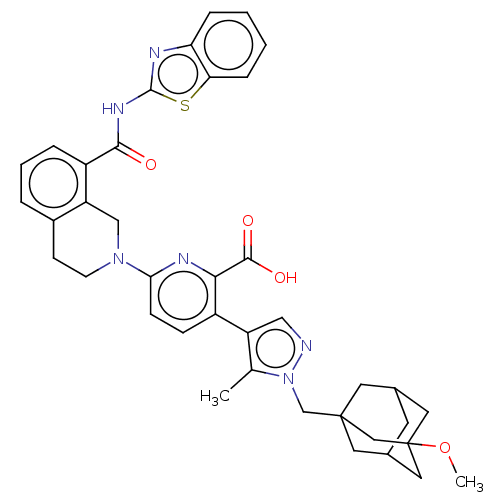
(CHEMBL4762875)Show SMILES COC12CC3CC(CC(Cn4ncc(c4C)-c4ccc(nc4C(O)=O)N4CCc5cccc(C(=O)Nc6nc7ccccc7s6)c5C4)(C3)C1)C2 |TLB:1:2:5.4.47:7,5:6:4.3.47:48,THB:5:4:6.49.7:48,3:4:7:49.2.48,3:2:5.4.47:7| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM159
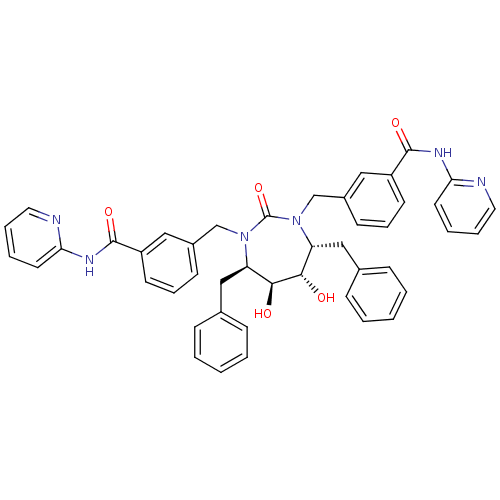
(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ccccn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ccccn2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C45H42N6O5/c52-41-37(27-31-13-3-1-4-14-31)50(29-33-17-11-19-35(25-33)43(54)48-39-21-7-9-23-46-39)45(56)51(38(42(41)53)28-32-15-5-2-6-16-32)30-34-18-12-20-36(26-34)44(55)49-40-22-8-10-24-47-40/h1-26,37-38,41-42,52-53H,27-30H2,(H,46,48,54)(H,47,49,55)/t37-,38-,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054156

((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...)Show SMILES Cc1ccc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4ccc(C)cn4)C3=O)c2)nc1 Show InChI InChI=1S/C47H46N6O5/c1-31-19-21-41(48-27-31)50-45(56)37-17-9-15-35(23-37)29-52-39(25-33-11-5-3-6-12-33)43(54)44(55)40(26-34-13-7-4-8-14-34)53(47(52)58)30-36-16-10-18-38(24-36)46(57)51-42-22-20-32(2)28-49-42/h3-24,27-28,39-40,43-44,54-55H,25-26,29-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054156

((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...)Show SMILES Cc1ccc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4ccc(C)cn4)C3=O)c2)nc1 Show InChI InChI=1S/C47H46N6O5/c1-31-19-21-41(48-27-31)50-45(56)37-17-9-15-35(23-37)29-52-39(25-33-11-5-3-6-12-33)43(54)44(55)40(26-34-13-7-4-8-14-34)53(47(52)58)30-36-16-10-18-38(24-36)46(57)51-42-22-20-32(2)28-49-42/h3-24,27-28,39-40,43-44,54-55H,25-26,29-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Affinity of the compound against HIV protease |
J Med Chem 40: 4079-88 (1998)
Article DOI: 10.1021/jm970288b
BindingDB Entry DOI: 10.7270/Q2XS5TG1 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
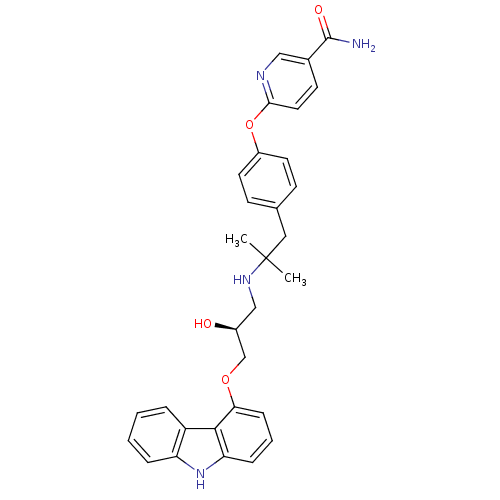
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054159

(5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ccc(Cl)cn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ccc(Cl)cn2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C45H40Cl2N6O5/c46-35-17-19-39(48-25-35)50-43(56)33-15-7-13-31(21-33)27-52-37(23-29-9-3-1-4-10-29)41(54)42(55)38(24-30-11-5-2-6-12-30)53(45(52)58)28-32-14-8-16-34(22-32)44(57)51-40-20-18-36(47)26-49-40/h1-22,25-26,37-38,41-42,54-55H,23-24,27-28H2,(H,48,50,56)(H,49,51,57)/t37-,38-,41+,42+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061409
(3-[(4R,5S,6S,7R)-3-(3-Amino-benzyl)-4,7-dibenzyl-5...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)Nc3ncc[nH]3)C2=O)c1 Show InChI InChI=1S/C37H38N6O4/c38-30-16-8-14-28(20-30)24-43-32(22-26-11-5-2-6-12-26)34(45)33(44)31(21-25-9-3-1-4-10-25)42(37(43)47)23-27-13-7-15-29(19-27)35(46)41-36-39-17-18-40-36/h1-20,31-34,44-45H,21-24,38H2,(H2,39,40,41,46)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Affinity of the compound against HIV protease |
J Med Chem 40: 4079-88 (1998)
Article DOI: 10.1021/jm970288b
BindingDB Entry DOI: 10.7270/Q2XS5TG1 |
More data for this
Ligand-Target Pair | |
Vesicular acetylcholine transporter
(Torpedo californica) | BDBM50039623
((2R,3R)-5-Amino-3-(4-phenyl-piperidin-1-yl)-1,2,3,...)Show SMILES Nc1cccc2C[C@@H](O)[C@@H](Cc12)N1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C21H26N2O/c22-19-8-4-7-17-13-21(24)20(14-18(17)19)23-11-9-16(10-12-23)15-5-2-1-3-6-15/h1-8,16,20-21,24H,9-14,22H2/t20-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmaceutical Cancer Research
Curated by ChEMBL
| Assay Description
Displacement of (-)-[3H]vesamicol from VAChT in Torpedo californica electric organ synaptic vesicles |
Eur J Med Chem 100: 50-67 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.033
BindingDB Entry DOI: 10.7270/Q28C9XZ1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM160
(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H40N8O5/c50-35-33(23-27-9-3-1-4-10-27)48(25-29-13-7-15-31(21-29)37(52)46-39-42-17-18-43-39)41(54)49(34(36(35)51)24-28-11-5-2-6-12-28)26-30-14-8-16-32(22-30)38(53)47-40-44-19-20-45-40/h1-22,33-36,50-51H,23-26H2,(H2,42,43,46,52)(H2,44,45,47,53)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054184
(2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(5-methyl-1,...)Show SMILES Cc1cnc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4ncc(C)s4)C3=O)c2)s1 Show InChI InChI=1S/C43H42N6O5S2/c1-27-23-44-41(55-27)46-39(52)33-17-9-15-31(19-33)25-48-35(21-29-11-5-3-6-12-29)37(50)38(51)36(22-30-13-7-4-8-14-30)49(43(48)54)26-32-16-10-18-34(20-32)40(53)47-42-45-24-28(2)56-42/h3-20,23-24,35-38,50-51H,21-22,25-26H2,1-2H3,(H,44,46,52)(H,45,47,53)/t35-,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061405
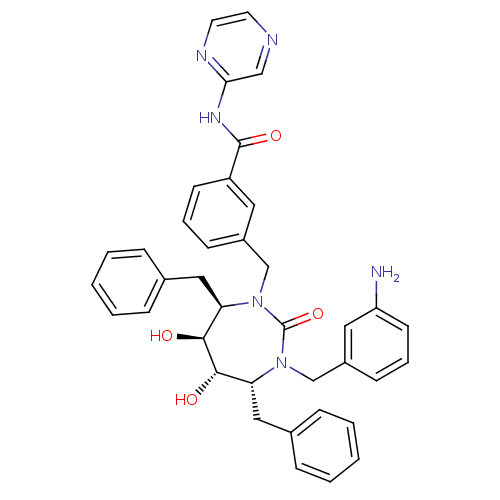
(3-[(4R,5S,6S,7R)-3-(3-Amino-benzyl)-4,7-dibenzyl-5...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)Nc3cnccn3)C2=O)c1 Show InChI InChI=1S/C38H38N6O4/c39-31-16-8-14-29(20-31)25-44-33(22-27-11-5-2-6-12-27)36(46)35(45)32(21-26-9-3-1-4-10-26)43(38(44)48)24-28-13-7-15-30(19-28)37(47)42-34-23-40-17-18-41-34/h1-20,23,32-33,35-36,45-46H,21-22,24-25,39H2,(H,41,42,47)/t32-,33-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Affinity of the compound against HIV protease |
J Med Chem 40: 4079-88 (1998)
Article DOI: 10.1021/jm970288b
BindingDB Entry DOI: 10.7270/Q2XS5TG1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054178
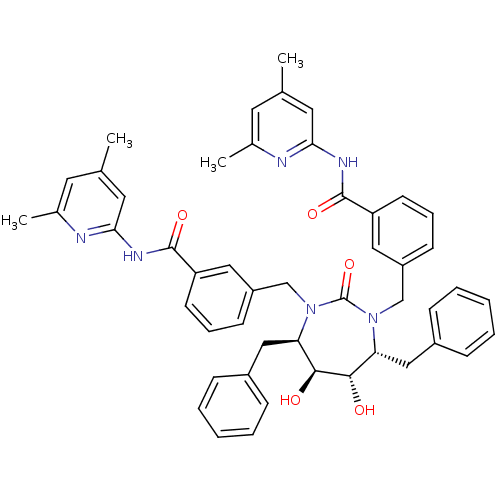
(2-{3-[4,7-dibenzyl-3-[3-(4,6-dimethyl-2-pyridylcar...)Show SMILES Cc1cc(C)nc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4cc(C)cc(C)n4)C3=O)c2)c1 Show InChI InChI=1S/C49H50N6O5/c1-31-21-33(3)50-43(23-31)52-47(58)39-19-11-17-37(25-39)29-54-41(27-35-13-7-5-8-14-35)45(56)46(57)42(28-36-15-9-6-10-16-36)55(49(54)60)30-38-18-12-20-40(26-38)48(59)53-44-24-32(2)22-34(4)51-44/h5-26,41-42,45-46,56-57H,27-30H2,1-4H3,(H,50,52,58)(H,51,53,59)/t41-,42-,45+,46+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054179
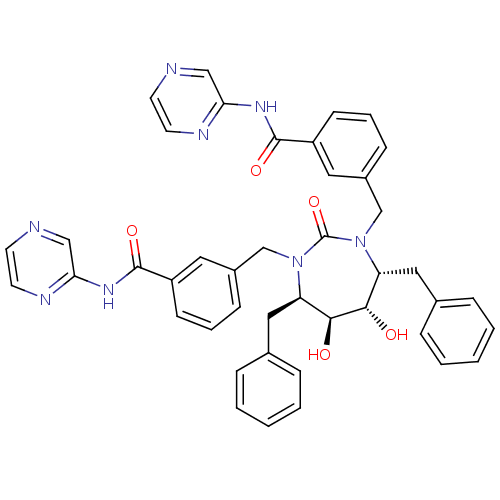
((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2cnccn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2cnccn2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H40N8O5/c52-39-35(23-29-9-3-1-4-10-29)50(27-31-13-7-15-33(21-31)41(54)48-37-25-44-17-19-46-37)43(56)51(36(40(39)53)24-30-11-5-2-6-12-30)28-32-14-8-16-34(22-32)42(55)49-38-26-45-18-20-47-38/h1-22,25-26,35-36,39-40,52-53H,23-24,27-28H2,(H,46,48,54)(H,47,49,55)/t35-,36-,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054174
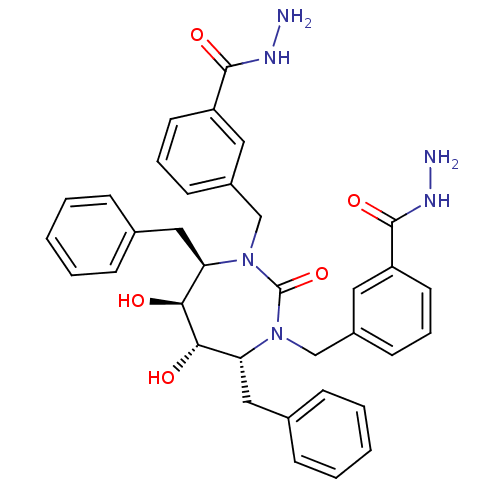
(3-[4,7-dibenzyl-3-(3-hydrazinocarbonylbenzyl)-5,6-...)Show SMILES NNC(=O)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)NN)C2=O)c1 Show InChI InChI=1S/C35H38N6O5/c36-38-33(44)27-15-7-13-25(17-27)21-40-29(19-23-9-3-1-4-10-23)31(42)32(43)30(20-24-11-5-2-6-12-24)41(35(40)46)22-26-14-8-16-28(18-26)34(45)39-37/h1-18,29-32,42-43H,19-22,36-37H2,(H,38,44)(H,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054174

(3-[4,7-dibenzyl-3-(3-hydrazinocarbonylbenzyl)-5,6-...)Show SMILES NNC(=O)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)NN)C2=O)c1 Show InChI InChI=1S/C35H38N6O5/c36-38-33(44)27-15-7-13-25(17-27)21-40-29(19-23-9-3-1-4-10-23)31(42)32(43)30(20-24-11-5-2-6-12-24)41(35(40)46)22-26-14-8-16-28(18-26)34(45)39-37/h1-18,29-32,42-43H,19-22,36-37H2,(H,38,44)(H,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054179

((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2cnccn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2cnccn2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H40N8O5/c52-39-35(23-29-9-3-1-4-10-29)50(27-31-13-7-15-33(21-31)41(54)48-37-25-44-17-19-46-37)43(56)51(36(40(39)53)24-30-11-5-2-6-12-30)28-32-14-8-16-34(22-32)42(55)49-38-26-45-18-20-47-38/h1-22,25-26,35-36,39-40,52-53H,23-24,27-28H2,(H,46,48,54)(H,47,49,55)/t35-,36-,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Affinity of the compound against HIV protease |
J Med Chem 40: 4079-88 (1998)
Article DOI: 10.1021/jm970288b
BindingDB Entry DOI: 10.7270/Q2XS5TG1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054178

(2-{3-[4,7-dibenzyl-3-[3-(4,6-dimethyl-2-pyridylcar...)Show SMILES Cc1cc(C)nc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4cc(C)cc(C)n4)C3=O)c2)c1 Show InChI InChI=1S/C49H50N6O5/c1-31-21-33(3)50-43(23-31)52-47(58)39-19-11-17-37(25-39)29-54-41(27-35-13-7-5-8-14-35)45(56)46(57)42(28-36-15-9-6-10-16-36)55(49(54)60)30-38-18-12-20-40(26-38)48(59)53-44-24-32(2)22-34(4)51-44/h5-26,41-42,45-46,56-57H,27-30H2,1-4H3,(H,50,52,58)(H,51,53,59)/t41-,42-,45+,46+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50368642

(ACETYLPEPSTATIN)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(C)=O)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C31H57N5O9/c1-15(2)11-21(35-30(44)28(18(7)8)36-31(45)27(17(5)6)33-20(10)37)23(38)13-25(40)32-19(9)29(43)34-22(12-16(3)4)24(39)14-26(41)42/h15-19,21-24,27-28,38-39H,11-14H2,1-10H3,(H,32,40)(H,33,37)(H,34,43)(H,35,44)(H,36,45)(H,41,42)/t19-,21-,22-,23-,24-,27-,28-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 55: 1424-44 (2012)
Article DOI: 10.1021/jm2010332
BindingDB Entry DOI: 10.7270/Q2D79F84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054168
((4alpha,5alpha,6beta,7beta)-3,3'-[Tetrahydro-5,6-d...)Show SMILES ONC(=O)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)NO)C2=O)c1 Show InChI InChI=1S/C35H36N4O7/c40-31-29(19-23-9-3-1-4-10-23)38(21-25-13-7-15-27(17-25)33(42)36-45)35(44)39(22-26-14-8-16-28(18-26)34(43)37-46)30(32(31)41)20-24-11-5-2-6-12-24/h1-18,29-32,40-41,45-46H,19-22H2,(H,36,42)(H,37,43)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054180
(2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(6-methyl-2-...)Show SMILES Cc1cccc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4cccc(C)n4)C3=O)c2)n1 Show InChI InChI=1S/C47H46N6O5/c1-31-13-9-23-41(48-31)50-45(56)37-21-11-19-35(25-37)29-52-39(27-33-15-5-3-6-16-33)43(54)44(55)40(28-34-17-7-4-8-18-34)53(47(52)58)30-36-20-12-22-38(26-36)46(57)51-42-24-10-14-32(2)49-42/h3-26,39-40,43-44,54-55H,27-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50063698

(4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccc2cc(ccc2c1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C19H17N5O2/c20-17(21)14-2-1-13-10-16(8-5-12(13)9-14)26-18(25)11-3-6-15(7-4-11)24-19(22)23/h1-10H,(H3,20,21)(H4,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 5: 1219-24 (2014)
Article DOI: 10.1021/ml500254r
BindingDB Entry DOI: 10.7270/Q2VM4DV8 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030756
(CHEMBL3342197)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C35H33N5O4S2/c1-39(2)19-6-8-23-14-16-25(17-15-23)44-21-7-13-30-31(33(42)43)37-35(46-30)40-20-18-24-9-5-10-26(27(24)22-40)32(41)38-34-36-28-11-3-4-12-29(28)45-34/h3-5,9-12,14-17H,7,13,18-22H2,1-2H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054168

((4alpha,5alpha,6beta,7beta)-3,3'-[Tetrahydro-5,6-d...)Show SMILES ONC(=O)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)NO)C2=O)c1 Show InChI InChI=1S/C35H36N4O7/c40-31-29(19-23-9-3-1-4-10-23)38(21-25-13-7-15-27(17-25)33(42)36-45)35(44)39(22-26-14-8-16-28(18-26)34(43)37-46)30(32(31)41)20-24-11-5-2-6-12-24/h1-18,29-32,40-41,45-46H,19-22H2,(H,36,42)(H,37,43)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054180

(2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(6-methyl-2-...)Show SMILES Cc1cccc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4cccc(C)n4)C3=O)c2)n1 Show InChI InChI=1S/C47H46N6O5/c1-31-13-9-23-41(48-31)50-45(56)37-21-11-19-35(25-37)29-52-39(27-33-15-5-3-6-16-33)43(54)44(55)40(28-34-17-7-4-8-18-34)53(47(52)58)30-36-20-12-22-38(26-36)46(57)51-42-24-10-14-32(2)49-42/h3-26,39-40,43-44,54-55H,27-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054179

((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2cnccn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2cnccn2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C43H40N8O5/c52-39-35(23-29-9-3-1-4-10-29)50(27-31-13-7-15-33(21-31)41(54)48-37-25-44-17-19-46-37)43(56)51(36(40(39)53)24-30-11-5-2-6-12-30)28-32-14-8-16-34(22-32)42(55)49-38-26-45-18-20-47-38/h1-22,25-26,35-36,39-40,52-53H,23-24,27-28H2,(H,46,48,54)(H,47,49,55)/t35-,36-,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061410
(3-[(4R,5S,6S,7R)-3-(3-Amino-benzyl)-4,7-dibenzyl-5...)Show SMILES Nc1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=O)Nc3nc4ccccc4[nH]3)C2=O)c1 Show InChI InChI=1S/C41H40N6O4/c42-32-18-10-16-30(22-32)26-47-36(24-28-13-5-2-6-14-28)38(49)37(48)35(23-27-11-3-1-4-12-27)46(41(47)51)25-29-15-9-17-31(21-29)39(50)45-40-43-33-19-7-8-20-34(33)44-40/h1-22,35-38,48-49H,23-26,42H2,(H2,43,44,45,50)/t35-,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Affinity of the compound against HIV protease |
J Med Chem 40: 4079-88 (1998)
Article DOI: 10.1021/jm970288b
BindingDB Entry DOI: 10.7270/Q2XS5TG1 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086

(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM155
(CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nc3ccccc3[nH]2)C(=O)N(Cc2cccc(c2)C(=O)Nc2nc3ccccc3[nH]2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C49H44N8O5/c58-43-41(27-31-13-3-1-4-14-31)56(29-33-17-11-19-35(25-33)45(60)54-47-50-37-21-7-8-22-38(37)51-47)49(62)57(42(44(43)59)28-32-15-5-2-6-16-32)30-34-18-12-20-36(26-34)46(61)55-48-52-39-23-9-10-24-40(39)53-48/h1-26,41-44,58-59H,27-30H2,(H2,50,51,54,60)(H2,52,53,55,61)/t41-,42-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061408
((3-{(4R,5S,6S,7R)-4,7-Dibenzyl-3-[3-(cyanomethyl-c...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)NCC(O)=O)C(=O)N(Cc2cccc(c2)C(=O)NCC#N)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C39H39N5O7/c40-17-18-41-37(49)30-15-7-13-28(19-30)24-43-32(21-26-9-3-1-4-10-26)35(47)36(48)33(22-27-11-5-2-6-12-27)44(39(43)51)25-29-14-8-16-31(20-29)38(50)42-23-34(45)46/h1-16,19-20,32-33,35-36,47-48H,18,21-25H2,(H,41,49)(H,42,50)(H,45,46)/t32-,33-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Affinity of the compound against HIV protease |
J Med Chem 40: 4079-88 (1998)
Article DOI: 10.1021/jm970288b
BindingDB Entry DOI: 10.7270/Q2XS5TG1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054181
(2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(4-methyl-1,...)Show SMILES Cc1csc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4nc(C)cs4)C3=O)c2)n1 Show InChI InChI=1S/C43H42N6O5S2/c1-27-25-55-41(44-27)46-39(52)33-17-9-15-31(19-33)23-48-35(21-29-11-5-3-6-12-29)37(50)38(51)36(22-30-13-7-4-8-14-30)49(43(48)54)24-32-16-10-18-34(20-32)40(53)47-42-45-28(2)26-56-42/h3-20,25-26,35-38,50-51H,21-24H2,1-2H3,(H,44,46,52)(H,45,47,53)/t35-,36-,37+,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM209074
(US9266877, 20)Show SMILES Cc1c(cnn1Cc1ccccc1)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C34H28N6O3S/c1-21-26(18-35-40(21)19-22-8-3-2-4-9-22)24-14-15-30(37-31(24)33(42)43)39-17-16-23-10-7-11-25(27(23)20-39)32(41)38-34-36-28-12-5-6-13-29(28)44-34/h2-15,18H,16-17,19-20H2,1H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM154

(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nccs2)C(=O)N(Cc2cccc(c2)C(=O)Nc2nccs2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H38N6O5S2/c48-35-33(23-27-9-3-1-4-10-27)46(25-29-13-7-15-31(21-29)37(50)44-39-42-17-19-53-39)41(52)47(34(36(35)49)24-28-11-5-2-6-12-28)26-30-14-8-16-32(22-30)38(51)45-40-43-18-20-54-40/h1-22,33-36,48-49H,23-26H2,(H,42,44,50)(H,43,45,51)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054183
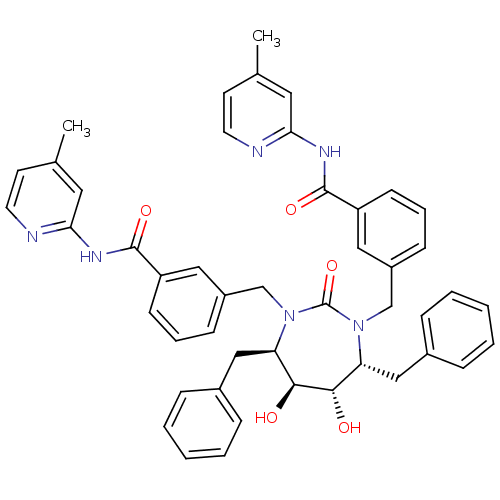
(2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(4-methyl-2-...)Show SMILES Cc1ccnc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4cc(C)ccn4)C3=O)c2)c1 Show InChI InChI=1S/C47H46N6O5/c1-31-19-21-48-41(23-31)50-45(56)37-17-9-15-35(25-37)29-52-39(27-33-11-5-3-6-12-33)43(54)44(55)40(28-34-13-7-4-8-14-34)53(47(52)58)30-36-16-10-18-38(26-36)46(57)51-42-24-32(2)20-22-49-42/h3-26,39-40,43-44,54-55H,27-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50207116

(CHEMBL3905247 | US9550741, I-4)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2[nH]1 |r,wU:6.6,wD:3.2,(25.87,-26.54,;26.59,-25.21,;25.82,-23.88,;24.29,-23.88,;23.52,-22.54,;21.97,-22.54,;21.2,-23.88,;19.67,-23.88,;18.9,-22.54,;17.36,-22.54,;16.59,-23.88,;15.06,-23.88,;14.29,-22.54,;15.06,-21.21,;16.59,-21.21,;12.75,-22.54,;11.83,-23.78,;10.4,-23.31,;10.4,-21.77,;9.27,-20.75,;9.58,-19.21,;11.07,-18.75,;12.13,-19.78,;11.83,-21.26,;21.97,-25.21,;23.52,-25.21,;28.13,-25.11,;28.95,-23.83,;30.43,-24.24,;31.72,-23.36,;33.1,-24.08,;33.15,-25.62,;31.87,-26.44,;30.48,-25.77,;29.05,-26.29,)| Show InChI InChI=1S/C28H33N5OS/c34-28(25-19-21-5-1-3-7-24(21)30-25)29-22-11-9-20(10-12-22)13-14-32-15-17-33(18-16-32)27-23-6-2-4-8-26(23)35-31-27/h1-8,19-20,22,30H,9-18H2,(H,29,34)/t20-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... |
Eur J Med Chem 123: 332-353 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.038
BindingDB Entry DOI: 10.7270/Q2GH9KXP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592
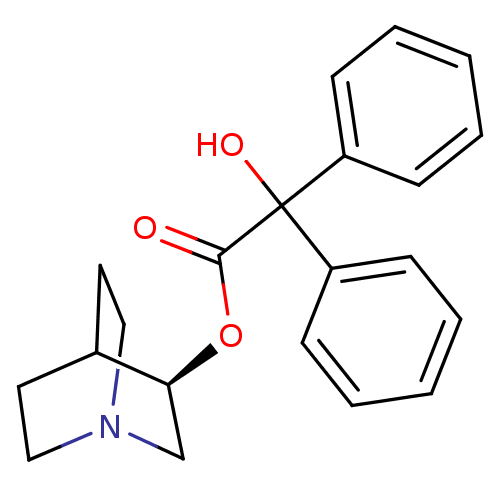
(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393725
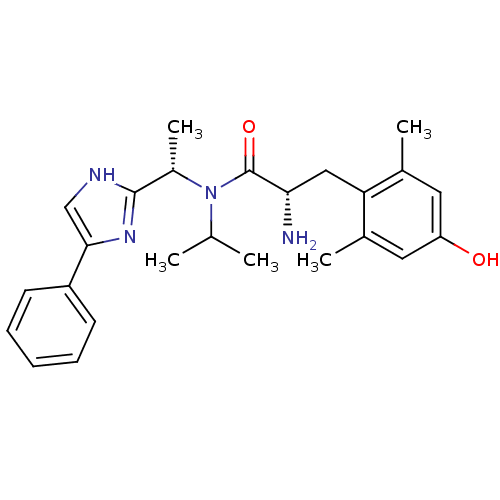
(CHEMBL2159117)Show SMILES CC(C)N([C@@H](C)c1nc(c[nH]1)-c1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C25H32N4O2/c1-15(2)29(18(5)24-27-14-23(28-24)19-9-7-6-8-10-19)25(31)22(26)13-21-16(3)11-20(30)12-17(21)4/h6-12,14-15,18,22,30H,13,26H2,1-5H3,(H,27,28)/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030751
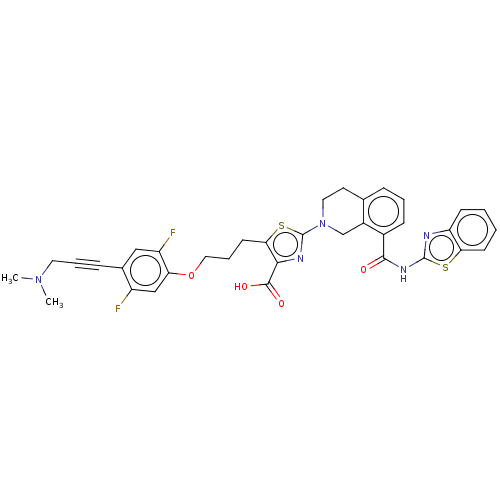
(CHEMBL3342334)Show SMILES CN(C)CC#Cc1cc(F)c(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H31F2N5O4S2/c1-41(2)15-6-9-22-18-26(37)28(19-25(22)36)46-17-7-13-30-31(33(44)45)39-35(48-30)42-16-14-21-8-5-10-23(24(21)20-42)32(43)40-34-38-27-11-3-4-12-29(27)47-34/h3-5,8,10-12,18-19H,7,13-17,20H2,1-2H3,(H,44,45)(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054183

(2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(4-methyl-2-...)Show SMILES Cc1ccnc(NC(=O)c2cccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4cccc(c4)C(=O)Nc4cc(C)ccn4)C3=O)c2)c1 Show InChI InChI=1S/C47H46N6O5/c1-31-19-21-48-41(23-31)50-45(56)37-17-9-15-35(25-37)29-52-39(27-33-11-5-3-6-12-33)43(54)44(55)40(28-34-13-7-4-8-14-34)53(47(52)58)30-36-16-10-18-38(26-36)46(57)51-42-24-32(2)20-22-49-42/h3-26,39-40,43-44,54-55H,27-30H2,1-2H3,(H,48,50,56)(H,49,51,57)/t39-,40-,43+,44+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity for HIV-1 protease |
J Med Chem 39: 4299-312 (1996)
Article DOI: 10.1021/jm9602773
BindingDB Entry DOI: 10.7270/Q2T152QJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50393719
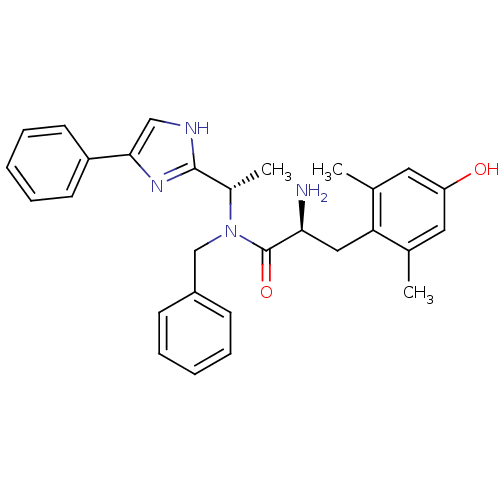
(CHEMBL2159118)Show SMILES C[C@H](N(Cc1ccccc1)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C29H32N4O2/c1-19-14-24(34)15-20(2)25(19)16-26(30)29(35)33(18-22-10-6-4-7-11-22)21(3)28-31-17-27(32-28)23-12-8-5-9-13-23/h4-15,17,21,26,34H,16,18,30H2,1-3H3,(H,31,32)/t21-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat mu opioid receptor |
Bioorg Med Chem Lett 22: 4869-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.042
BindingDB Entry DOI: 10.7270/Q2F76DP8 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757

(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data