

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50366780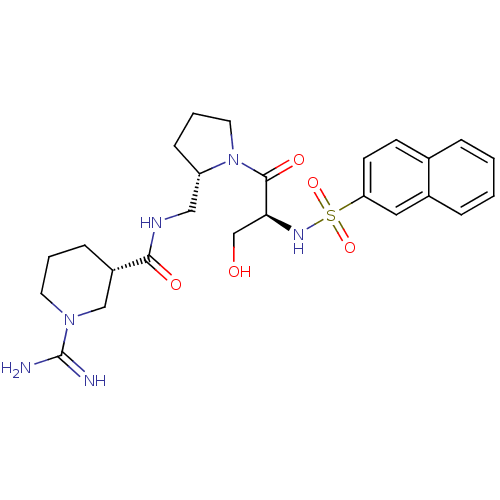 (BMS-189090 | CHEMBL138877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro reversible inhibition of thrombin catalytic activity | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50039010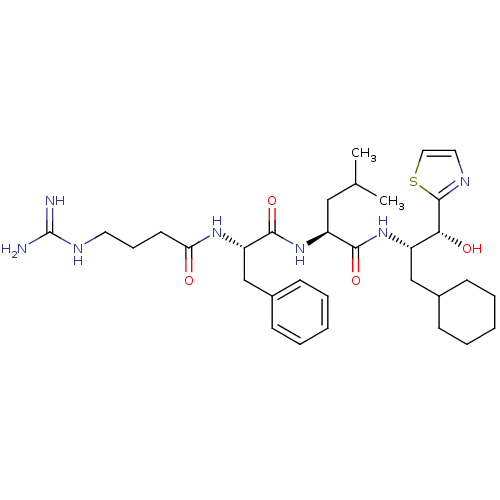 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for thrombin was reported | J Med Chem 37: 2122-4 (1994) BindingDB Entry DOI: 10.7270/Q2FQ9VPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087518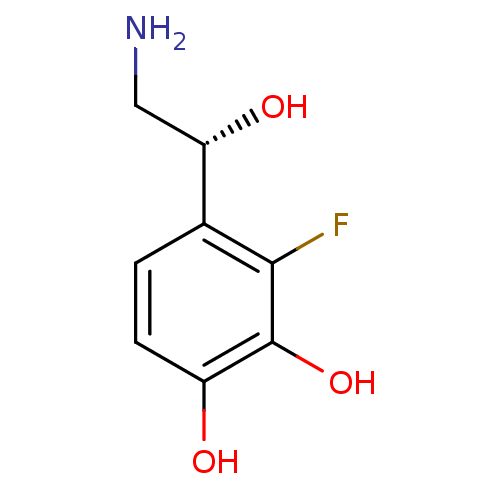 ((R)4-(2-Amino-1-hydroxy-ethyl)-3-fluoro-benzene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087520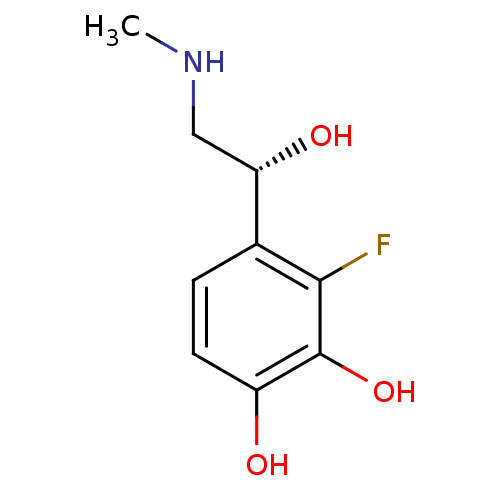 ((R)3-Fluoro-4-(1-hydroxy-2-methylamino-ethyl)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087518 ((R)4-(2-Amino-1-hydroxy-ethyl)-3-fluoro-benzene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50019060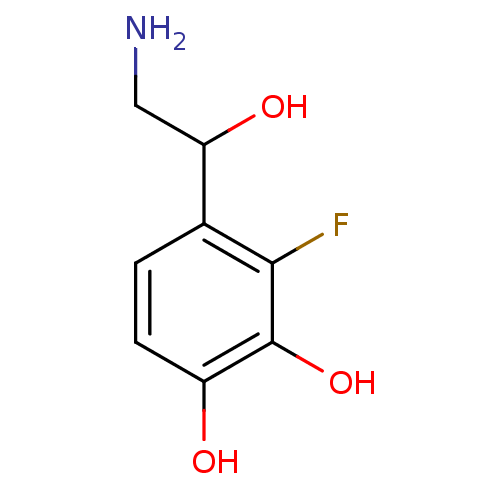 ((+/-)4-(2-Amino-1-hydroxy-ethyl)-3-fluoro-benzene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50019060 ((+/-)4-(2-Amino-1-hydroxy-ethyl)-3-fluoro-benzene-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50019059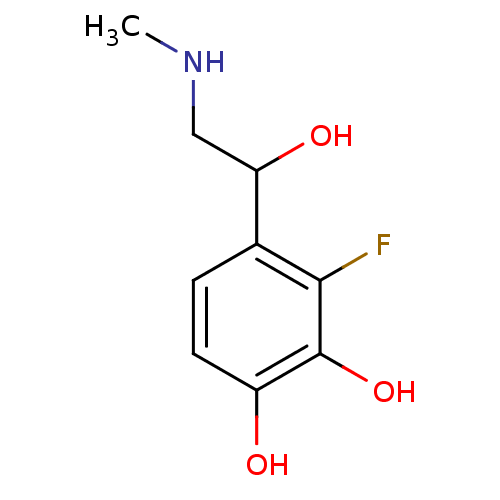 ((+/-)3-Fluoro-4-(1-hydroxy-2-methylamino-ethyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287156 (2-Benzyl-1H-indole-5-carboxamidine | CHEMBL287401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of human alpha-thrombin catalytic activity | Bioorg Med Chem Lett 6: 1339-1344 (1996) Article DOI: 10.1016/0960-894X(96)00229-6 BindingDB Entry DOI: 10.7270/Q2TM7B2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087520 ((R)3-Fluoro-4-(1-hydroxy-2-methylamino-ethyl)-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50029050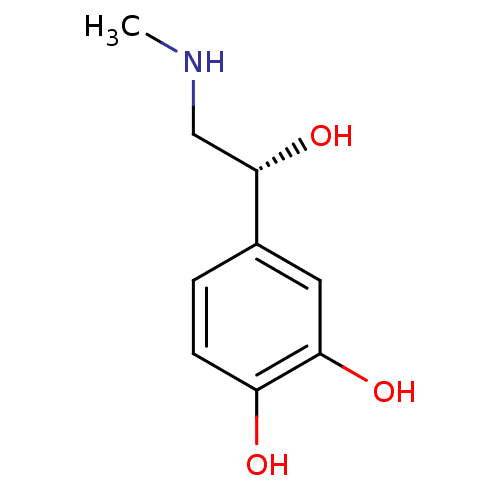 ((-)-(R)-epinephrine | (-)-3,4-dihydroxy-alpha-((me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50029050 ((-)-(R)-epinephrine | (-)-3,4-dihydroxy-alpha-((me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50019059 ((+/-)3-Fluoro-4-(1-hydroxy-2-methylamino-ethyl)-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50029051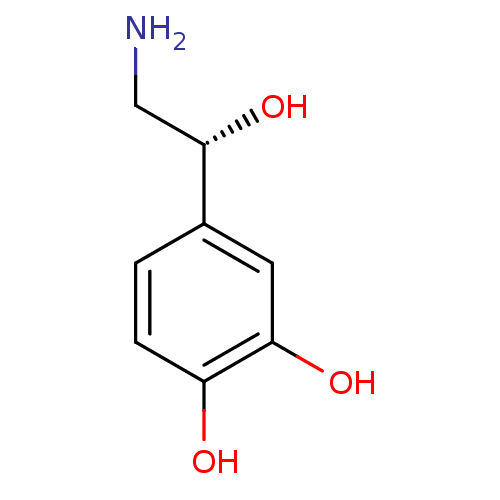 ((-)-arterenol | (-)-noradrenaline | (-)-norepineph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087512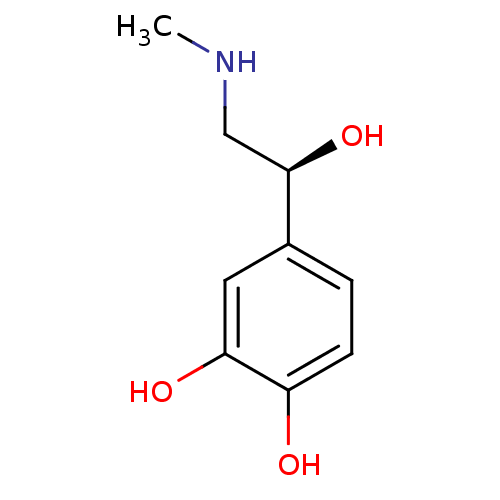 ((+)-adrenaline | (S)-adrenaline | CHEMBL42280) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50029051 ((-)-arterenol | (-)-noradrenaline | (-)-norepineph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087512 ((+)-adrenaline | (S)-adrenaline | CHEMBL42280) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087517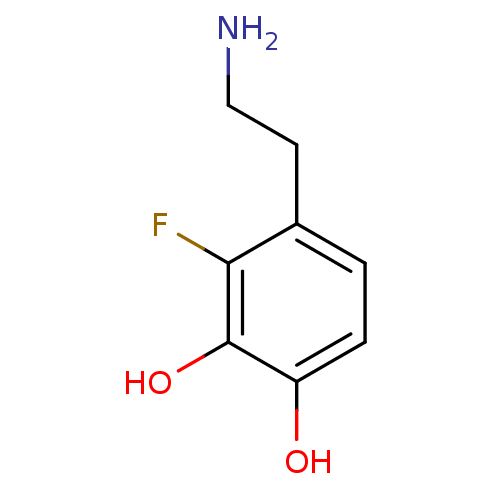 (4-(2-Amino-ethyl)-3-fluoro-benzene-1,2-diol | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087514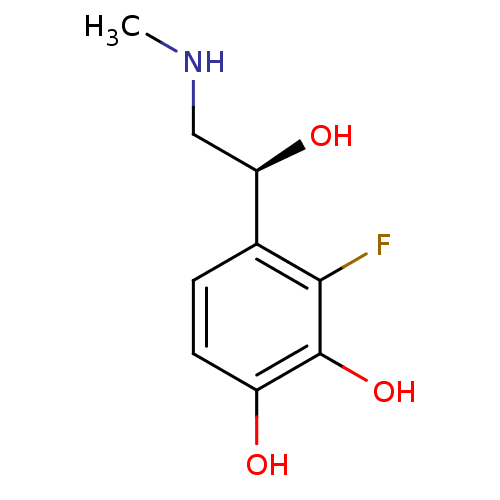 ((S)3-Fluoro-4-(1-hydroxy-2-methylamino-ethyl)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087513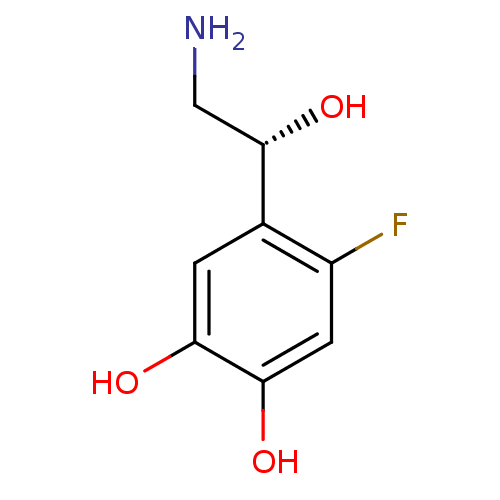 ((R)4-(2-Amino-1-hydroxy-ethyl)-5-fluoro-benzene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087516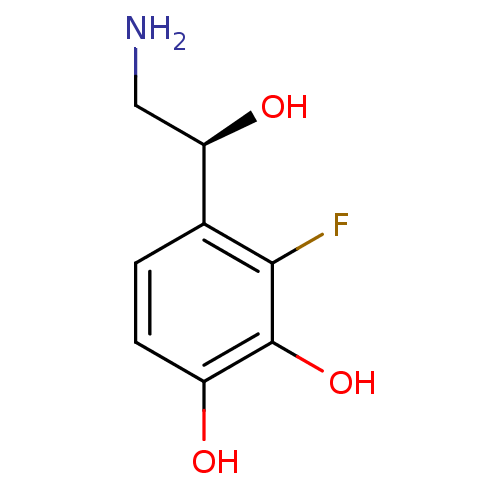 ((S)4-(2-Amino-1-hydroxy-ethyl)-3-fluoro-benzene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087513 ((R)4-(2-Amino-1-hydroxy-ethyl)-5-fluoro-benzene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50087515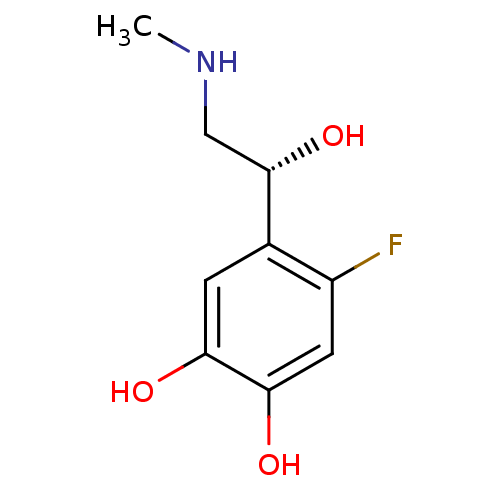 ((R)4-Fluoro-5-(1-hydroxy-2-methylamino-ethyl)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087510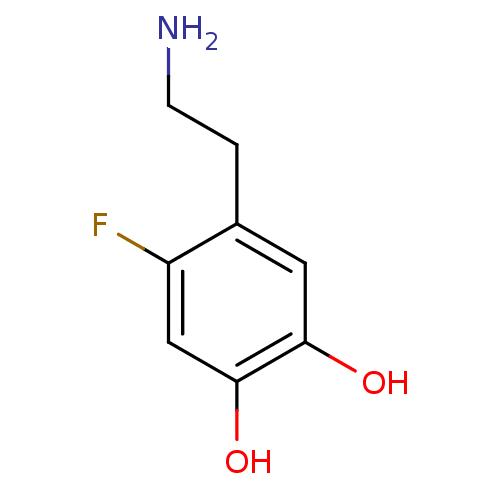 (4-(2-Amino-ethyl)-5-fluoro-benzene-1,2-diol | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50019057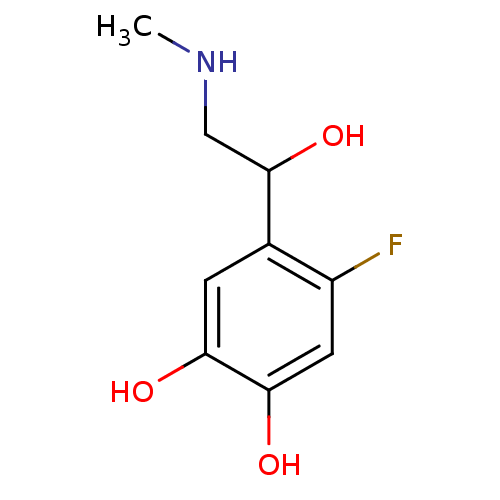 ((+/-)4-Fluoro-5-(1-hydroxy-2-methylamino-ethyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50087516 ((S)4-(2-Amino-1-hydroxy-ethyl)-3-fluoro-benzene-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50019061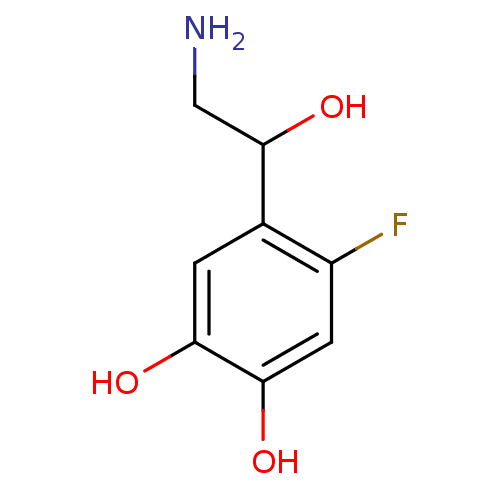 ((+/-)4-(2-Amino-1-hydroxy-ethyl)-5-fluoro-benzene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-2 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50019061 ((+/-)4-(2-Amino-1-hydroxy-ethyl)-5-fluoro-benzene-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding of [3H]-dihydroalprenolol to beta-1 adrenergic receptor of rat cerebral cortical membranes | J Med Chem 43: 1611-9 (2000) BindingDB Entry DOI: 10.7270/Q25M64ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM84007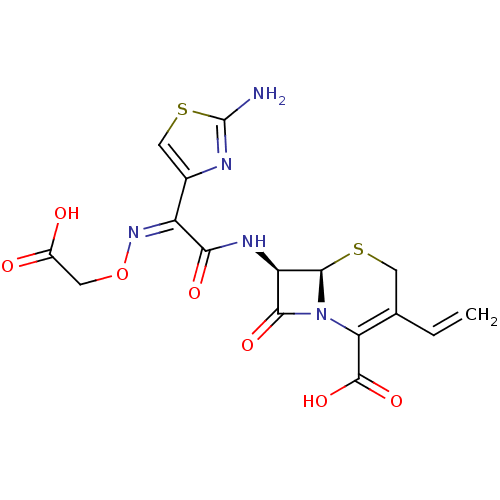 (CEFIXIME | Cefiximum [Latin] | MLS002222332 | SMR0...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PubMed | 1.31E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Giessen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Cefadroxil uptake in Caco-2 cells | J Pharmacol Exp Ther 277: 831-9 (1996) BindingDB Entry DOI: 10.7270/Q20G3P0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50350467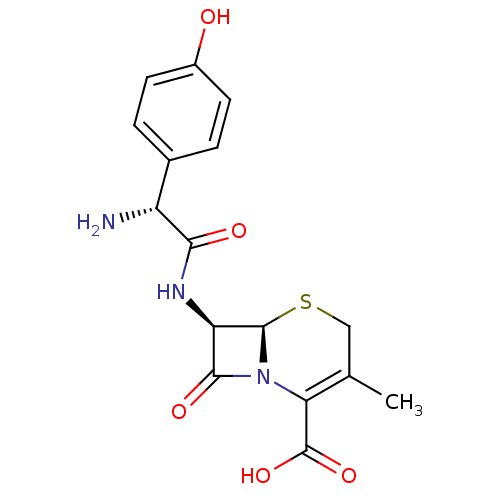 (BL-S578 | CEFADROXIL | Cefadrops) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 1.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Giessen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Cefixim uptake in Caco-2 cells | J Pharmacol Exp Ther 277: 831-9 (1996) BindingDB Entry DOI: 10.7270/Q20G3P0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Trypsin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Trypsin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Plasmin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244556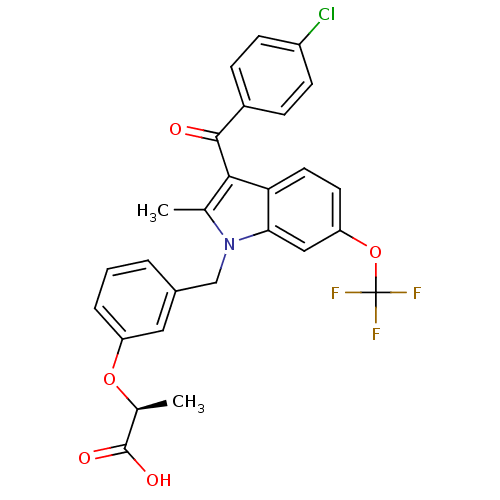 ((2S)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244702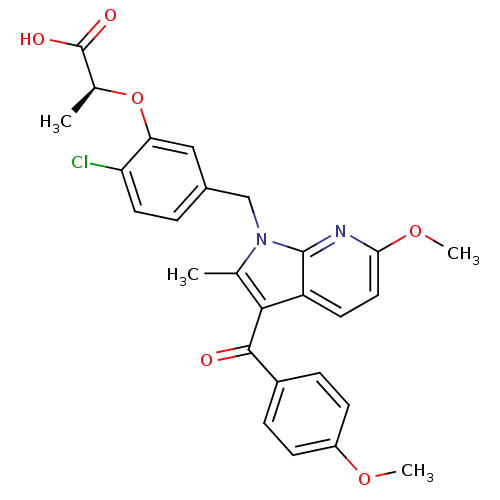 ((S)-2-(2-chloro-5-((6-methoxy-3-(4-methoxybenzoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50125393 (CHEMBL57014) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase activity in H4 human neuroglioma cells by measuring amyloid beta production | Bioorg Med Chem Lett 14: 1917-21 (2004) BindingDB Entry DOI: 10.7270/Q2TT4T4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50223758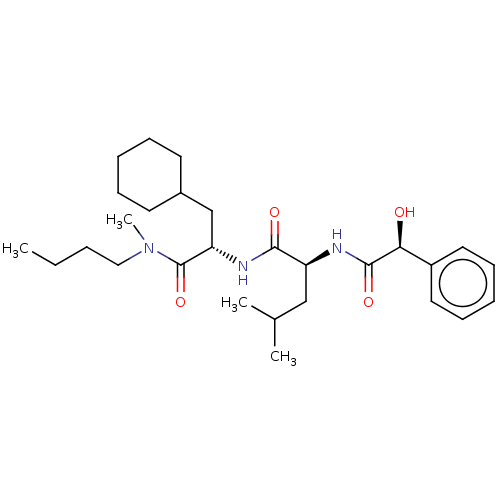 (CHEMBL58925) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase activity in H4 human neuroglioma cells by measuring amyloid beta production | Bioorg Med Chem Lett 14: 1917-21 (2004) BindingDB Entry DOI: 10.7270/Q2TT4T4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244700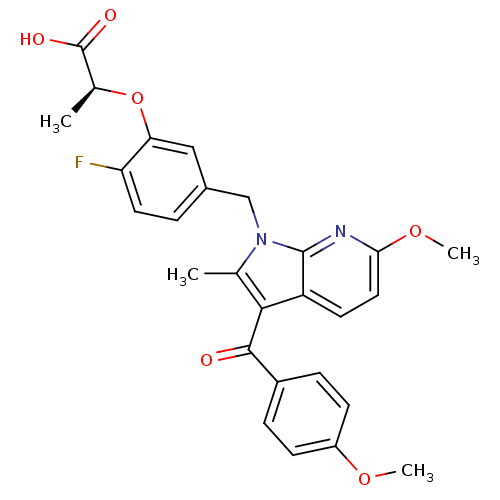 ((S)-2-(2-fluoro-5-((6-methoxy-3-(4-methoxybenzoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244699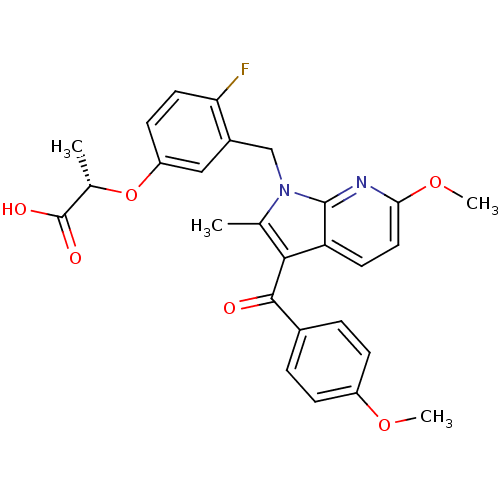 ((S)-2-(4-fluoro-3-((6-methoxy-3-(4-methoxybenzoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244701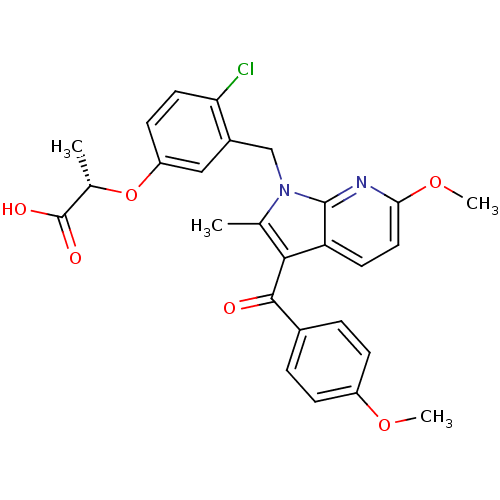 ((S)-2-(4-chloro-3-((6-methoxy-3-(4-methoxybenzoyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244748 ((S)-2-(2-chloro-5-((3-(4-chlorophenoxy)-6-fluoro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244750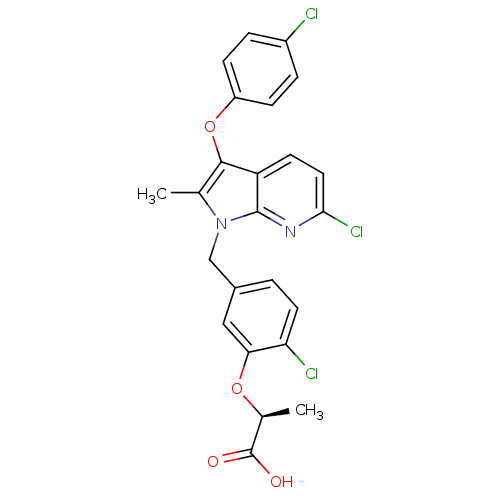 ((S)-2-(2-chloro-5-((6-chloro-3-(4-chlorophenoxy)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50244657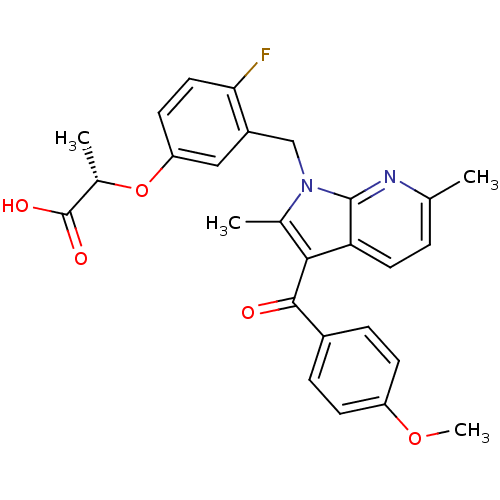 ((S)-2-(4-fluoro-3-((3-(4-methoxybenzoyl)-2,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476941 (CHEMBL233363) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1275 total ) | Next | Last >> |