 Found 336 hits with Last Name = 'schuler' and Initial = 'w'
Found 336 hits with Last Name = 'schuler' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of PKCbeta1 (unknown origin) |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of PKCalpha (unknown origin) |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235283
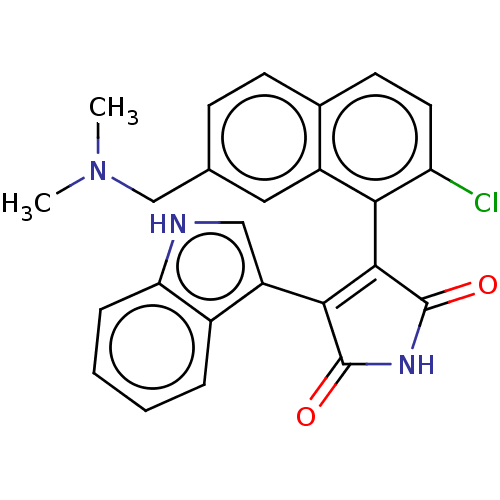
(CHEMBL4102228)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-8-15-9-10-19(26)21(17(15)11-14)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-16(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235288

(CHEMBL4072879)Show SMILES CNCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:12| Show InChI InChI=1S/C25H20ClN3O2/c1-27-12-14-7-8-15-9-10-19(26)21(17(15)11-14)23-22(24(30)28-25(23)31)18-13-29(2)20-6-4-3-5-16(18)20/h3-11,13,27H,12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609
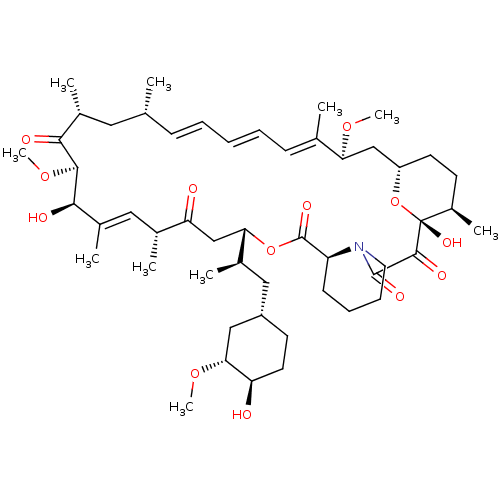
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its ability to compete with immobilized FK506 for binding to biotinylated FK506 binding protein 12 in a competitive ... |
Bioorg Med Chem Lett 9: 459-62 (1999)
BindingDB Entry DOI: 10.7270/Q23N23WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235283

(CHEMBL4102228)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-8-15-9-10-19(26)21(17(15)11-14)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-16(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50030448
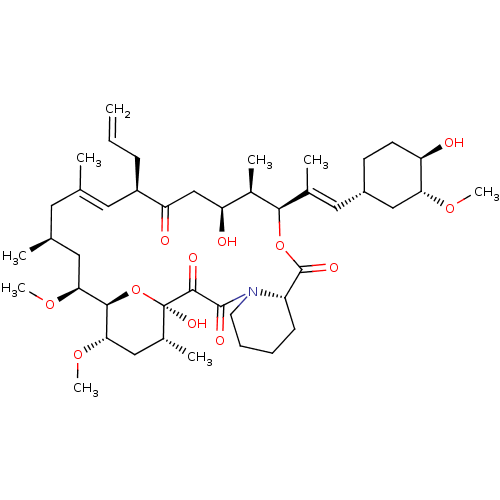
(8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |r,t:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235288

(CHEMBL4072879)Show SMILES CNCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:12| Show InChI InChI=1S/C25H20ClN3O2/c1-27-12-14-7-8-15-9-10-19(26)21(17(15)11-14)23-22(24(30)28-25(23)31)18-13-29(2)20-6-4-3-5-16(18)20/h3-11,13,27H,12H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50153090

(CHEMBL411735 | Macrolide derivative)Show SMILES COC1C[C@H](CC(C)[C@H]2OC(=O)C3CCCCN3C(=O)C(=O)[C@]3(O)CC([C@H](C[C@H]3C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)CC[C@H]1CC(=O)Nc1ccc(CCCC(=O)OC)cc1 |t:40| Show InChI InChI=1S/C58H88N2O13/c1-11-15-42-27-35(2)26-36(3)28-50(70-8)45-34-58(68,38(5)30-51(45)71-9)55(65)56(66)60-25-13-12-17-46(60)57(67)73-54(39(6)47(61)33-48(42)62)37(4)29-41-19-22-43(49(31-41)69-7)32-52(63)59-44-23-20-40(21-24-44)16-14-18-53(64)72-10/h11,20-21,23-24,27,36-39,41-43,45-47,49-51,54,61,68H,1,12-19,22,25-26,28-34H2,2-10H3,(H,59,63)/b35-27+/t36-,37?,38+,39+,41-,42+,43-,45?,46?,47-,49?,50-,51-,54+,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235292

(CHEMBL4085837)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-9-16-15(11-14)8-10-19(26)21(16)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-17(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50153091
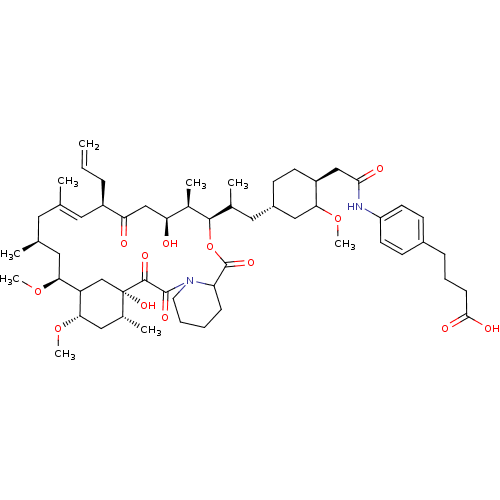
(CHEMBL265123 | Macrolide derivative)Show SMILES COC1C[C@H](CC(C)[C@H]2OC(=O)C3CCCCN3C(=O)C(=O)[C@]3(O)CC([C@H](C[C@H]3C)OC)[C@H](C[C@@H](C)C\C(C)=C\[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)OC)CC[C@H]1CC(=O)Nc1ccc(CCCC(O)=O)cc1 |t:40| Show InChI InChI=1S/C57H86N2O13/c1-10-14-41-26-34(2)25-35(3)27-49(70-8)44-33-57(68,37(5)29-50(44)71-9)54(65)55(66)59-24-12-11-16-45(59)56(67)72-53(38(6)46(60)32-47(41)61)36(4)28-40-18-21-42(48(30-40)69-7)31-51(62)58-43-22-19-39(20-23-43)15-13-17-52(63)64/h10,19-20,22-23,26,35-38,40-42,44-46,48-50,53,60,68H,1,11-18,21,24-25,27-33H2,2-9H3,(H,58,62)(H,63,64)/b34-26+/t35-,36?,37+,38+,40-,41+,42-,44?,45?,46-,48?,49-,50-,53+,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research and Novartis Pharma Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against macrophilin (FKBP-12) |
J Med Chem 47: 4950-7 (2004)
Article DOI: 10.1021/jm031101l
BindingDB Entry DOI: 10.7270/Q21N81WK |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235287
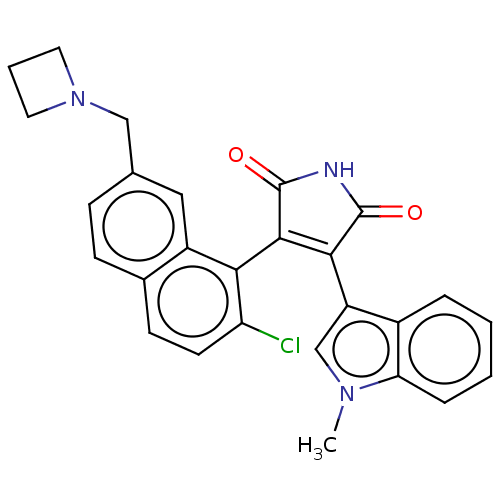
(CHEMBL4089217)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c(Cl)ccc3ccc(CN4CCC4)cc23)c2ccccc12 |t:4| Show InChI InChI=1S/C27H22ClN3O2/c1-30-15-20(18-5-2-3-6-22(18)30)24-25(27(33)29-26(24)32)23-19-13-16(14-31-11-4-12-31)7-8-17(19)9-10-21(23)28/h2-3,5-10,13,15H,4,11-12,14H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391897
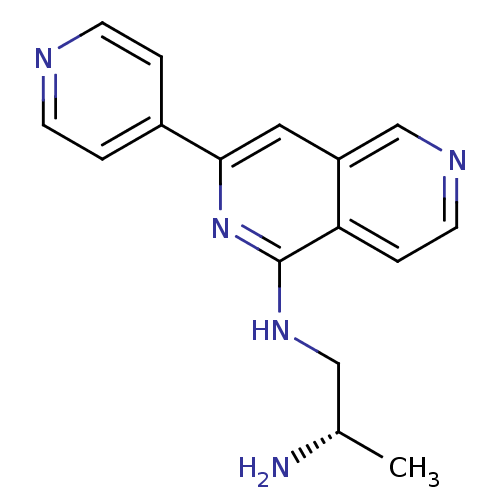
(CHEMBL2147537)Show InChI InChI=1S/C16H17N5/c1-11(17)9-20-16-14-4-7-19-10-13(14)8-15(21-16)12-2-5-18-6-3-12/h2-8,10-11H,9,17H2,1H3,(H,20,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235286

(CHEMBL4083319)Show SMILES CC(C)NCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:14| Show InChI InChI=1S/C27H24ClN3O2/c1-15(2)29-13-16-8-9-17-10-11-21(28)23(19(17)12-16)25-24(26(32)30-27(25)33)20-14-31(3)22-7-5-4-6-18(20)22/h4-12,14-15,29H,13H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235285

(CHEMBL4101316)Show SMILES COCCNCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:15| Show InChI InChI=1S/C27H24ClN3O3/c1-31-15-20(18-5-3-4-6-22(18)31)24-25(27(33)30-26(24)32)23-19-13-16(14-29-11-12-34-2)7-8-17(19)9-10-21(23)28/h3-10,13,15,29H,11-12,14H2,1-2H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235286

(CHEMBL4083319)Show SMILES CC(C)NCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:14| Show InChI InChI=1S/C27H24ClN3O2/c1-15(2)29-13-16-8-9-17-10-11-21(28)23(19(17)12-16)25-24(26(32)30-27(25)33)20-14-31(3)22-7-5-4-6-18(20)22/h4-12,14-15,29H,13H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235285

(CHEMBL4101316)Show SMILES COCCNCc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:15| Show InChI InChI=1S/C27H24ClN3O3/c1-31-15-20(18-5-3-4-6-22(18)31)24-25(27(33)30-26(24)32)23-19-13-16(14-29-11-12-34-2)7-8-17(19)9-10-21(23)28/h3-10,13,15,29H,11-12,14H2,1-2H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235281
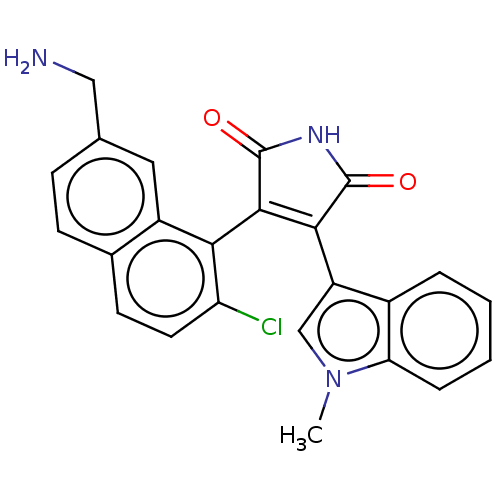
(CHEMBL4089665)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c(Cl)ccc3ccc(CN)cc23)c2ccccc12 |t:4| Show InChI InChI=1S/C24H18ClN3O2/c1-28-12-17(15-4-2-3-5-19(15)28)21-22(24(30)27-23(21)29)20-16-10-13(11-26)6-7-14(16)8-9-18(20)25/h2-10,12H,11,26H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]mazindol from dopamine transporter |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235292

(CHEMBL4085837)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-9-16-15(11-14)8-10-19(26)21(16)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-17(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235287

(CHEMBL4089217)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c(Cl)ccc3ccc(CN4CCC4)cc23)c2ccccc12 |t:4| Show InChI InChI=1S/C27H22ClN3O2/c1-30-15-20(18-5-2-3-6-22(18)30)24-25(27(33)29-26(24)32)23-19-13-16(14-31-11-4-12-31)7-8-17(19)9-10-21(23)28/h2-3,5-10,13,15H,4,11-12,14H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50075184

(28-O-methylrapamycin | CHEMBL140442)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H](CCO)CCC(=O)OC |c:33,t:48,50,52| Show InChI InChI=1S/C51H79NO14/c1-31-16-12-11-13-17-32(2)42(62-8)29-39-21-19-37(7)51(61,66-39)48(58)49(59)52-24-15-14-18-40(52)50(60)65-43(34(4)28-38(23-25-53)20-22-44(55)63-9)30-41(54)33(3)27-36(6)46(57)47(64-10)45(56)35(5)26-31/h11-13,16-17,27,31,33-35,37-40,42-43,46-47,53,57,61H,14-15,18-26,28-30H2,1-10H3/b13-11+,16-12+,32-17+,36-27+/t31-,33-,34-,35-,37-,38-,39+,40+,42+,43+,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its ability to compete with immobilized FK506 for binding to biotinylated FK506 binding protein 12 in a competitive ... |
Bioorg Med Chem Lett 9: 459-62 (1999)
BindingDB Entry DOI: 10.7270/Q23N23WR |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235284
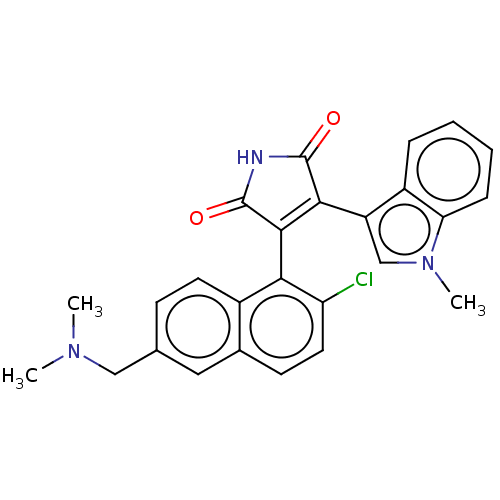
(CHEMBL4064291)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-10-17-16(12-15)9-11-20(27)22(17)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-18(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391902
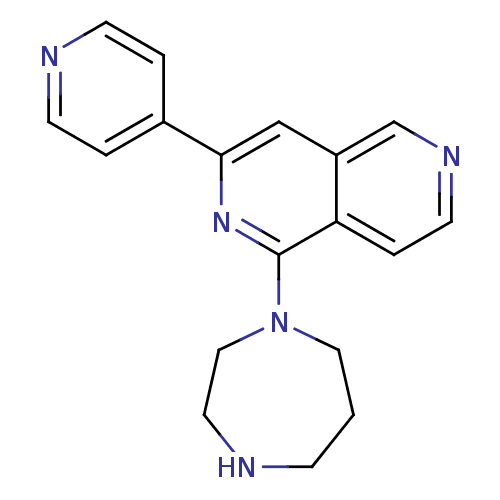
(CHEMBL2147542)Show InChI InChI=1S/C18H19N5/c1-5-19-9-11-23(10-1)18-16-4-8-21-13-15(16)12-17(22-18)14-2-6-20-7-3-14/h2-4,6-8,12-13,19H,1,5,9-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235282

(CHEMBL4077967)Show SMILES CN(C)Cc1ccc2ccc(Cl)c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c2c1 |t:13| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-9-16-10-11-20(27)22(18(16)12-15)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-17(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [3H]dopamine uptake |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50235292

(CHEMBL4085837)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-9-16-15(11-14)8-10-19(26)21(16)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-17(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCepsilon (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50235281

(CHEMBL4089665)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2c(Cl)ccc3ccc(CN)cc23)c2ccccc12 |t:4| Show InChI InChI=1S/C24H18ClN3O2/c1-28-12-17(15-4-2-3-5-19(15)28)21-22(24(30)27-23(21)29)20-16-10-13(11-26)6-7-14(16)8-9-18(20)25/h2-10,12H,11,26H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14028

((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(C)c12 |r| Show InChI InChI=1S/C16H21N3O2S/c1-12-9-18-11-14-5-3-6-15(16(12)14)22(20,21)19-8-4-7-17-10-13(19)2/h3,5-6,9,11,13,17H,4,7-8,10H2,1-2H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ROCK-2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK as substrate after 40 mins by scintillation counting analysis |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50324316
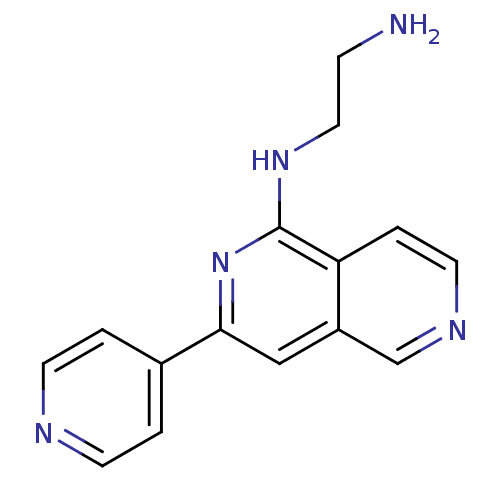
(CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...)Show InChI InChI=1S/C15H15N5/c16-4-8-19-15-13-3-7-18-10-12(13)9-14(20-15)11-1-5-17-6-2-11/h1-3,5-7,9-10H,4,8,16H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKC-eta after 60 mins by 33P-ATP incorporation assay |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14028

((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(C)c12 |r| Show InChI InChI=1S/C16H21N3O2S/c1-12-9-18-11-14-5-3-6-15(16(12)14)22(20,21)19-8-4-7-17-10-13(19)2/h3,5-6,9,11,13,17H,4,7-8,10H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of ROCK-1 (unknown origin) |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391897

(CHEMBL2147537)Show InChI InChI=1S/C16H17N5/c1-11(17)9-20-16-14-4-7-19-10-13(14)8-15(21-16)12-2-5-18-6-3-12/h2-8,10-11H,9,17H2,1H3,(H,20,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50324316

(CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...)Show InChI InChI=1S/C15H15N5/c16-4-8-19-15-13-3-7-18-10-12(13)9-14(20-15)11-1-5-17-6-2-11/h1-3,5-7,9-10H,4,8,16H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50324316

(CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...)Show InChI InChI=1S/C15H15N5/c16-4-8-19-15-13-3-7-18-10-12(13)9-14(20-15)11-1-5-17-6-2-11/h1-3,5-7,9-10H,4,8,16H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50324319
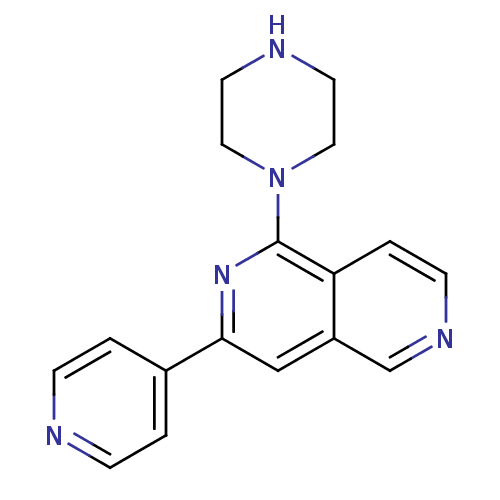
(1-Piperazin-1-yl-3-pyridin-4-yl[2,6]naphthyridine ...)Show InChI InChI=1S/C17H17N5/c1-4-18-5-2-13(1)16-11-14-12-20-6-3-15(14)17(21-16)22-9-7-19-8-10-22/h1-6,11-12,19H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM33971

(AEB071 | Sotrastaurin | med.21724, Compound 190)Show SMILES CN1CCN(CC1)c1nc(C2=C(C(=O)NC2=O)c2c[nH]c3ccccc23)c2ccccc2n1 |t:11| Show InChI InChI=1S/C25H22N6O2/c1-30-10-12-31(13-11-30)25-27-19-9-5-3-7-16(19)22(28-25)21-20(23(32)29-24(21)33)17-14-26-18-8-4-2-6-15(17)18/h2-9,14,26H,10-13H2,1H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCepsilon (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50235284

(CHEMBL4064291)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3cn(C)c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C26H22ClN3O2/c1-29(2)13-15-8-10-17-16(12-15)9-11-20(27)22(17)24-23(25(31)28-26(24)32)19-14-30(3)21-7-5-4-6-18(19)21/h4-12,14H,13H2,1-3H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta1 (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50365218
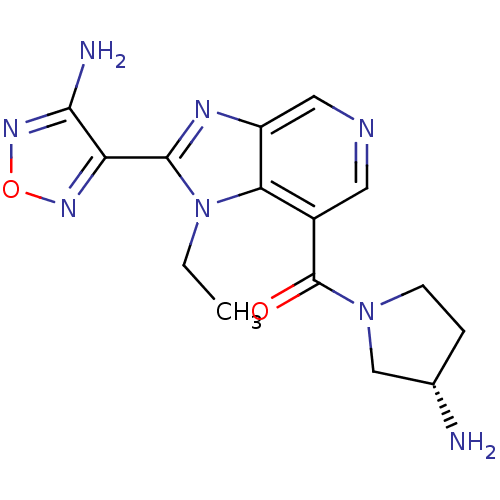
(CHEMBL1956071 | GSK screening, 29)Show SMILES CCn1c(nc2cncc(C(=O)N3CC[C@H](N)C3)c12)-c1nonc1N Show InChI InChI=1S/C15H18N8O2/c1-2-23-12-9(15(24)22-4-3-8(16)7-22)5-18-6-10(12)19-14(23)11-13(17)21-25-20-11/h5-6,8H,2-4,7,16H2,1H3,(H2,17,21)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ROCK-2 using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK as substrate after 40 mins by scintillation counting analysis |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N1
(Homo sapiens (Human)) | BDBM50324316

(CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...)Show InChI InChI=1S/C15H15N5/c16-4-8-19-15-13-3-7-18-10-12(13)9-14(20-15)11-1-5-17-6-2-11/h1-3,5-7,9-10H,4,8,16H2,(H,19,20) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of PKN-1 (unknown origin) |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391902

(CHEMBL2147542)Show InChI InChI=1S/C18H19N5/c1-5-19-9-11-23(10-1)18-16-4-8-21-13-15(16)12-17(22-18)14-2-6-20-7-3-14/h2-4,6-8,12-13,19H,1,5,9-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N2
(Homo sapiens (Human)) | BDBM50324316

(CHEMBL1214929 | N*1*-(3-Pyridin-4-yl[2,6]naphthyri...)Show InChI InChI=1S/C15H15N5/c16-4-8-19-15-13-3-7-18-10-12(13)9-14(20-15)11-1-5-17-6-2-11/h1-3,5-7,9-10H,4,8,16H2,(H,19,20) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKN-2 using AKRRRLSSLRA as substrate after 40 mins by scintillation counting analysis |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50235292

(CHEMBL4085837)Show SMILES CN(C)Cc1ccc2c(C3=C(C(=O)NC3=O)c3c[nH]c4ccccc34)c(Cl)ccc2c1 |t:9| Show InChI InChI=1S/C25H20ClN3O2/c1-29(2)13-14-7-9-16-15(11-14)8-10-19(26)21(16)23-22(24(30)28-25(23)31)18-12-27-20-6-4-3-5-17(18)20/h3-12,27H,13H2,1-2H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta (unknown origin) after 60 mins in presence of [gamma33P]ATP by scintillation proximity assay |
Bioorg Med Chem Lett 27: 781-786 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.038
BindingDB Entry DOI: 10.7270/Q2930WF1 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50391897

(CHEMBL2147537)Show InChI InChI=1S/C16H17N5/c1-11(17)9-20-16-14-4-7-19-10-13(14)8-15(21-16)12-2-5-18-6-3-12/h2-8,10-11H,9,17H2,1H3,(H,20,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCdelta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity a... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50391903
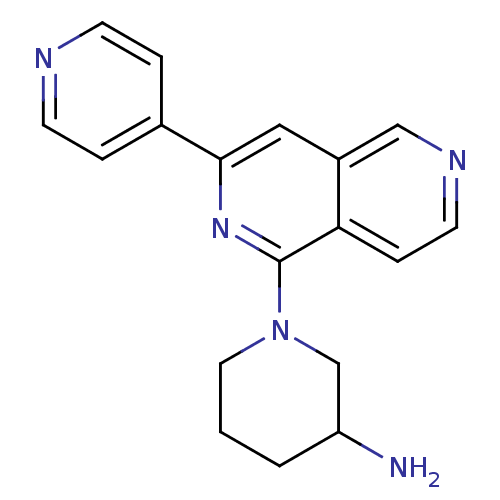
(CHEMBL2147543)Show InChI InChI=1S/C18H19N5/c19-15-2-1-9-23(12-15)18-16-5-8-21-11-14(16)10-17(22-18)13-3-6-20-7-4-13/h3-8,10-11,15H,1-2,9,12,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50324319

(1-Piperazin-1-yl-3-pyridin-4-yl[2,6]naphthyridine ...)Show InChI InChI=1S/C17H17N5/c1-4-18-5-2-13(1)16-11-14-12-20-6-3-15(14)17(21-16)22-9-7-19-8-10-22/h1-6,11-12,19H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCepsilon assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391898
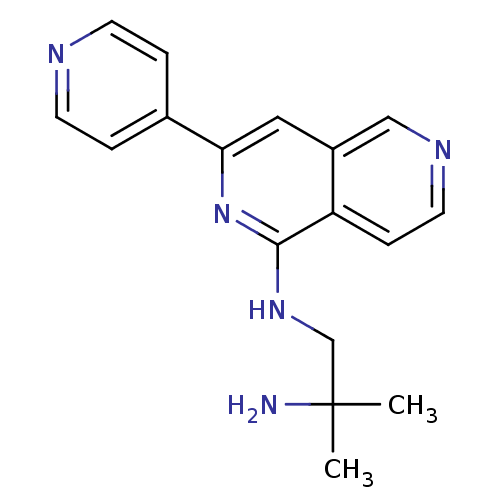
(CHEMBL2147538)Show InChI InChI=1S/C17H19N5/c1-17(2,18)11-21-16-14-5-8-20-10-13(14)9-15(22-16)12-3-6-19-7-4-12/h3-10H,11,18H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PKC-eta after 60 mins by 33P-ATP incorporation assay |
Bioorg Med Chem Lett 24: 4812-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.002
BindingDB Entry DOI: 10.7270/Q2MP54VV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data