 Found 730 hits with Last Name = 'han' and Initial = 'wc'
Found 730 hits with Last Name = 'han' and Initial = 'wc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50107460
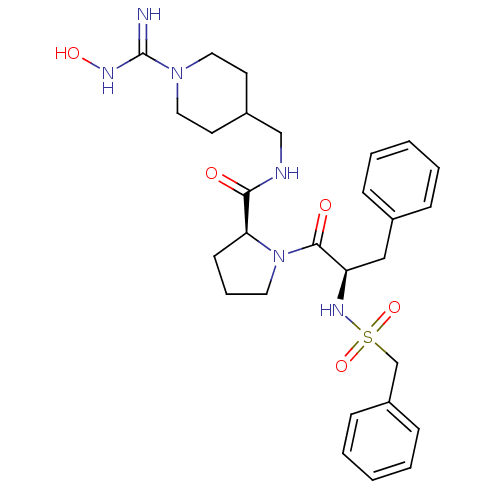
((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...)Show SMILES ONC(=N)N1CCC(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NS(=O)(=O)Cc2ccccc2)CC1 Show InChI InChI=1S/C28H38N6O5S/c29-28(31-37)33-16-13-22(14-17-33)19-30-26(35)25-12-7-15-34(25)27(36)24(18-21-8-3-1-4-9-21)32-40(38,39)20-23-10-5-2-6-11-23/h1-6,8-11,22,24-25,32,37H,7,12-20H2,(H2,29,31)(H,30,35)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Competitive kinetic for thrombin inhibition Ki was determined |
Bioorg Med Chem Lett 12: 45-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GX4C3P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323038
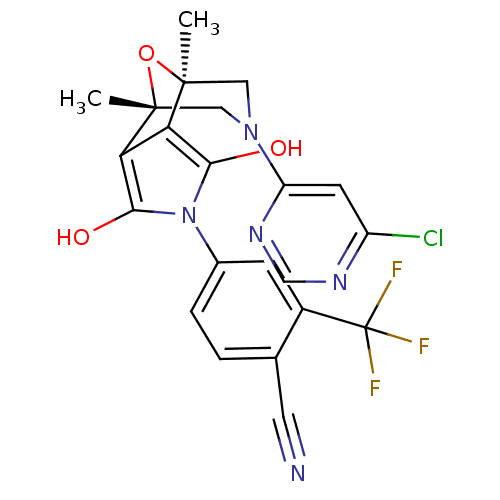
(4-[(1S,2S,6R,7R)-9-(6-Chloro-pyrimidin-4-yl)-1,7-d...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)c1cc(Cl)ncn1 |r| Show InChI InChI=1S/C22H17ClF3N5O3/c1-20-8-30(15-6-14(23)28-10-29-15)9-21(2,34-20)17-16(20)18(32)31(19(17)33)12-4-3-11(7-27)13(5-12)22(24,25)26/h3-6,10,32-33H,8-9H2,1-2H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323017
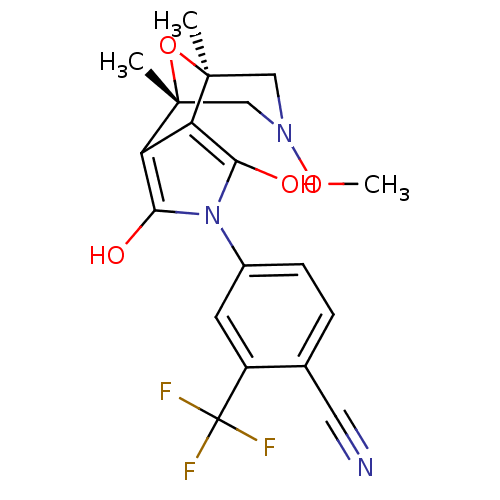
(4-((1S,2S,6R,7R)-9-Methoxy-1,7-dimethyl-3,5-dioxo-...)Show SMILES CON1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H18F3N3O4/c1-17-8-24(28-3)9-18(2,29-17)14-13(17)15(26)25(16(14)27)11-5-4-10(7-23)12(6-11)19(20,21)22/h4-6,26-27H,8-9H2,1-3H3/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323023
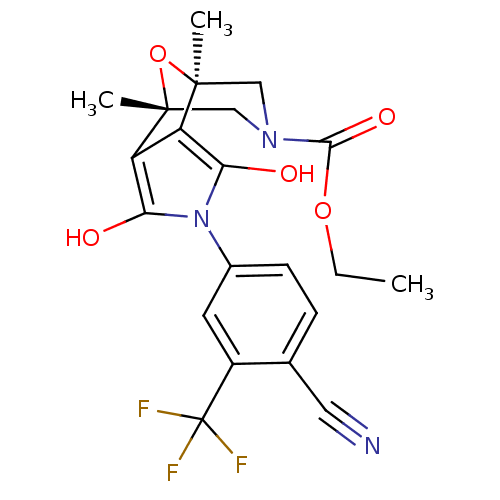
((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES CCOC(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N3O5/c1-4-31-18(30)26-9-19(2)14-15(20(3,10-26)32-19)17(29)27(16(14)28)12-6-5-11(8-25)13(7-12)21(22,23)24/h5-7,28-29H,4,9-10H2,1-3H3/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323039
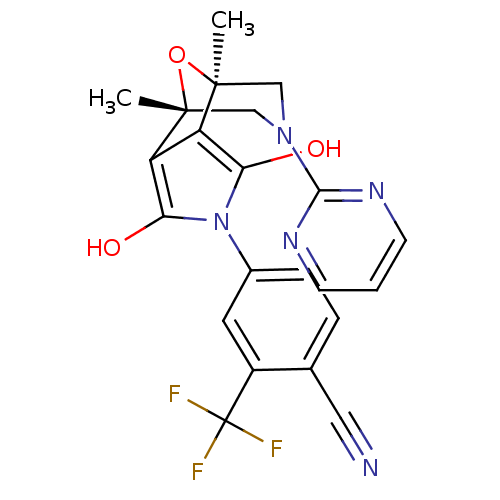
(4-((1S,2S,6R,7R)-1,7-Dimethyl-3,5-dioxo-9-pyrimidi...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)c1ncccn1 |r| Show InChI InChI=1S/C22H18F3N5O3/c1-20-10-29(19-27-6-3-7-28-19)11-21(2,33-20)16-15(20)17(31)30(18(16)32)13-5-4-12(9-26)14(8-13)22(23,24)25/h3-8,31-32H,10-11H2,1-2H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323024
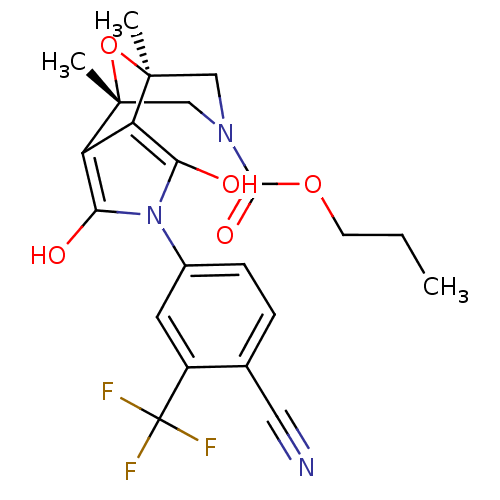
((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES CCCOC(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N3O5/c1-4-7-32-19(31)27-10-20(2)15-16(21(3,11-27)33-20)18(30)28(17(15)29)13-6-5-12(9-26)14(8-13)22(23,24)25/h5-6,8,29-30H,4,7,10-11H2,1-3H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228863

((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O Show InChI InChI=1S/C21H32N6O3/c1-24-17(13-15-7-3-2-4-8-15)20(30)27-12-6-10-18(27)19(29)26-16(14-28)9-5-11-25-21(22)23/h2-4,7-8,14,16-18,24H,5-6,9-13H2,1H3,(H,26,29)(H4,22,23,25)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Human alpha-thrombin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50107463
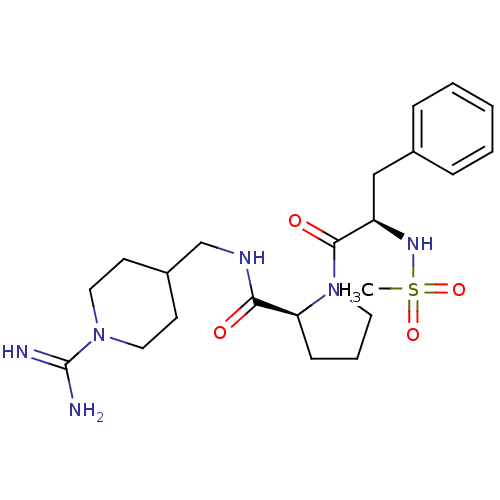
((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...)Show SMILES CS(=O)(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCN(CC1)C(N)=N Show InChI InChI=1S/C22H34N6O4S/c1-33(31,32)26-18(14-16-6-3-2-4-7-16)21(30)28-11-5-8-19(28)20(29)25-15-17-9-12-27(13-10-17)22(23)24/h2-4,6-7,17-19,26H,5,8-15H2,1H3,(H3,23,24)(H,25,29)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Competitive kinetic for human alpha thrombin inhibition Ki was determined |
Bioorg Med Chem Lett 12: 45-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GX4C3P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323035
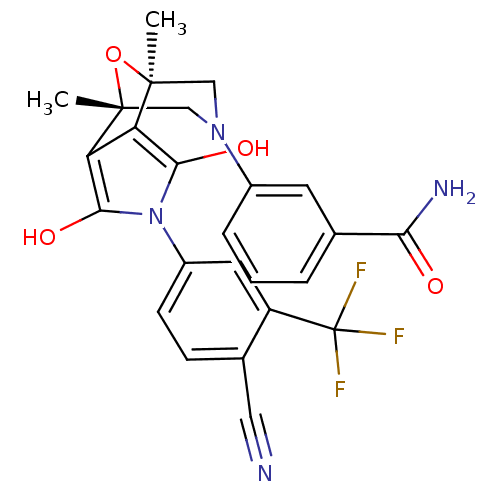
(3-[(1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phen...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(c1)C(N)=O |r| Show InChI InChI=1S/C25H21F3N4O4/c1-23-11-31(15-5-3-4-13(8-15)20(30)33)12-24(2,36-23)19-18(23)21(34)32(22(19)35)16-7-6-14(10-29)17(9-16)25(26,27)28/h3-9,34-35H,11-12H2,1-2H3,(H2,30,33)/t23-,24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323037

(4-[(1S,2S,6R,7R)-9-(3-Methanesulfonyl-phenyl)-1,7-...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C25H22F3N3O5S/c1-23-12-30(15-5-4-6-17(9-15)37(3,34)35)13-24(2,36-23)20-19(23)21(32)31(22(20)33)16-8-7-14(11-29)18(10-16)25(26,27)28/h4-10,32-33H,12-13H2,1-3H3/t23-,24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323025
((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES CC(C)OC(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N3O5/c1-11(2)32-19(31)27-9-20(3)15-16(21(4,10-27)33-20)18(30)28(17(15)29)13-6-5-12(8-26)14(7-13)22(23,24)25/h5-7,11,29-30H,9-10H2,1-4H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323032
(4-[(1R,2R,6S,7S)-3,5-dioxo-9-(phenylsulfonyl)-11-o...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C24H20F3N3O5S/c1-22-12-29(36(33,34)16-6-4-3-5-7-16)13-23(2,35-22)19-18(22)20(31)30(21(19)32)15-9-8-14(11-28)17(10-15)24(25,26)27/h3-10,31-32H,12-13H2,1-2H3/t22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323018
(4-((1S,2S,6R,7R)-1,7-Dimethyl-3,5-dioxo-9-propyl-1...)Show SMILES CCCN1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N3O3/c1-4-7-26-10-19(2)15-16(20(3,11-26)30-19)18(29)27(17(15)28)13-6-5-12(9-25)14(8-13)21(22,23)24/h5-6,8,28-29H,4,7,10-11H2,1-3H3/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323015
(4-((1S,2S,6R,7R)-9-Isopropyl-1,7-dimethyl-3,5,8,10...)Show SMILES CC(C)N1C(=O)[C@]2(C)O[C@@](C)(c3c(O)n(c(O)c23)-c2ccc(C#N)c(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C21H18F3N3O5/c1-9(2)26-17(30)19(3)13-14(20(4,32-19)18(26)31)16(29)27(15(13)28)11-6-5-10(8-25)12(7-11)21(22,23)24/h5-7,9,28-29H,1-4H3/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323031
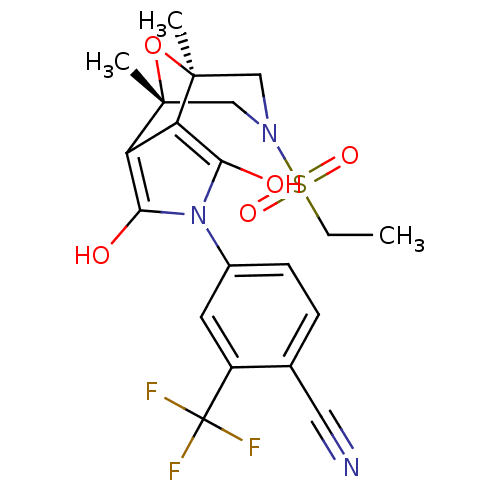
(4-((1S,2S,6R,7R)-9-Ethanesulfonyl-1,7-dimethyl-3,5...)Show SMILES CCS(=O)(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N3O5S/c1-4-32(29,30)25-9-18(2)14-15(19(3,10-25)31-18)17(28)26(16(14)27)12-6-5-11(8-24)13(7-12)20(21,22)23/h5-7,27-28H,4,9-10H2,1-3H3/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323012
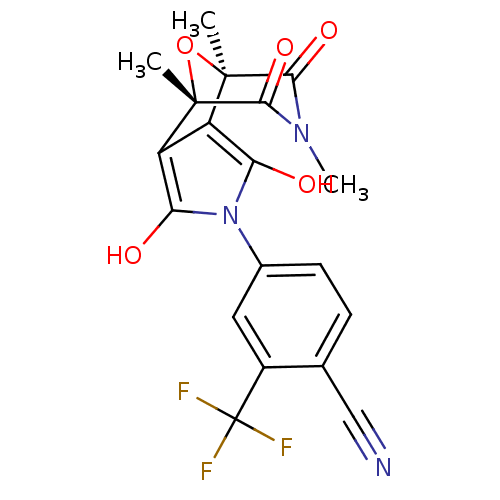
(2-Trifluoromethyl-4-((1S,2S,6R,7R)-1,7,9-trimethyl...)Show SMILES CN1C(=O)[C@]2(C)O[C@@](C)(c3c(O)n(c(O)c23)-c2ccc(C#N)c(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C19H14F3N3O5/c1-17-11-12(18(2,30-17)16(29)24(3)15(17)28)14(27)25(13(11)26)9-5-4-8(7-23)10(6-9)19(20,21)22/h4-6,26-27H,1-3H3/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323011

(4-((1S,2S,6R,7R)-1,7-Dimethyl-3,5-dioxo-11-oxa-4,9...)Show SMILES C[C@@]12CNC[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H16F3N3O3/c1-16-7-23-8-17(2,27-16)13-12(16)14(25)24(15(13)26)10-4-3-9(6-22)11(5-10)18(19,20)21/h3-5,23,25-26H,7-8H2,1-2H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323036
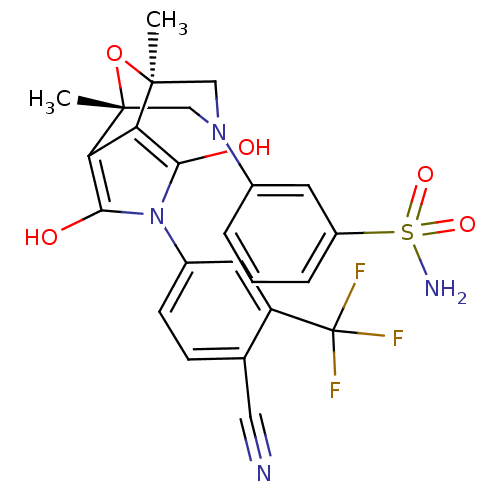
(3-[(1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phen...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)c1cccc(c1)S(N)(=O)=O |r| Show InChI InChI=1S/C24H21F3N4O5S/c1-22-11-30(14-4-3-5-16(8-14)37(29,34)35)12-23(2,36-22)19-18(22)20(32)31(21(19)33)15-7-6-13(10-28)17(9-15)24(25,26)27/h3-9,32-33H,11-12H2,1-2H3,(H2,29,34,35)/t22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323022
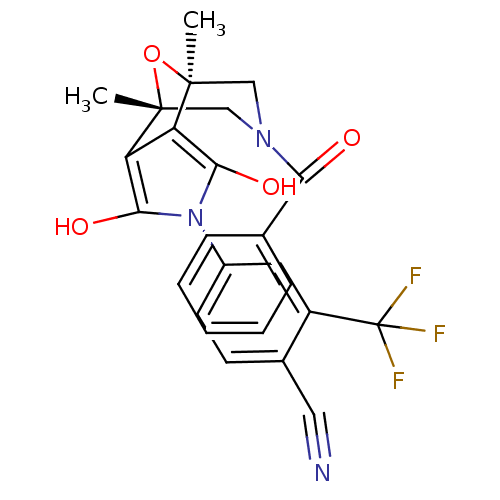
(4-((1S,2S,6R,7R)-9-Benzoyl-1,7-dimethyl-3,5-dioxo-...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H20F3N3O4/c1-23-12-30(20(32)14-6-4-3-5-7-14)13-24(2,35-23)19-18(23)21(33)31(22(19)34)16-9-8-15(11-29)17(10-16)25(26,27)28/h3-10,33-34H,12-13H2,1-2H3/t23-,24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323029
((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)C(=O)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C25H20F4N4O4/c1-23-11-32(22(36)31-15-6-4-14(26)5-7-15)12-24(2,37-23)19-18(23)20(34)33(21(19)35)16-8-3-13(10-30)17(9-16)25(27,28)29/h3-9,34-35H,11-12H2,1-2H3,(H,31,36)/t23-,24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323033

(4-[(1S,2S,6R,7R)-9-(4-Fluoro-benzenesulfonyl)-1,7-...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H19F4N3O5S/c1-22-11-30(37(34,35)16-7-4-14(25)5-8-16)12-23(2,36-22)19-18(22)20(32)31(21(19)33)15-6-3-13(10-29)17(9-15)24(26,27)28/h3-9,32-33H,11-12H2,1-2H3/t22-,23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323021
(4-((1S,2S,6R,7R)-9-Cyclopropanecarbonyl-1,7-dimeth...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)C(=O)C1CC1 |r| Show InChI InChI=1S/C22H20F3N3O4/c1-20-9-27(17(29)11-3-4-11)10-21(2,32-20)16-15(20)18(30)28(19(16)31)13-6-5-12(8-26)14(7-13)22(23,24)25/h5-7,11,30-31H,3-4,9-10H2,1-2H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323016
(4-((1S,2S,6R,7R)-9-Hydroxy-1,7-dimethyl-3,5-dioxo-...)Show SMILES C[C@@]12CN(O)C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H16F3N3O4/c1-16-7-23(27)8-17(2,28-16)13-12(16)14(25)24(15(13)26)10-4-3-9(6-22)11(5-10)18(19,20)21/h3-5,25-27H,7-8H2,1-2H3/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323020
(4-((1S,2S,6R,7R)-9-Isobutyryl-1,7-dimethyl-3,5-dio...)Show SMILES CC(C)C(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N3O4/c1-11(2)17(29)27-9-20(3)15-16(21(4,10-27)32-20)19(31)28(18(15)30)13-6-5-12(8-26)14(7-13)22(23,24)25/h5-7,11,30-31H,9-10H2,1-4H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323028

((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C25H21F3N4O4/c1-23-12-31(22(35)30-15-6-4-3-5-7-15)13-24(2,36-23)19-18(23)20(33)32(21(19)34)16-9-8-14(11-29)17(10-16)25(26,27)28/h3-10,33-34H,12-13H2,1-2H3,(H,30,35)/t23-,24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323013
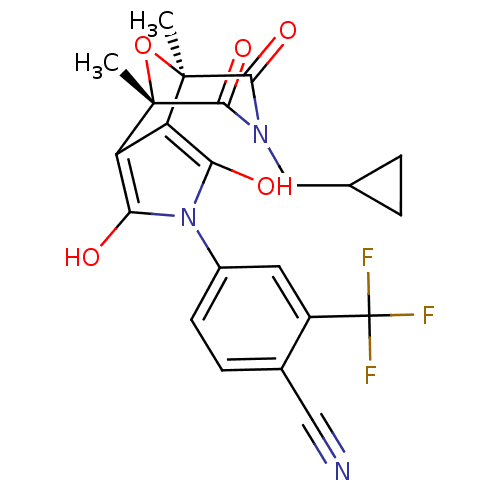
(4-((1S,2S,6R,7R)-9-Cyclopropylmethyl-1,7-dimethyl-...)Show SMILES C[C@@]12O[C@@](C)(c3c(O)n(c(O)c13)-c1ccc(C#N)c(c1)C(F)(F)F)C(=O)N(CC1CC1)C2=O |r| Show InChI InChI=1S/C22H18F3N3O5/c1-20-14-15(21(2,33-20)19(32)27(18(20)31)9-10-3-4-10)17(30)28(16(14)29)12-6-5-11(8-26)13(7-12)22(23,24)25/h5-7,10,29-30H,3-4,9H2,1-2H3/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323030
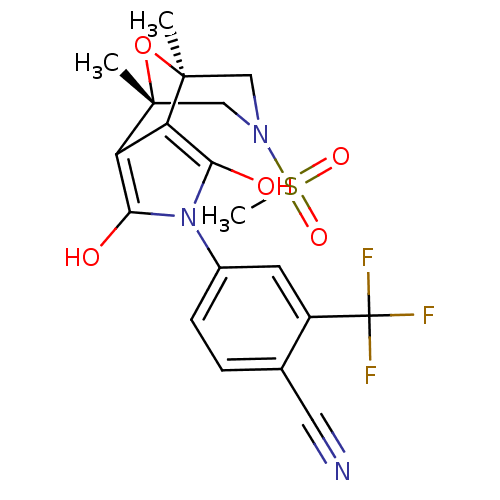
(4-((1S,2S,6R,7R)-9-Methanesulfonyl-1,7-dimethyl-3,...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)S(C)(=O)=O |r| Show InChI InChI=1S/C19H18F3N3O5S/c1-17-8-24(31(3,28)29)9-18(2,30-17)14-13(17)15(26)25(16(14)27)11-5-4-10(7-23)12(6-11)19(20,21)22/h4-6,26-27H,8-9H2,1-3H3/t17-,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525
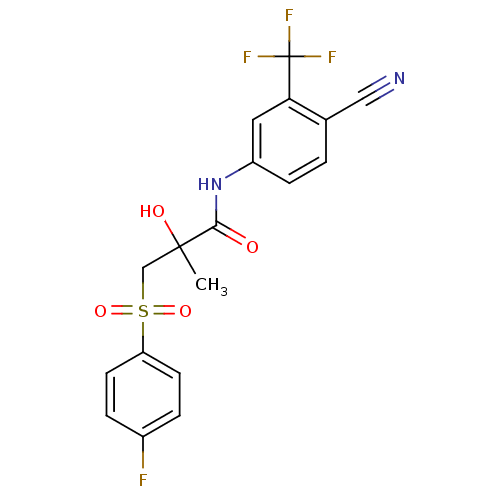
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323019
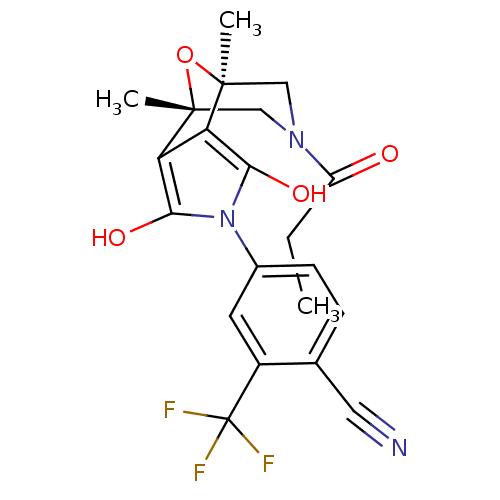
(4-[(1R,2R,6S,7S)-3,5-dioxo-9-propionyl-11-oxa-4,9-...)Show SMILES CCC(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N3O4/c1-4-14(28)26-9-19(2)15-16(20(3,10-26)31-19)18(30)27(17(15)29)12-6-5-11(8-25)13(7-12)21(22,23)24/h5-7,29-30H,4,9-10H2,1-3H3/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323034
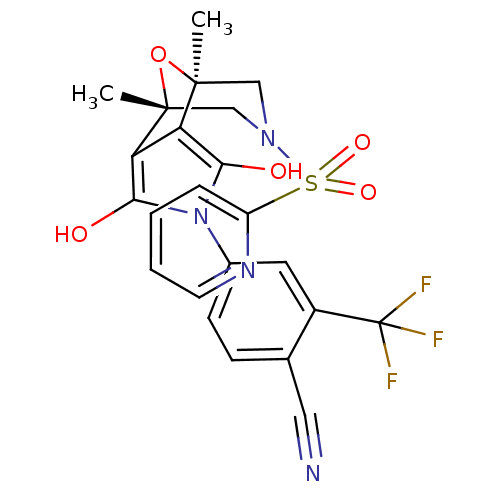
(4-[(1S,2S,6R,7R)-1,7-Dimethyl-3,5-dioxo-9-(pyridin...)Show SMILES C[C@@]12CN(C[C@@](C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C23H19F3N4O5S/c1-21-11-29(36(33,34)16-5-3-4-8-28-16)12-22(2,35-21)18-17(21)19(31)30(20(18)32)14-7-6-13(10-27)15(9-14)23(24,25)26/h3-9,31-32H,11-12H2,1-2H3/t21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323027
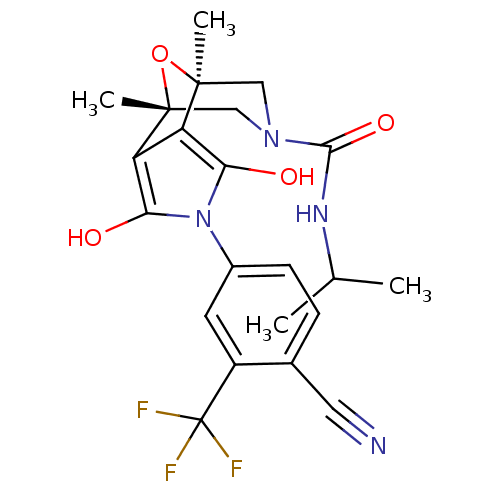
((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES CC(C)NC(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H23F3N4O4/c1-11(2)27-19(32)28-9-20(3)15-16(21(4,10-28)33-20)18(31)29(17(15)30)13-6-5-12(8-26)14(7-13)22(23,24)25/h5-7,11,30-31H,9-10H2,1-4H3,(H,27,32)/t20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323026
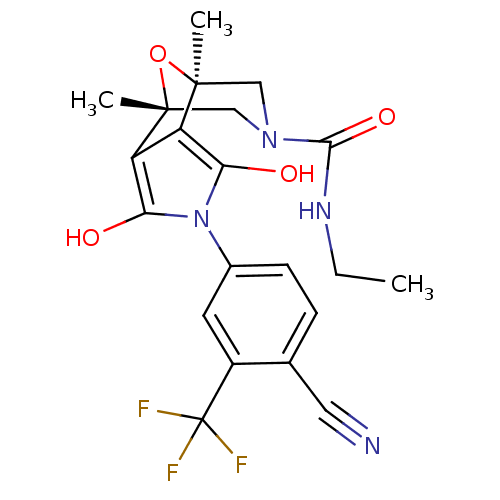
((1S,2S,6R,7R)-4-(4-Cyano-3-trifluoromethyl-phenyl)...)Show SMILES CCNC(=O)N1C[C@]2(C)O[C@](C)(C1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H21F3N4O4/c1-4-26-18(31)27-9-19(2)14-15(20(3,10-27)32-19)17(30)28(16(14)29)12-6-5-11(8-25)13(7-12)21(22,23)24/h5-7,29-30H,4,9-10H2,1-3H3,(H,26,31)/t19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50323014
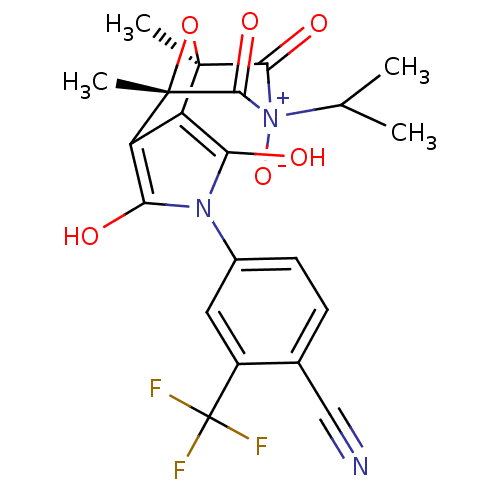
(4-((1S,2S,6R,7R)-9-Isopropyl-1,7-dimethyl-3,5,8,10...)Show SMILES CC(C)[N+]1([O-])C(=O)[C@]2(C)O[C@@](C)(c3c(O)n(c(O)c23)-c2ccc(C#N)c(c2)C(F)(F)F)C1=O |r| Show InChI InChI=1S/C21H18F3N3O6/c1-9(2)27(32)17(30)19(3)13-14(20(4,33-19)18(27)31)16(29)26(15(13)28)11-6-5-10(8-25)12(7-11)21(22,23)24/h5-7,9,28-29H,1-4H3/t19-,20+,27? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from AR in human MDA-MB-453 cells |
Bioorg Med Chem Lett 20: 4491-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.034
BindingDB Entry DOI: 10.7270/Q2MK6DVH |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50548231
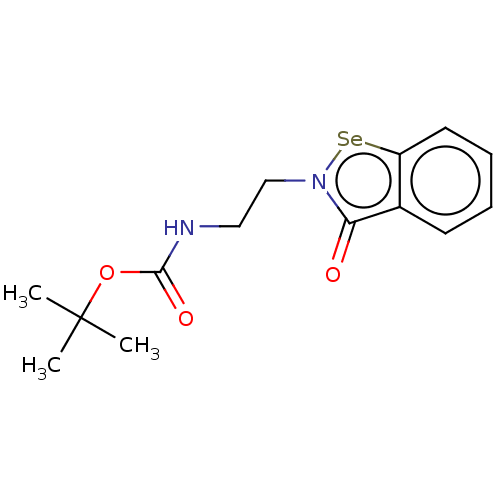
(CHEMBL4753599)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6]-[#6]-n1[se;v2]c2ccccc2c1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type N-terminal His6-tagged Klebsiella pneumoniae NDM-1 expressed in Escherichia coli BL21 assessed as inhibition constant using n... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.06.007
BindingDB Entry DOI: 10.7270/Q2SB49CC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50120225

(2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...)Show SMILES CC(O)C(N(C)C(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CCC[N-]C(N)=[NH2+])C(=O)NC(C)c1ccccc1 Show InChI InChI=1S/C27H37N7O6/c1-17(20-8-5-4-6-9-20)31-25(37)24(18(2)35)33(3)26(38)22(32-23(36)10-7-15-30-27(28)29)16-19-11-13-21(14-12-19)34(39)40/h4-6,8-9,11-14,17-18,22,24,35H,7,10,15-16H2,1-3H3,(H6,28,29,30,31,32,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit thrombin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50120225

(2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...)Show SMILES CC(O)C(N(C)C(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CCC[N-]C(N)=[NH2+])C(=O)NC(C)c1ccccc1 Show InChI InChI=1S/C27H37N7O6/c1-17(20-8-5-4-6-9-20)31-25(37)24(18(2)35)33(3)26(38)22(32-23(36)10-7-15-30-27(28)29)16-19-11-13-21(14-12-19)34(39)40/h4-6,8-9,11-14,17-18,22,24,35H,7,10,15-16H2,1-3H3,(H6,28,29,30,31,32,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Trypsin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179969
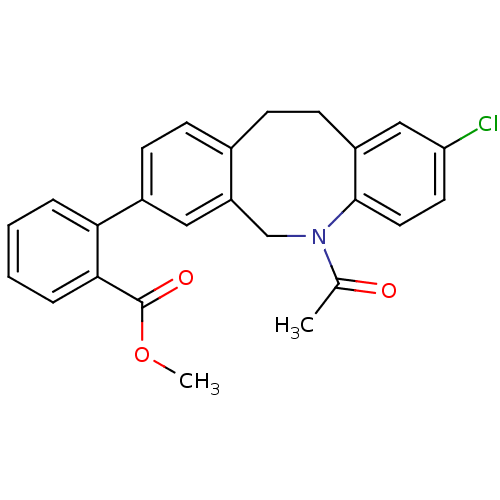
(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179935
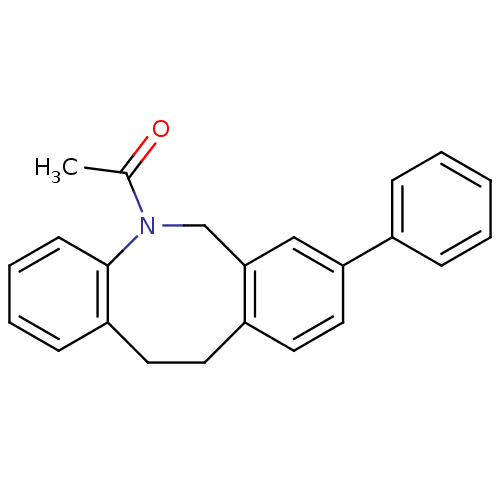
(1-(8-Phenyl-11,12-dihydro-6H-dibenzo[b,f]azocin-5-...)Show InChI InChI=1S/C23H21NO/c1-17(25)24-16-22-15-21(18-7-3-2-4-8-18)14-12-19(22)11-13-20-9-5-6-10-23(20)24/h2-10,12,14-15H,11,13,16H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179960

(1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C(C)=O Show InChI InChI=1S/C25H22ClNO2/c1-16(28)23-5-3-4-6-24(23)19-9-7-18-8-10-20-14-22(26)11-12-25(20)27(17(2)29)15-21(18)13-19/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50228863

((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O Show InChI InChI=1S/C21H32N6O3/c1-24-17(13-15-7-3-2-4-8-15)20(30)27-12-6-10-18(27)19(29)26-16(14-28)9-5-11-25-21(22)23/h2-4,7-8,14,16-18,24H,5-6,9-13H2,1H3,(H,26,29)(H4,22,23,25)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit thrombin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50228863

((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O Show InChI InChI=1S/C21H32N6O3/c1-24-17(13-15-7-3-2-4-8-15)20(30)27-12-6-10-18(27)19(29)26-16(14-28)9-5-11-25-21(22)23/h2-4,7-8,14,16-18,24H,5-6,9-13H2,1H3,(H,26,29)(H4,22,23,25)/t16-,17+,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Trypsin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50120225

(2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...)Show SMILES CC(O)C(N(C)C(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CCC[N-]C(N)=[NH2+])C(=O)NC(C)c1ccccc1 Show InChI InChI=1S/C27H37N7O6/c1-17(20-8-5-4-6-9-20)31-25(37)24(18(2)35)33(3)26(38)22(32-23(36)10-7-15-30-27(28)29)16-19-11-13-21(14-12-19)34(39)40/h4-6,8-9,11-14,17-18,22,24,35H,7,10,15-16H2,1-3H3,(H6,28,29,30,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Plasmin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Sensory histidine kinase DcuS
(Staphylococcus aureus) | BDBM50151871
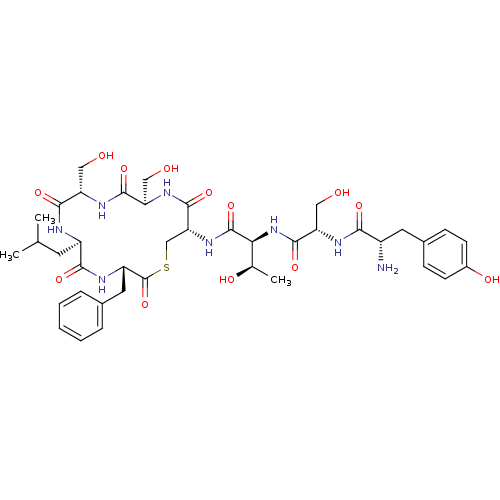
(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...)Show SMILES CC(C)C[C@H]1NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC(=O)[C@@H](CSC(=O)[C@H](Cc2ccccc2)NC1=O)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O Show InChI InChI=1S/C40H56N8O13S/c1-20(2)13-26-34(55)43-27(15-22-7-5-4-6-8-22)40(61)62-19-31(38(59)46-29(17-50)36(57)45-28(16-49)35(56)42-26)47-39(60)32(21(3)52)48-37(58)30(18-51)44-33(54)25(41)14-23-9-11-24(53)12-10-23/h4-12,20-21,25-32,49-53H,13-19,41H2,1-3H3,(H,42,56)(H,43,55)(H,44,54)(H,45,57)(H,46,59)(H,47,60)(H,48,58)/t21-,25+,26-,27+,28+,29-,30+,31-,32+/m1/s1 | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Concentration required to inhibit quorum sensor of Staphylococcus aureus |
J Med Chem 47: 4633-41 (2004)
Article DOI: 10.1021/jm0400754
BindingDB Entry DOI: 10.7270/Q2P26XM1 |
More data for this
Ligand-Target Pair | |
Sensory histidine kinase DcuS
(Staphylococcus aureus) | BDBM50151874
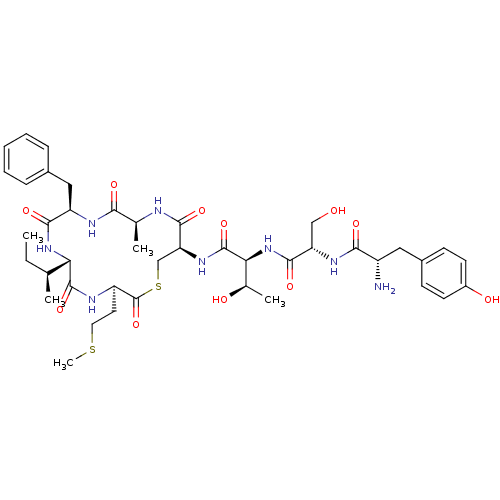
(CHEMBL362838 | Tyr-Ser-Thr-cyclo(Cys-Ala-Phe-Ile-M...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@H](CSC(=O)[C@@H](CCSC)NC1=O)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)O Show InChI InChI=1S/C42H60N8O11S2/c1-6-22(2)33-40(59)45-29(16-17-62-5)42(61)63-21-32(39(58)44-23(3)35(54)46-30(37(56)49-33)19-25-10-8-7-9-11-25)48-41(60)34(24(4)52)50-38(57)31(20-51)47-36(55)28(43)18-26-12-14-27(53)15-13-26/h7-15,22-24,28-34,51-53H,6,16-21,43H2,1-5H3,(H,44,58)(H,45,59)(H,46,54)(H,47,55)(H,48,60)(H,49,56)(H,50,57)/t22-,23-,24+,28-,29+,30+,31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Concentration required to inhibit quorum sensor of Staphylococcus aureus |
J Med Chem 47: 4633-41 (2004)
Article DOI: 10.1021/jm0400754
BindingDB Entry DOI: 10.7270/Q2P26XM1 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179960

(1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C(C)=O Show InChI InChI=1S/C25H22ClNO2/c1-16(28)23-5-3-4-6-24(23)19-9-7-18-8-10-20-14-22(26)11-12-25(20)27(17(2)29)15-21(18)13-19/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179969

(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50120225

(2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...)Show SMILES CC(O)C(N(C)C(=O)C(Cc1ccc(cc1)[N+]([O-])=O)NC(=O)CCC[N-]C(N)=[NH2+])C(=O)NC(C)c1ccccc1 Show InChI InChI=1S/C27H37N7O6/c1-17(20-8-5-4-6-9-20)31-25(37)24(18(2)35)33(3)26(38)22(32-23(36)10-7-15-30-27(28)29)16-19-11-13-21(14-12-19)34(39)40/h4-6,8-9,11-14,17-18,22,24,35H,7,10,15-16H2,1-3H3,(H6,28,29,30,31,32,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179940

(2-(5-acetyl-2-bromo-5,6,11,12-tetrahydro-dibenzo[b...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Br)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22BrNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179964
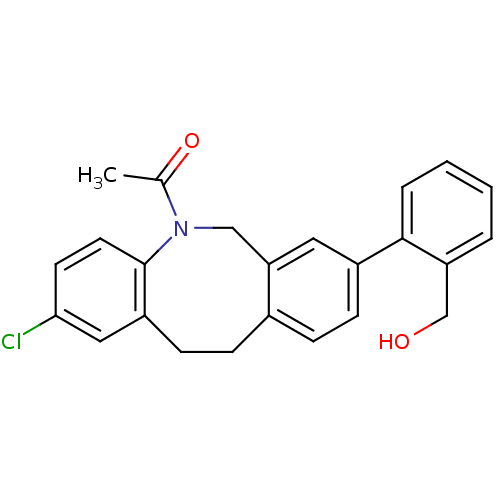
(1-[2-chloro-8-(2-hydroxymethyl-phenyl)-11,12-dihyd...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1CO Show InChI InChI=1S/C24H22ClNO2/c1-16(28)26-14-21-12-18(23-5-3-2-4-20(23)15-27)8-6-17(21)7-9-19-13-22(25)10-11-24(19)26/h2-6,8,10-13,27H,7,9,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Neurogenic locus notch homolog protein 1
(Homo sapiens (Human)) | BDBM50074796

(CHEMBL3410169)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)[C@H](CCC(F)(F)F)[C@@H](C(N)=O)c2ccc(F)cc2F)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C29H25F5N4O3/c1-38-22-10-6-5-9-19(22)24(16-7-3-2-4-8-16)36-26(28(38)41)37-27(40)20(13-14-29(32,33)34)23(25(35)39)18-12-11-17(30)15-21(18)31/h2-12,15,20,23,26H,13-14H2,1H3,(H2,35,39)(H,37,40)/t20-,23+,26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... |
Bioorg Med Chem Lett 25: 1905-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.038
BindingDB Entry DOI: 10.7270/Q2X92D18 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data