 Found 2842 hits with Last Name = 'di' and Initial = 'x'
Found 2842 hits with Last Name = 'di' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from dopamine D3 receptor (unknown origin) |
J Med Chem 60: 6480-6515 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00010
BindingDB Entry DOI: 10.7270/Q2PK0JDP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015266
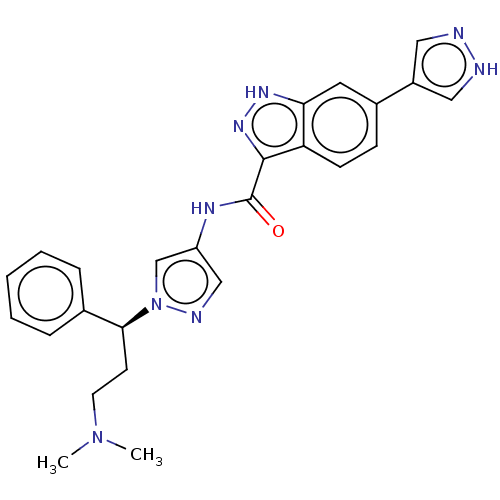
(CHEMBL3263053)Show SMILES CN(C)CC[C@@H](c1ccccc1)n1cc(NC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)cn1 |r| Show InChI InChI=1S/C25H26N8O/c1-32(2)11-10-23(17-6-4-3-5-7-17)33-16-20(15-28-33)29-25(34)24-21-9-8-18(12-22(21)30-31-24)19-13-26-27-14-19/h3-9,12-16,23H,10-11H2,1-2H3,(H,26,27)(H,29,34)(H,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015268
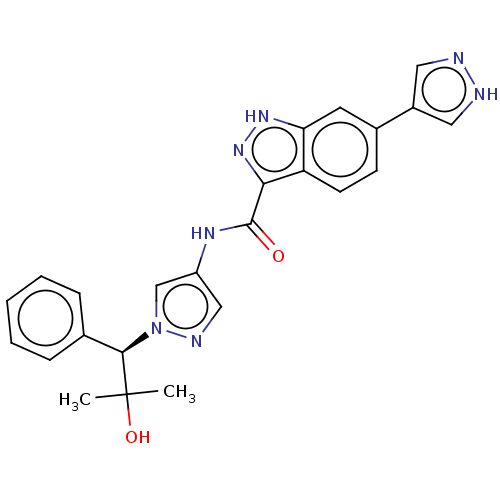
(CHEMBL3263055)Show SMILES CC(C)(O)[C@@H](c1ccccc1)n1cc(NC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)cn1 |r| Show InChI InChI=1S/C24H23N7O2/c1-24(2,33)22(15-6-4-3-5-7-15)31-14-18(13-27-31)28-23(32)21-19-9-8-16(10-20(19)29-30-21)17-11-25-26-12-17/h3-14,22,33H,1-2H3,(H,25,26)(H,28,32)(H,29,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428131
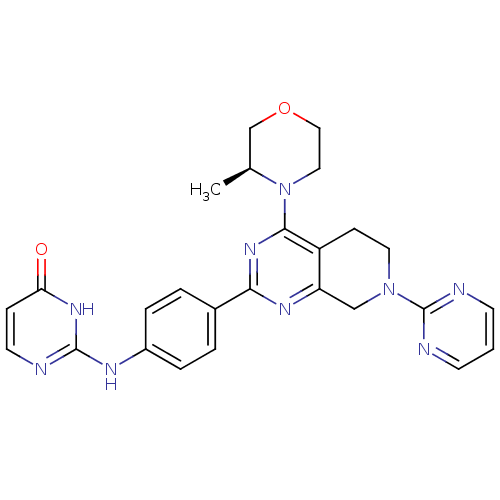
(CHEMBL2331687)Show SMILES C[C@H]1COCCN1c1nc(nc2CN(CCc12)c1ncccn1)-c1ccc(Nc2nccc(=O)[nH]2)cc1 |r| Show InChI InChI=1S/C26H27N9O2/c1-17-16-37-14-13-35(17)24-20-8-12-34(26-28-9-2-10-29-26)15-21(20)31-23(33-24)18-3-5-19(6-4-18)30-25-27-11-7-22(36)32-25/h2-7,9-11,17H,8,12-16H2,1H3,(H2,27,30,32,36)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human dopamine D2L receptor expressed in CHO cells |
J Med Chem 60: 6480-6515 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00010
BindingDB Entry DOI: 10.7270/Q2PK0JDP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443182
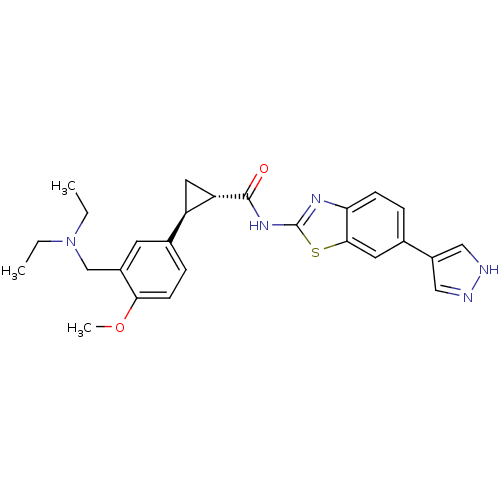
(CHEMBL3086538)Show SMILES CCN(CC)Cc1cc(ccc1OC)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C26H29N5O2S/c1-4-31(5-2)15-18-10-17(7-9-23(18)33-3)20-12-21(20)25(32)30-26-29-22-8-6-16(11-24(22)34-26)19-13-27-28-14-19/h6-11,13-14,20-21H,4-5,12,15H2,1-3H3,(H,27,28)(H,29,30,32)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015263

(CHEMBL3263050)Show SMILES OC[C@@H](c1ccccc1)n1cc(NC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)cn1 |r| Show InChI InChI=1S/C22H19N7O2/c30-13-20(14-4-2-1-3-5-14)29-12-17(11-25-29)26-22(31)21-18-7-6-15(8-19(18)27-28-21)16-9-23-24-10-16/h1-12,20,30H,13H2,(H,23,24)(H,26,31)(H,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443181

(CHEMBL3086535)Show SMILES CN(C)Cc1ccc(cc1)C1CC1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 Show InChI InChI=1S/C23H23N5OS/c1-28(2)13-14-3-5-15(6-4-14)18-10-19(18)22(29)27-23-26-20-8-7-16(9-21(20)30-23)17-11-24-25-12-17/h3-9,11-12,18-19H,10,13H2,1-2H3,(H,24,25)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiperone from human D2S receptor expressed in CHO cells |
J Med Chem 60: 6480-6515 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00010
BindingDB Entry DOI: 10.7270/Q2PK0JDP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443180

(CHEMBL3086536)Show SMILES CN(C)Cc1ccc(cc1)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C23H23N5OS/c1-28(2)13-14-3-5-15(6-4-14)18-10-19(18)22(29)27-23-26-20-8-7-16(9-21(20)30-23)17-11-24-25-12-17/h3-9,11-12,18-19H,10,13H2,1-2H3,(H,24,25)(H,26,27,29)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015267
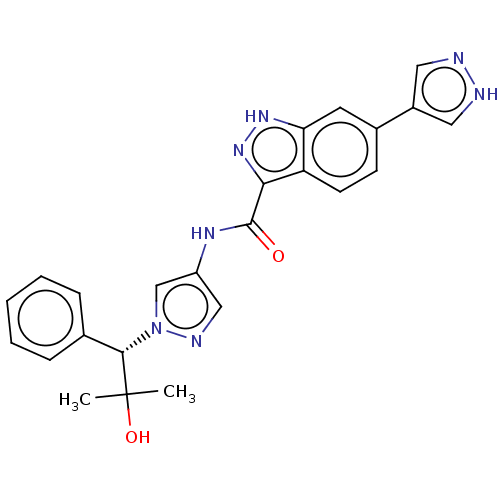
(CHEMBL3263054)Show SMILES CC(C)(O)[C@H](c1ccccc1)n1cc(NC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)cn1 |r| Show InChI InChI=1S/C24H23N7O2/c1-24(2,33)22(15-6-4-3-5-7-15)31-14-18(13-27-31)28-23(32)21-19-9-8-16(10-20(19)29-30-21)17-11-25-26-12-17/h3-14,22,33H,1-2H3,(H,25,26)(H,28,32)(H,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015271
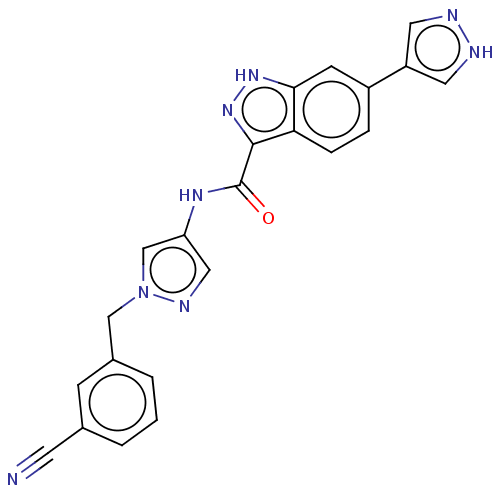
(CHEMBL3263036)Show SMILES O=C(Nc1cnn(Cc2cccc(c2)C#N)c1)c1n[nH]c2cc(ccc12)-c1cn[nH]c1 Show InChI InChI=1S/C22H16N8O/c23-8-14-2-1-3-15(6-14)12-30-13-18(11-26-30)27-22(31)21-19-5-4-16(7-20(19)28-29-21)17-9-24-25-10-17/h1-7,9-11,13H,12H2,(H,24,25)(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015265
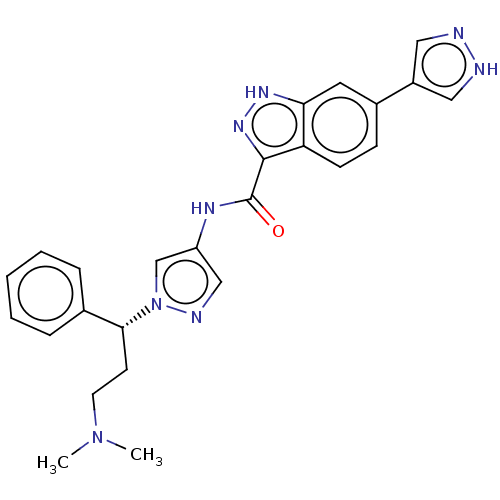
(CHEMBL3263052)Show SMILES CN(C)CC[C@H](c1ccccc1)n1cc(NC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)cn1 |r| Show InChI InChI=1S/C25H26N8O/c1-32(2)11-10-23(17-6-4-3-5-7-17)33-16-20(15-28-33)29-25(34)24-21-9-8-18(12-22(21)30-31-24)19-13-26-27-14-19/h3-9,12-16,23H,10-11H2,1-2H3,(H,26,27)(H,29,34)(H,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430787
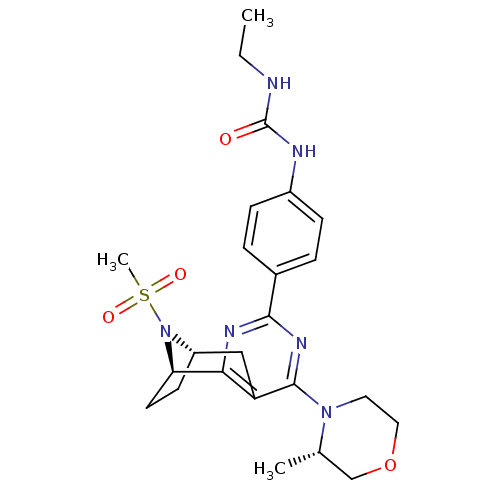
(CHEMBL2334764)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3S(C)(=O)=O |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C24H32N6O4S/c1-4-25-24(31)26-17-7-5-16(6-8-17)22-27-21-19(23(28-22)29-11-12-34-14-15(29)2)13-18-9-10-20(21)30(18)35(3,32)33/h5-8,15,18,20H,4,9-14H2,1-3H3,(H2,25,26,31)/t15-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398668
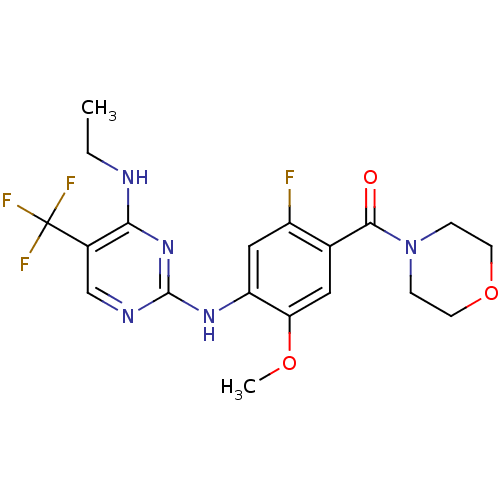
(CHEMBL2178134 | US8802674, 256)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H21F4N5O3/c1-3-24-16-12(19(21,22)23)10-25-18(27-16)26-14-9-13(20)11(8-15(14)30-2)17(29)28-4-6-31-7-5-28/h8-10H,3-7H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398662
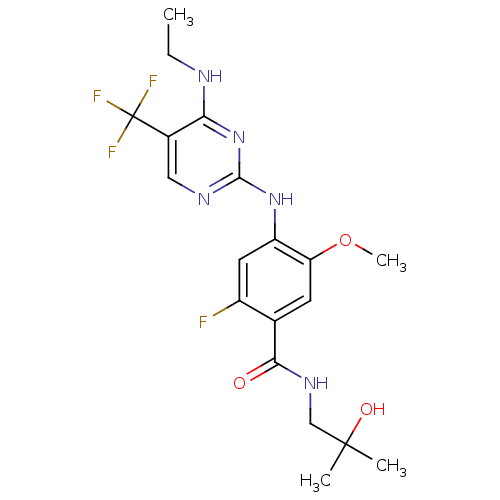
(CHEMBL2178140)Show SMILES CCNc1nc(Nc2cc(F)c(cc2OC)C(=O)NCC(C)(C)O)ncc1C(F)(F)F Show InChI InChI=1S/C19H23F4N5O3/c1-5-24-15-11(19(21,22)23)8-25-17(28-15)27-13-7-12(20)10(6-14(13)31-4)16(29)26-9-18(2,3)30/h6-8,30H,5,9H2,1-4H3,(H,26,29)(H2,24,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398667

(CHEMBL2178135)Show SMILES COc1cc(C(=O)N2CCOCC2)c(F)cc1Nc1ncc(c(NC2CC2)n1)C(F)(F)F Show InChI InChI=1S/C20H21F4N5O3/c1-31-16-8-12(18(30)29-4-6-32-7-5-29)14(21)9-15(16)27-19-25-10-13(20(22,23)24)17(28-19)26-11-2-3-11/h8-11H,2-7H2,1H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015262
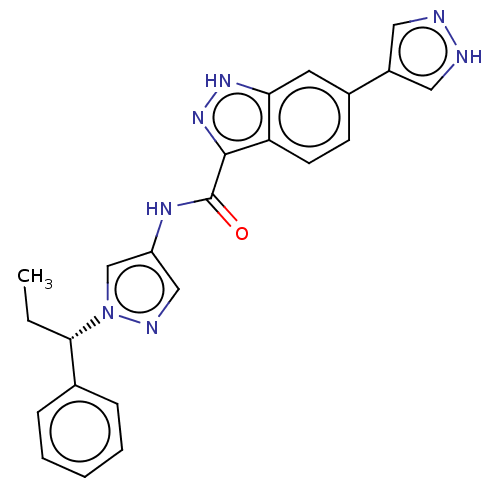
(CHEMBL3263049)Show SMILES CC[C@@H](c1ccccc1)n1cc(NC(=O)c2n[nH]c3cc(ccc23)-c2cn[nH]c2)cn1 |r| Show InChI InChI=1S/C23H21N7O/c1-2-21(15-6-4-3-5-7-15)30-14-18(13-26-30)27-23(31)22-19-9-8-16(10-20(19)28-29-22)17-11-24-25-12-17/h3-14,21H,2H2,1H3,(H,24,25)(H,27,31)(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398676
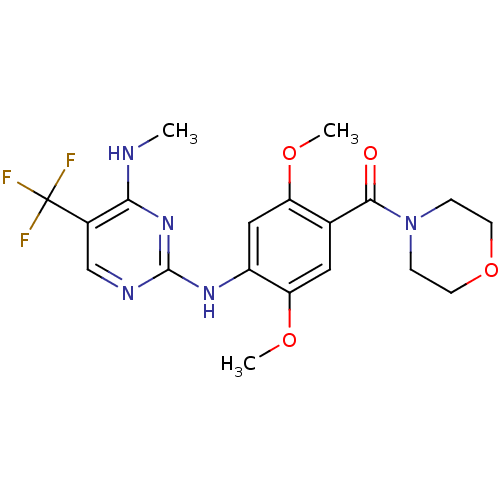
(CHEMBL2178125)Show SMILES CNc1nc(Nc2cc(OC)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O4/c1-23-16-12(19(20,21)22)10-24-18(26-16)25-13-9-14(29-2)11(8-15(13)30-3)17(28)27-4-6-31-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50315426
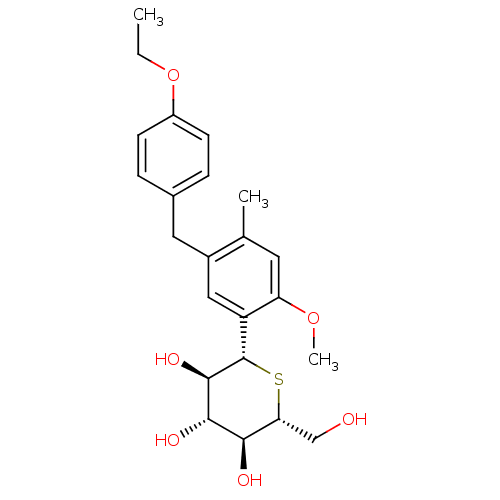
((1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4...)Show SMILES CCOc1ccc(Cc2cc([C@@H]3S[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(OC)cc2C)cc1 |r| Show InChI InChI=1S/C23H30O6S/c1-4-29-16-7-5-14(6-8-16)10-15-11-17(18(28-3)9-13(15)2)23-22(27)21(26)20(25)19(12-24)30-23/h5-9,11,19-27H,4,10,12H2,1-3H3/t19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human SGLT2 expressed in CHO-K1 cells assessed as inhibition of [14C]-alpha-methylglucoside uptake |
Bioorg Med Chem 24: 1937-80 (2016)
Article DOI: 10.1016/j.bmc.2016.03.004
BindingDB Entry DOI: 10.7270/Q22B90WR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430784
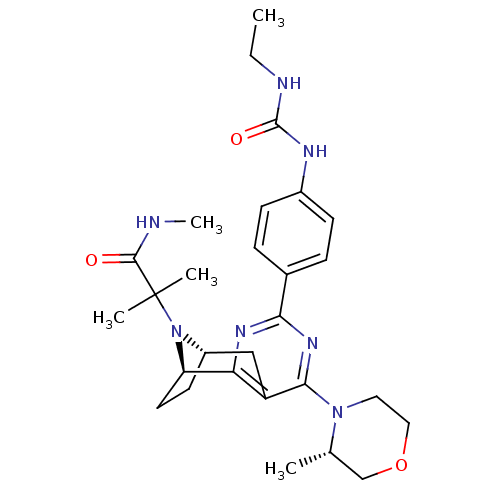
(CHEMBL2334767)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3C(C)(C)C(=O)NC |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C28H39N7O3/c1-6-30-27(37)31-19-9-7-18(8-10-19)24-32-23-21(25(33-24)34-13-14-38-16-17(34)2)15-20-11-12-22(23)35(20)28(3,4)26(36)29-5/h7-10,17,20,22H,6,11-16H2,1-5H3,(H,29,36)(H2,30,31,37)/t17-,20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430790

(CHEMBL2334761)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3C |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C24H32N6O2/c1-4-25-24(31)26-17-7-5-16(6-8-17)22-27-21-19(13-18-9-10-20(21)29(18)3)23(28-22)30-11-12-32-14-15(30)2/h5-8,15,18,20H,4,9-14H2,1-3H3,(H2,25,26,31)/t15-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430786

(CHEMBL2334765)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3C(C)=O |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C25H32N6O3/c1-4-26-25(33)27-18-7-5-17(6-8-18)23-28-22-20(13-19-9-10-21(22)31(19)16(3)32)24(29-23)30-11-12-34-14-15(30)2/h5-8,15,19,21H,4,9-14H2,1-3H3,(H2,26,27,33)/t15-,19-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400337
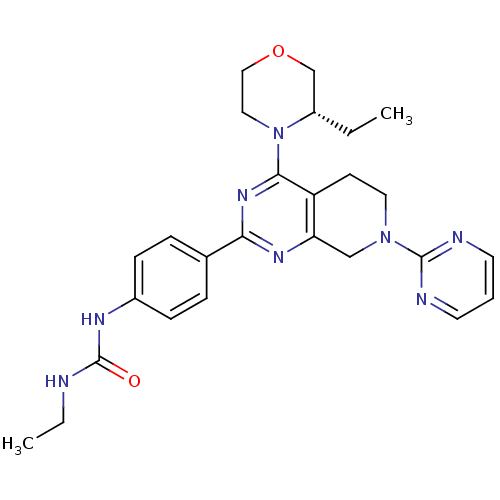
(CHEMBL2181523)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1CC)c1ncccn1 |r| Show InChI InChI=1S/C26H32N8O2/c1-3-20-17-36-15-14-34(20)24-21-10-13-33(25-28-11-5-12-29-25)16-22(21)31-23(32-24)18-6-8-19(9-7-18)30-26(35)27-4-2/h5-9,11-12,20H,3-4,10,13-17H2,1-2H3,(H2,27,30,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428125

(CHEMBL2331677)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1nccn1C |r| Show InChI InChI=1S/C25H32N8O2/c1-4-26-24(34)28-19-7-5-18(6-8-19)22-29-21-15-32(25-27-10-12-31(25)3)11-9-20(21)23(30-22)33-13-14-35-16-17(33)2/h5-8,10,12,17H,4,9,11,13-16H2,1-3H3,(H2,26,28,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400335

(CHEMBL2181525)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1cccnn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)28-19-8-6-18(7-9-19)23-29-21-15-32(22-5-4-11-27-31-22)12-10-20(21)24(30-23)33-13-14-35-16-17(33)2/h4-9,11,17H,3,10,12-16H2,1-2H3,(H2,26,28,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313984
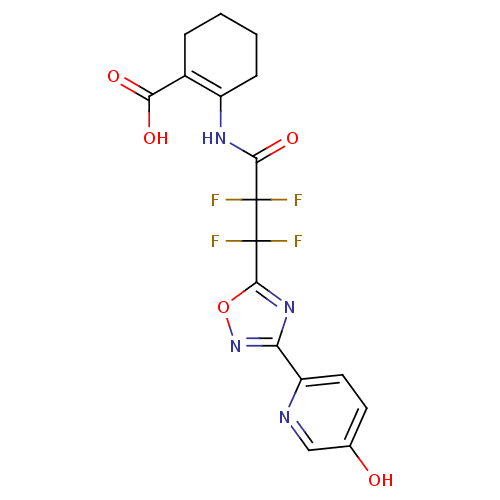
(2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)C(F)(F)C(F)(F)c1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H14F4N4O5/c18-16(19,14(29)23-10-4-2-1-3-9(10)13(27)28)17(20,21)15-24-12(25-30-15)11-6-5-8(26)7-22-11/h5-7,26H,1-4H2,(H,23,29)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015275
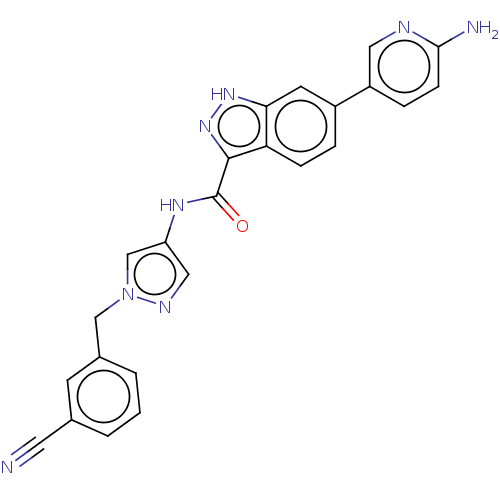
(CHEMBL3263035)Show SMILES Nc1ccc(cn1)-c1ccc2c(n[nH]c2c1)C(=O)Nc1cnn(Cc2cccc(c2)C#N)c1 Show InChI InChI=1S/C24H18N8O/c25-10-15-2-1-3-16(8-15)13-32-14-19(12-28-32)29-24(33)23-20-6-4-17(9-21(20)30-31-23)18-5-7-22(26)27-11-18/h1-9,11-12,14H,13H2,(H2,26,27)(H,29,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400338
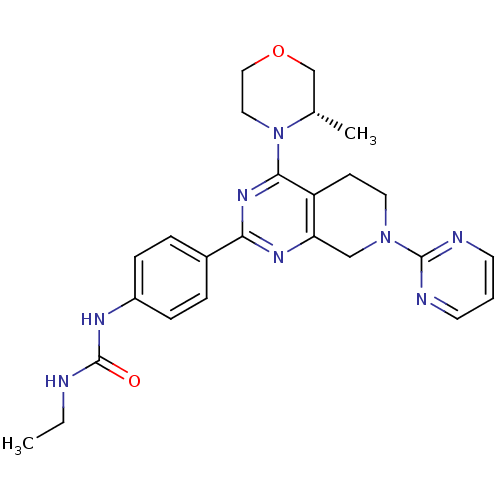
(CHEMBL2181522)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1ncccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)29-19-7-5-18(6-8-19)22-30-21-15-32(24-27-10-4-11-28-24)12-9-20(21)23(31-22)33-13-14-35-16-17(33)2/h4-8,10-11,17H,3,9,12-16H2,1-2H3,(H2,26,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430788
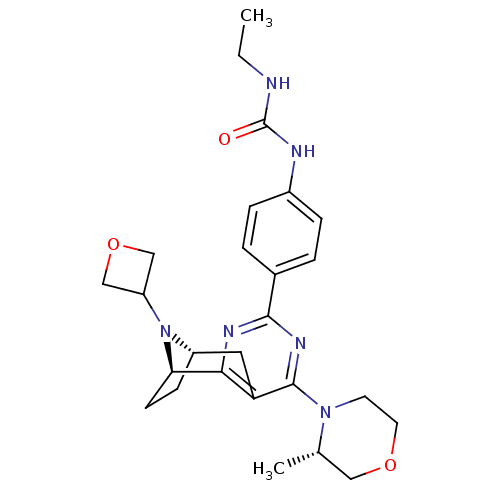
(CHEMBL2334763)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3C1COC1 |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C26H34N6O3/c1-3-27-26(33)28-18-6-4-17(5-7-18)24-29-23-21(25(30-24)31-10-11-34-13-16(31)2)12-19-8-9-22(23)32(19)20-14-35-15-20/h4-7,16,19-20,22H,3,8-15H2,1-2H3,(H2,27,28,33)/t16-,19-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400338

(CHEMBL2181522)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1ncccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)29-19-7-5-18(6-8-19)22-30-21-15-32(24-27-10-4-11-28-24)12-9-20(21)23(31-22)33-13-14-35-16-17(33)2/h4-8,10-11,17H,3,9,12-16H2,1-2H3,(H2,26,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50015252

(CHEMBL3263039)Show InChI InChI=1S/C18H14N4OS/c23-18(17-13-8-4-5-9-14(13)21-22-17)20-16-11-19-15(24-16)10-12-6-2-1-3-7-12/h1-9,11H,10H2,(H,20,23)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis |
Bioorg Med Chem Lett 24: 2448-52 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.023
BindingDB Entry DOI: 10.7270/Q2W097G8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400336
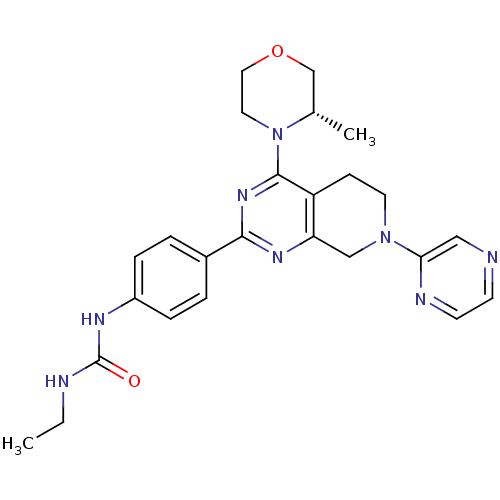
(CHEMBL2181524)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1cnccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-27-25(34)29-19-6-4-18(5-7-19)23-30-21-15-32(22-14-26-9-10-28-22)11-8-20(21)24(31-23)33-12-13-35-16-17(33)2/h4-7,9-10,14,17H,3,8,11-13,15-16H2,1-2H3,(H2,27,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443178
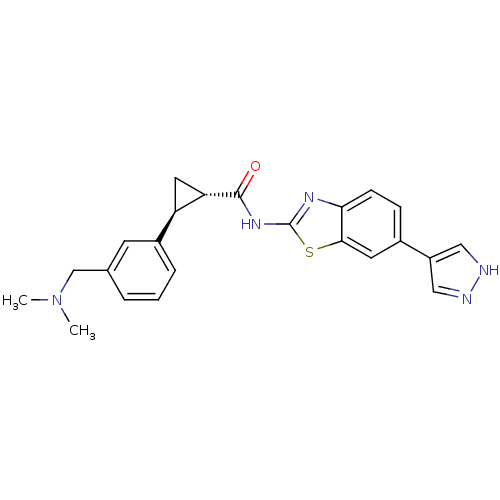
(CHEMBL3086537)Show SMILES CN(C)Cc1cccc(c1)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C23H23N5OS/c1-28(2)13-14-4-3-5-16(8-14)18-10-19(18)22(29)27-23-26-20-7-6-15(9-21(20)30-23)17-11-24-25-12-17/h3-9,11-12,18-19H,10,13H2,1-2H3,(H,24,25)(H,26,27,29)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396148

(CHEMBL2171745 | US8802674, 50)Show SMILES CNc1nc(Nc2ccc(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H20F3N5O3/c1-22-15-12(18(19,20)21)10-23-17(25-15)24-13-4-3-11(9-14(13)28-2)16(27)26-5-7-29-8-6-26/h3-4,9-10H,5-8H2,1-2H3,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50396150
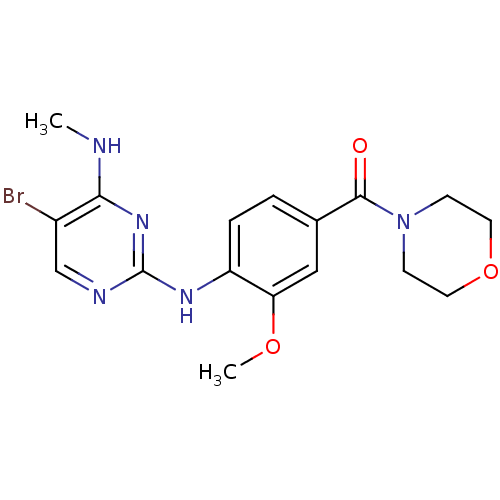
(CHEMBL2171743)Show InChI InChI=1S/C17H20BrN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398665
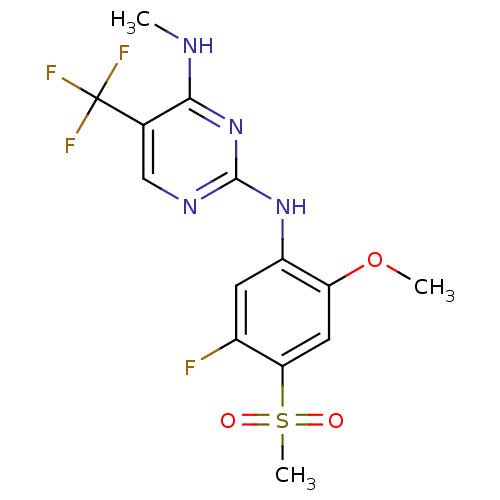
(CHEMBL2178137 | US9145402, 15)Show SMILES CNc1nc(Nc2cc(F)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C14H14F4N4O3S/c1-19-12-7(14(16,17)18)6-20-13(22-12)21-9-4-8(15)11(26(3,23)24)5-10(9)25-2/h4-6H,1-3H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398672
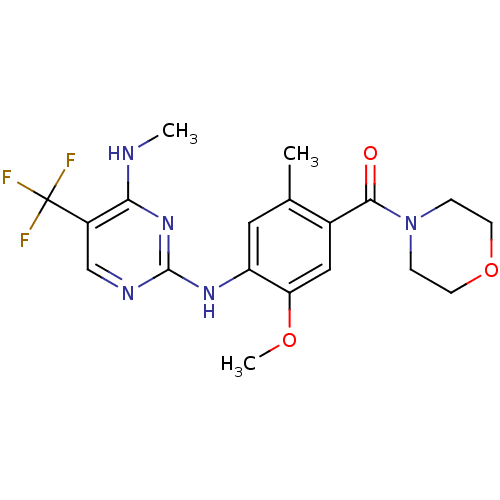
(CHEMBL2178130)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C19H22F3N5O3/c1-11-8-14(25-18-24-10-13(19(20,21)22)16(23-2)26-18)15(29-3)9-12(11)17(28)27-4-6-30-7-5-27/h8-10H,4-7H2,1-3H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50398677
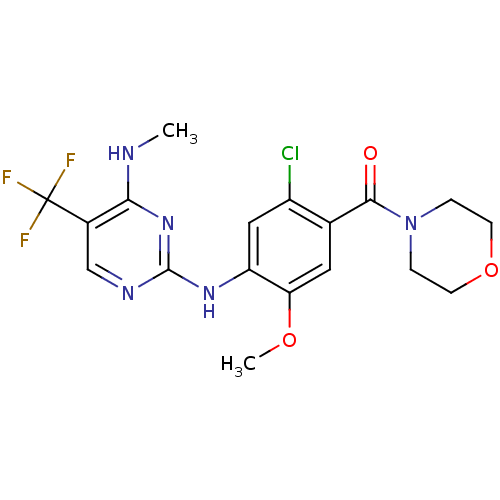
(CHEMBL2178124 | US8802674, 292)Show SMILES CNc1nc(Nc2cc(Cl)c(cc2OC)C(=O)N2CCOCC2)ncc1C(F)(F)F Show InChI InChI=1S/C18H19ClF3N5O3/c1-23-15-11(18(20,21)22)9-24-17(26-15)25-13-8-12(19)10(7-14(13)29-2)16(28)27-3-5-30-6-4-27/h7-9H,3-6H2,1-2H3,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430785

(CHEMBL2334766)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3C(=O)COC |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C26H34N6O4/c1-4-27-26(34)28-18-7-5-17(6-8-18)24-29-23-20(25(30-24)31-11-12-36-14-16(31)2)13-19-9-10-21(23)32(19)22(33)15-35-3/h5-8,16,19,21H,4,9-15H2,1-3H3,(H2,27,28,34)/t16-,19-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400349

(CHEMBL2181651)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CCN(Cc2c(n1)N1CCOC[C@@H]1C)c1ncccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-26-25(34)29-19-7-5-18(6-8-19)22-30-21-9-12-32(24-27-10-4-11-28-24)15-20(21)23(31-22)33-13-14-35-16-17(33)2/h4-8,10-11,17H,3,9,12-16H2,1-2H3,(H2,26,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400347

(CHEMBL2181653)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CCN(Cc2c(n1)N1CCOC[C@@H]1C)c1cnccn1 |r| Show InChI InChI=1S/C25H30N8O2/c1-3-27-25(34)29-19-6-4-18(5-7-19)23-30-21-8-11-32(22-14-26-9-10-28-22)15-20(21)24(31-23)33-12-13-35-16-17(33)2/h4-7,9-10,14,17H,3,8,11-13,15-16H2,1-2H3,(H2,27,29,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428127
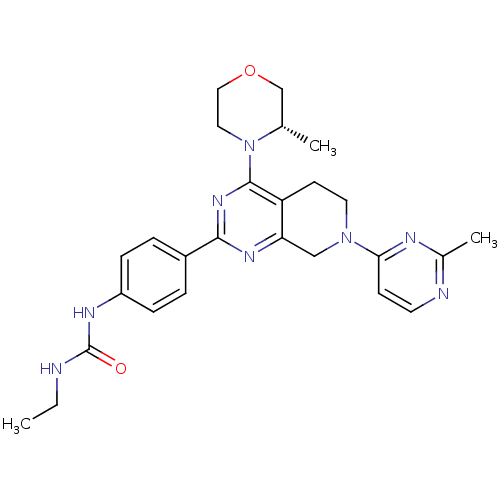
(CHEMBL2331689)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1ccnc(C)n1 |r| Show InChI InChI=1S/C26H32N8O2/c1-4-27-26(35)30-20-7-5-19(6-8-20)24-31-22-15-33(23-9-11-28-18(3)29-23)12-10-21(22)25(32-24)34-13-14-36-16-17(34)2/h5-9,11,17H,4,10,12-16H2,1-3H3,(H2,27,30,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443177
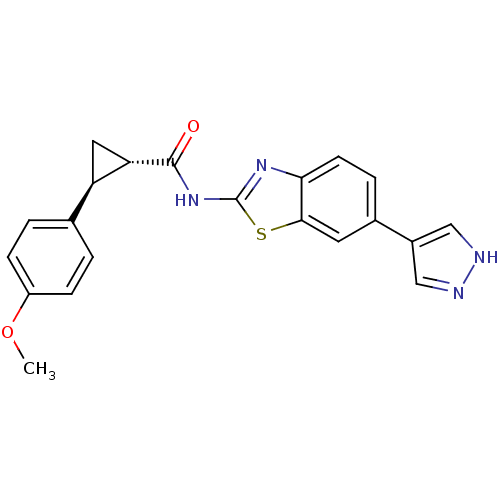
(CHEMBL3086533)Show SMILES COc1ccc(cc1)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C21H18N4O2S/c1-27-15-5-2-12(3-6-15)16-9-17(16)20(26)25-21-24-18-7-4-13(8-19(18)28-21)14-10-22-23-11-14/h2-8,10-11,16-17H,9H2,1H3,(H,22,23)(H,24,25,26)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430791
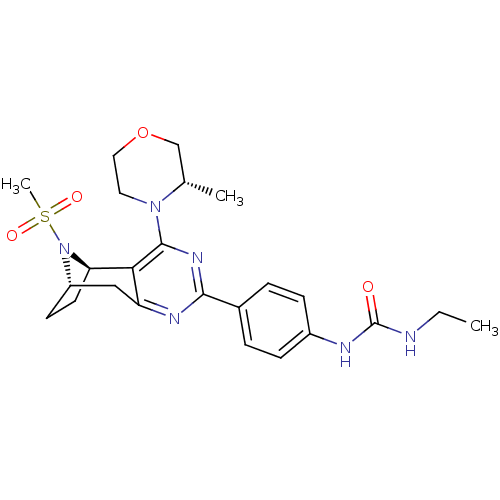
(CHEMBL2334758)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2C[C@@H]3CC[C@@H](N3S(C)(=O)=O)c2c(n1)N1CCOC[C@@H]1C |r,THB:21:20:14.25.15:18.17| Show InChI InChI=1S/C24H32N6O4S/c1-4-25-24(31)26-17-7-5-16(6-8-17)22-27-19-13-18-9-10-20(30(18)35(3,32)33)21(19)23(28-22)29-11-12-34-14-15(29)2/h5-8,15,18,20H,4,9-14H2,1-3H3,(H2,25,26,31)/t15-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428136
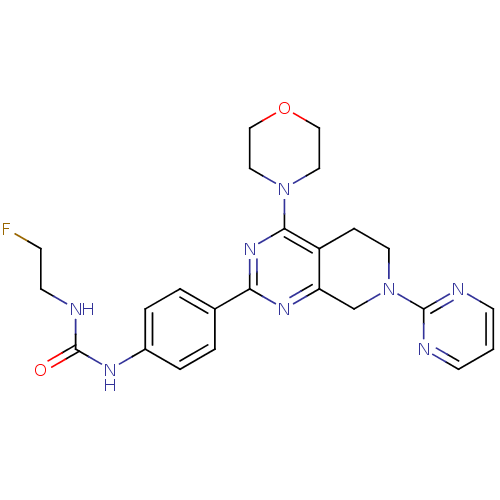
(CHEMBL2331682)Show SMILES FCCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOCC1)c1ncccn1 Show InChI InChI=1S/C24H27FN8O2/c25-7-10-28-24(34)29-18-4-2-17(3-5-18)21-30-20-16-33(23-26-8-1-9-27-23)11-6-19(20)22(31-21)32-12-14-35-15-13-32/h1-5,8-9H,6-7,10-16H2,(H2,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428126
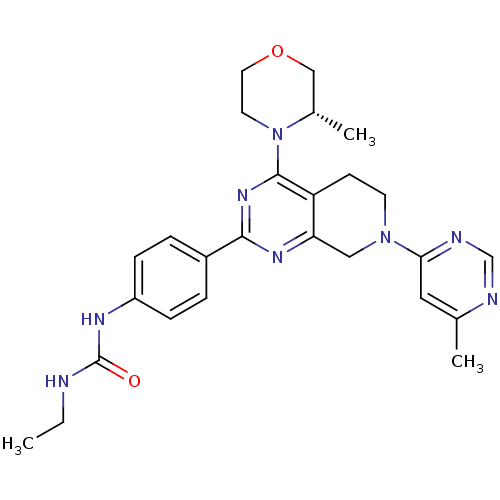
(CHEMBL2331676)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1cc(C)ncn1 |r| Show InChI InChI=1S/C26H32N8O2/c1-4-27-26(35)30-20-7-5-19(6-8-20)24-31-22-14-33(23-13-17(2)28-16-29-23)10-9-21(22)25(32-24)34-11-12-36-15-18(34)3/h5-8,13,16,18H,4,9-12,14-15H2,1-3H3,(H2,27,30,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428134
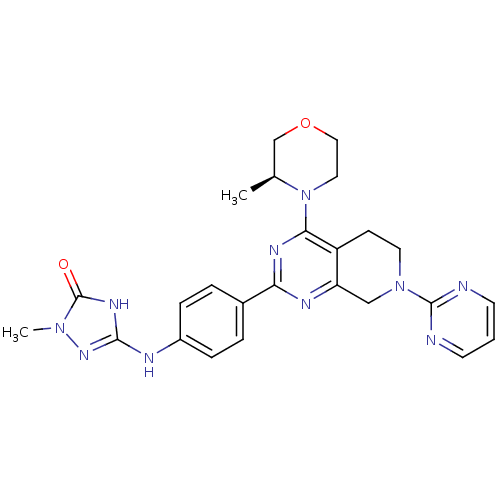
(CHEMBL2331684)Show SMILES C[C@H]1COCCN1c1nc(nc2CN(CCc12)c1ncccn1)-c1ccc(Nc2nn(C)c(=O)[nH]2)cc1 |r| Show InChI InChI=1S/C25H28N10O2/c1-16-15-37-13-12-35(16)22-19-8-11-34(24-26-9-3-10-27-24)14-20(19)29-21(30-22)17-4-6-18(7-5-17)28-23-31-25(36)33(2)32-23/h3-7,9-10,16H,8,11-15H2,1-2H3,(H2,28,31,32,36)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50400359

(CHEMBL2181664)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(Cc2c(n1)N1CCOCC1)c1ncccn1 Show InChI InChI=1S/C23H26N8O2/c1-2-24-23(32)27-17-6-4-16(5-7-17)20-28-19-15-31(22-25-8-3-9-26-22)14-18(19)21(29-20)30-10-12-33-13-11-30/h3-9H,2,10-15H2,1H3,(H2,24,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR by FRET assay |
J Med Chem 55: 10958-71 (2012)
Article DOI: 10.1021/jm301389h
BindingDB Entry DOI: 10.7270/Q2125TSR |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM482160
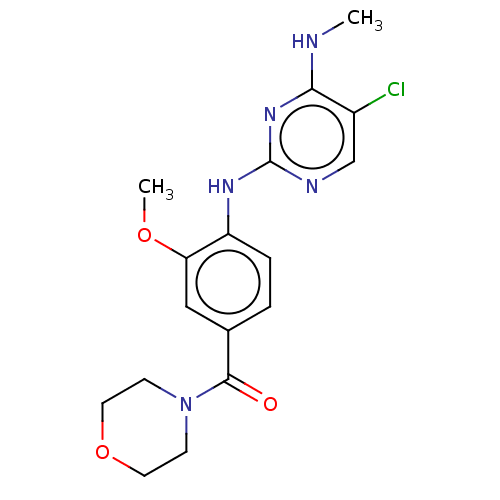
(BDBM50396041 | HG-10-102-01)Show InChI InChI=1S/C17H20ClN5O3/c1-19-15-12(18)10-20-17(22-15)21-13-4-3-11(9-14(13)25-2)16(24)23-5-7-26-8-6-23/h3-4,9-10H,5-8H2,1-2H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay |
J Med Chem 55: 9416-33 (2012)
Article DOI: 10.1021/jm301020q
BindingDB Entry DOI: 10.7270/Q2P55PN7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data