

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077930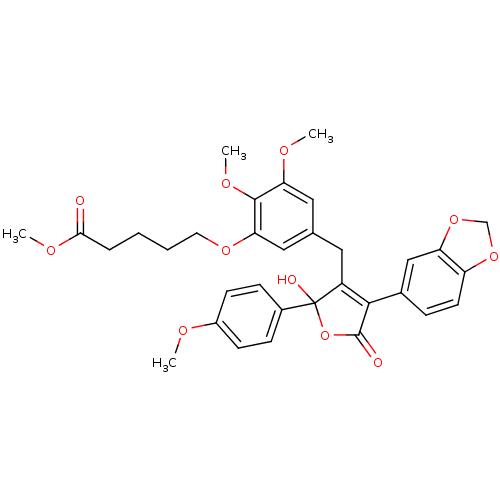 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077933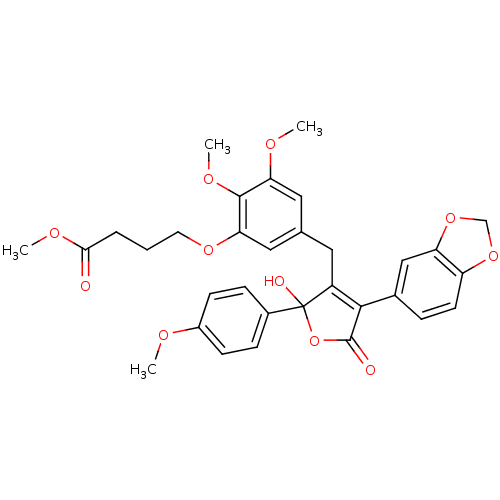 (4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18033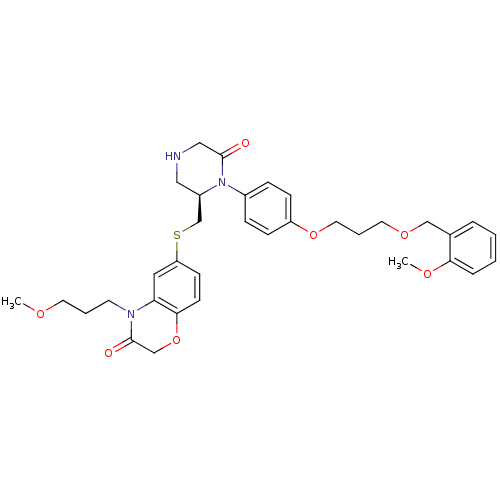 (Ketopiperazine-based inhibitor, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077934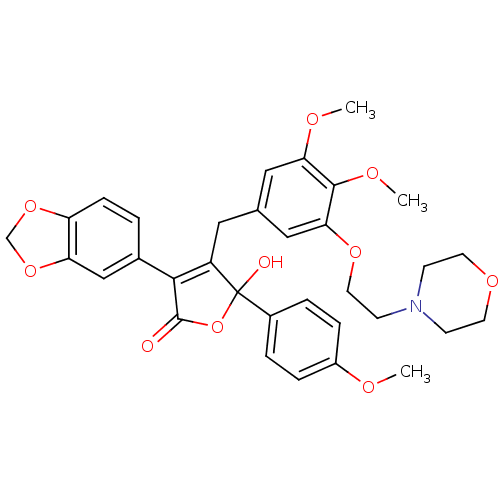 (3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(2-morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215692 (CHEMBL400874 | sodium (3R,5R)-7-(5-((4-cyanobenzyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077935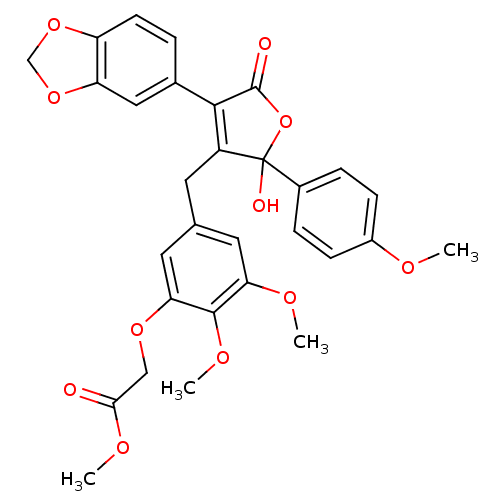 (CHEMBL308646 | {5-[4-Benzo[1,3]dioxol-5-yl-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077936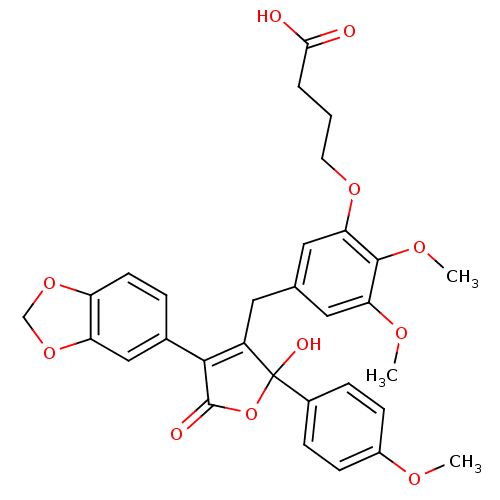 (4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267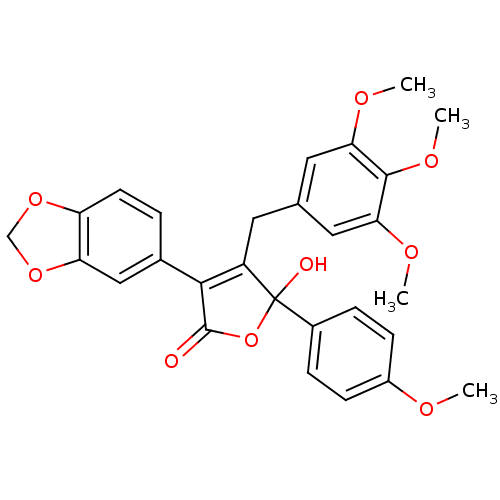 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077946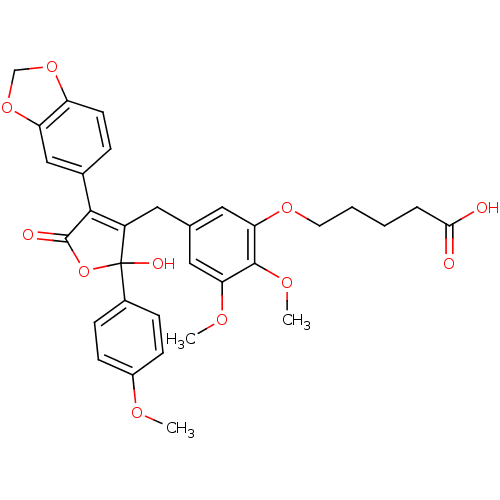 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Antagonistic activity against human Endothelin A receptor expressed in LtK | J Med Chem 38: 1259-63 (1995) BindingDB Entry DOI: 10.7270/Q2WH2P1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215689 (CHEMBL250317 | sodium (3R,5R)-7-(5-((4-carbamoylph...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215688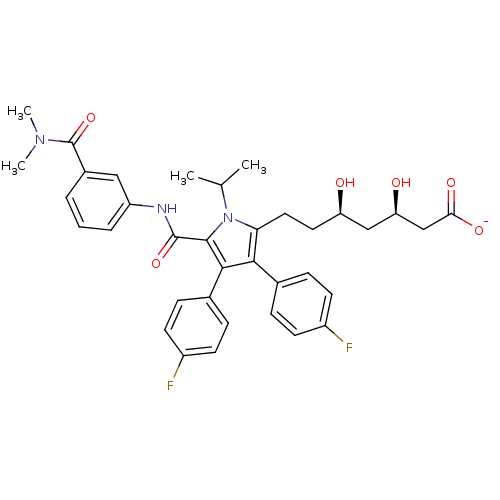 (CHEMBL399773 | sodium (3R,5R)-7-(5-((3-(dimethylca...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215693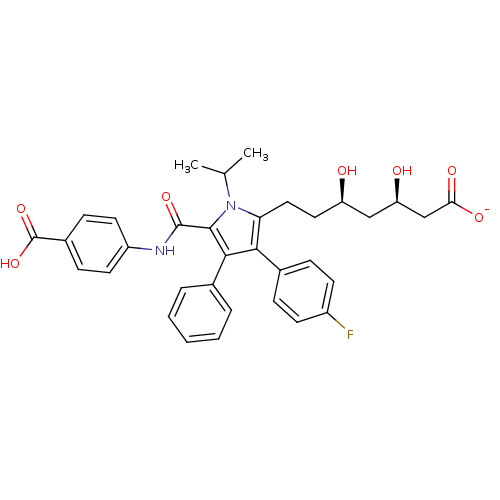 (CHEMBL437774 | sodium (3R,5R)-7-(5-((4-carboxyphen...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034263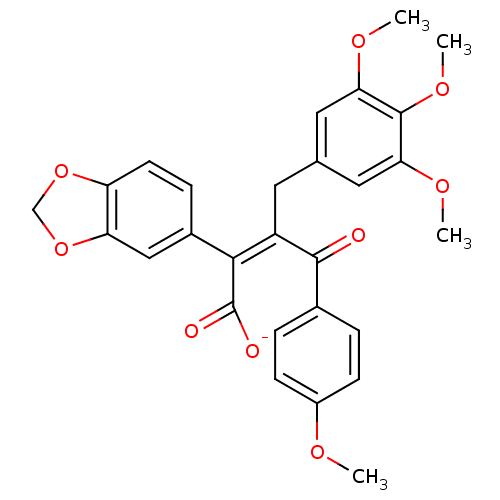 (CHEMBL25438 | PD-156707 | Sodium; (Z)-2-benzo[1,3]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Antagonistic activity against human Endothelin A receptor expressed in LtK | J Med Chem 38: 1259-63 (1995) BindingDB Entry DOI: 10.7270/Q2WH2P1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077944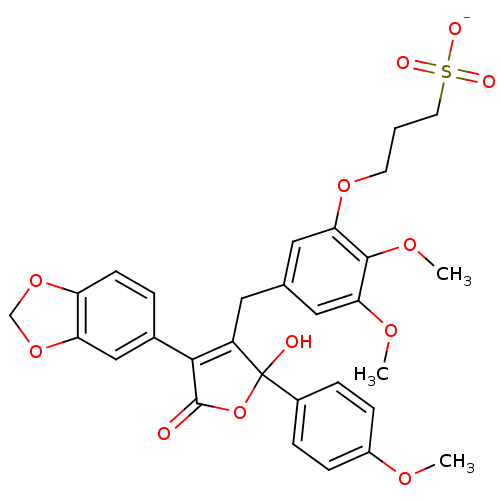 (CHEMBL408055 | Sodium; 3-{5-[4-benzo[1,3]dioxol-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215700 (CHEMBL400973 | sodium (3R,5R)-7-(3-(4-fluorophenyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18025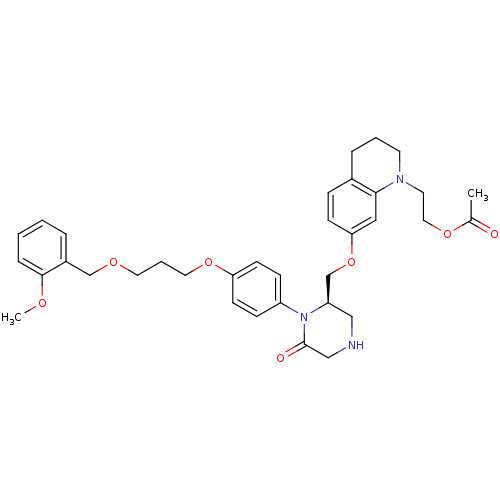 (2-(7-{[(2R)-1-(4-{3-[(2-methoxyphenyl)methoxy]prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215704 (CHEMBL398551 | sodium (3R,5R)-7-(5-(((1,5-dimethyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM18032 (Ketopiperazine-based inhibitor, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem Lett 16: 2500-4 (2006) Article DOI: 10.1016/j.bmcl.2006.01.084 BindingDB Entry DOI: 10.7270/Q26D5R7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215707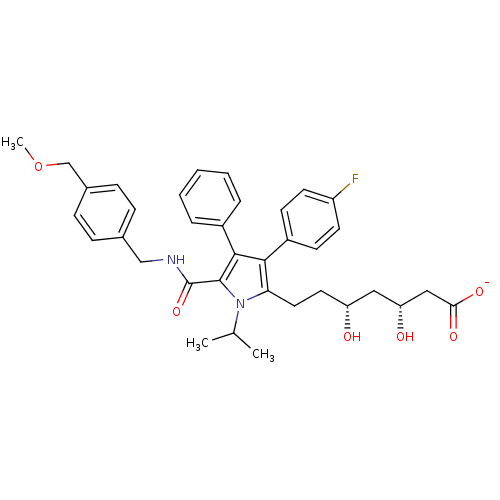 (CHEMBL250749 | sodium (3R,5R)-7-(5-((4-(methoxymet...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077941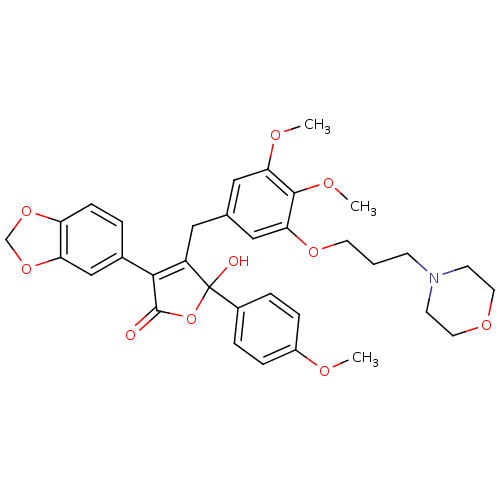 (3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(3-morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215699 (CHEMBL400747 | sodium (3R,5R)-7-(3,4-bis(4-fluorop...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215690 (CHEMBL399313 | sodium (3R,5R)-7-(5-(benzylcarbamoy...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215684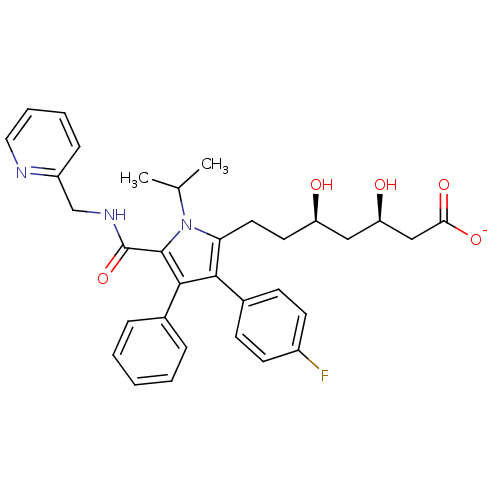 (CHEMBL398240 | sodium (3R,5R)-7-(3-(4-fluorophenyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215708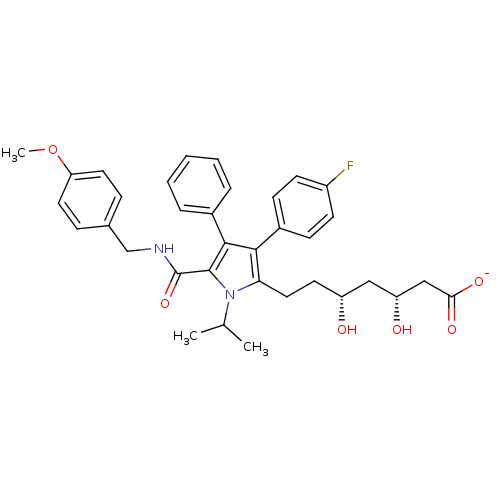 (CHEMBL249724 | sodium (3R,5R)-7-(5-((4-methoxybenz...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18425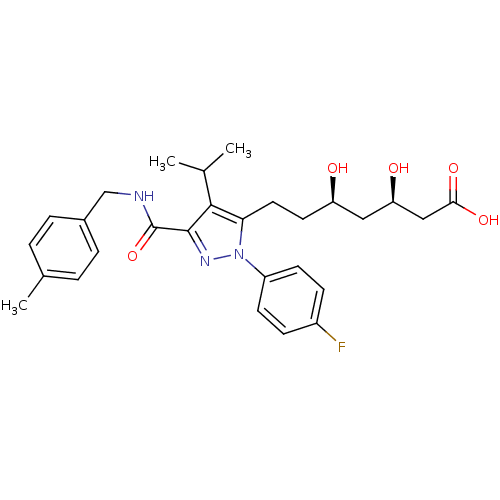 ((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(4-methylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077943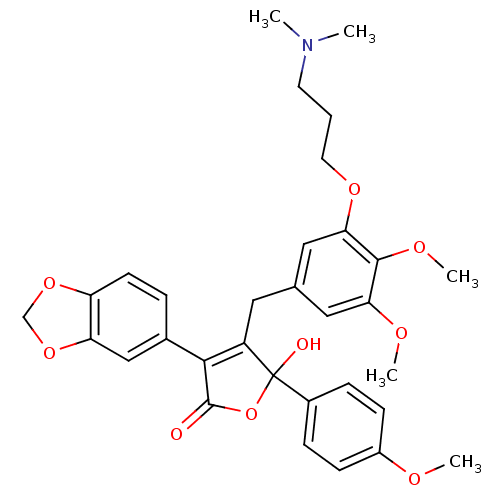 (3-Benzo[1,3]dioxol-5-yl-4-[3-(3-dimethylamino-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215698 (CHEMBL251499 | sodium (3R,5R)-7-(5-((4-(dimethylca...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346283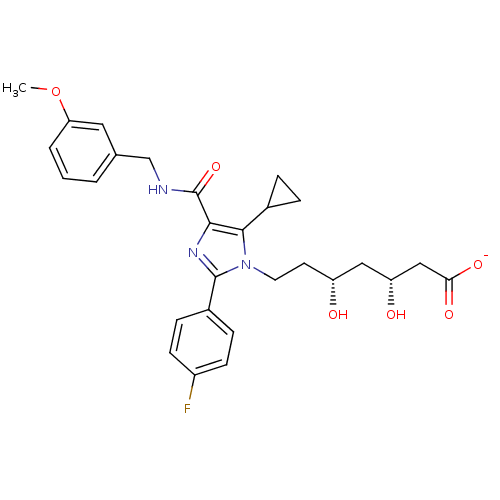 (CHEMBL1782556 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215694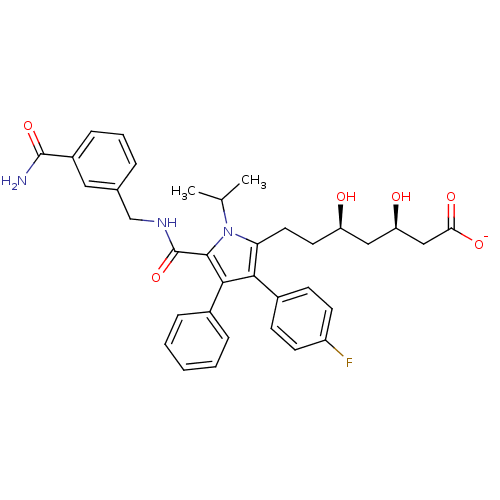 (CHEMBL249906 | sodium (3R,5R)-7-(5-((3-carbamoylbe...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346289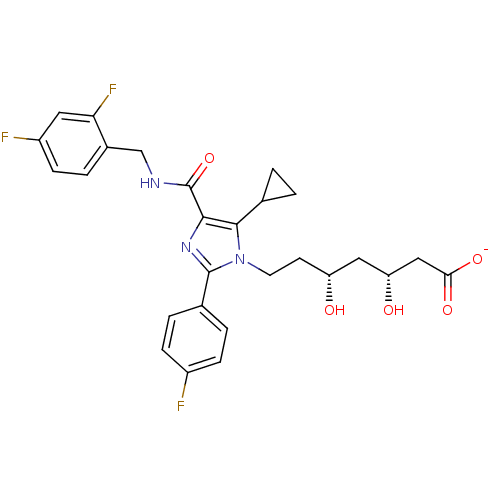 (CHEMBL1782561 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346288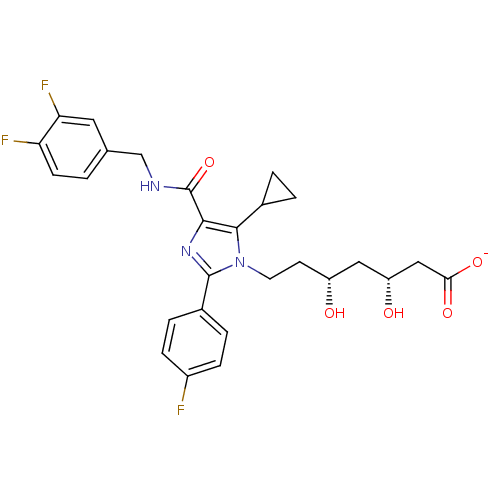 (CHEMBL1782560 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346286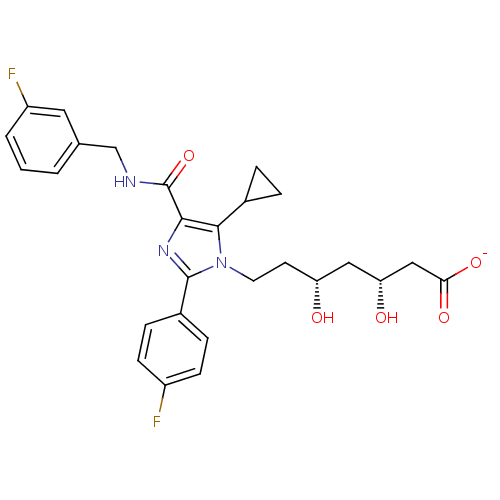 (CHEMBL1782559 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346282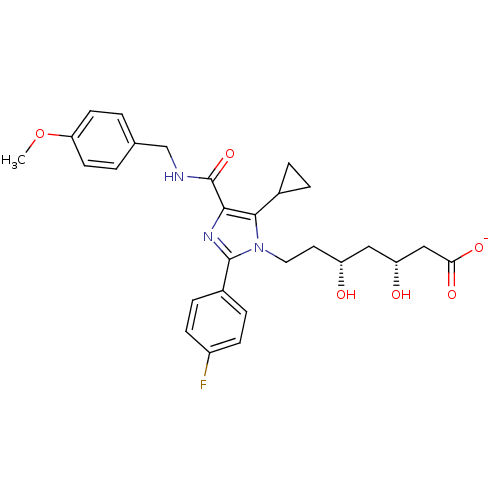 (CHEMBL1782555 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215683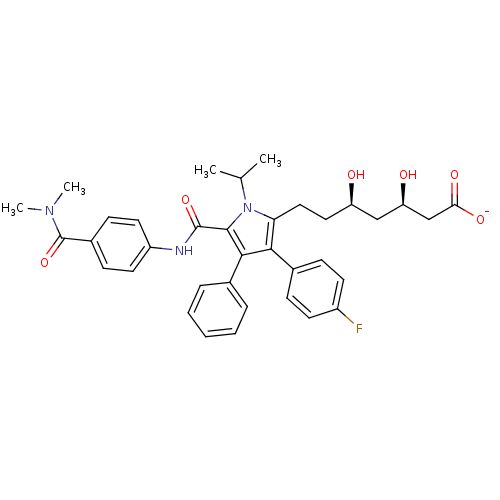 (CHEMBL403127 | sodium (3R,5R)-7-(5-((4-(dimethylca...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346278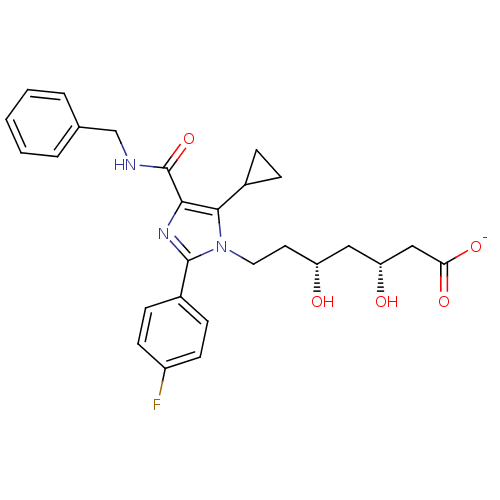 (CHEMBL1782551 | sodium(3R,5R)-7-(4-(benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215691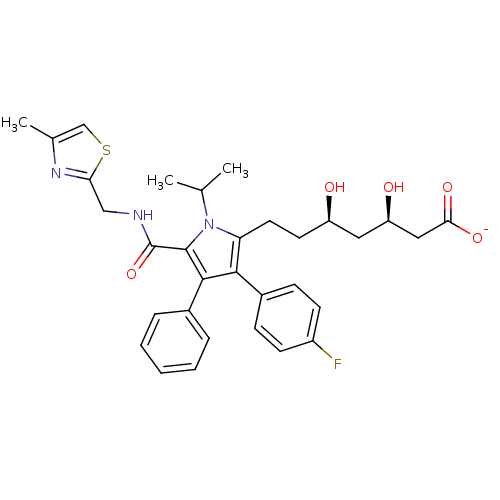 (CHEMBL398239 | sodium (3R,5R)-7-(3-(4-fluorophenyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346287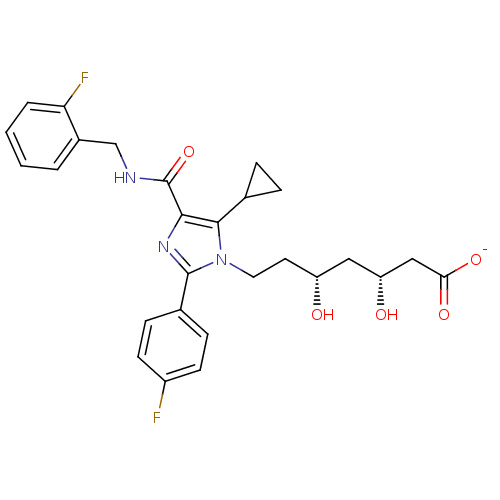 (CHEMBL1782062 | sodium(3R,5R)-7-(5-cyclopropyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50346279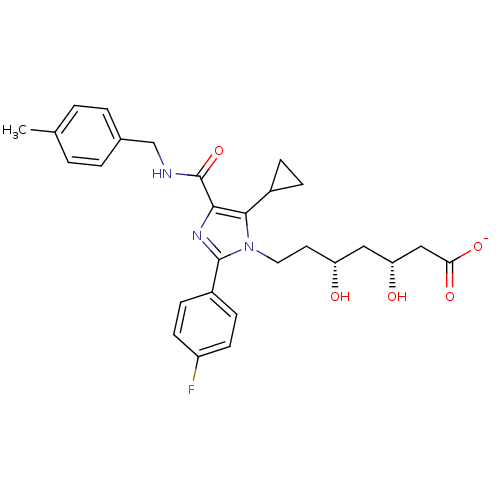 (CHEMBL1782552 | sodium(3R,5R)-7-(5-cyclopropyl-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase in Sprague-Dawley rat liver microsomes using using [14C]HMG-CoA as substrate preincubated for 0.5 hrs before substrat... | Bioorg Med Chem Lett 21: 2725-31 (2011) Article DOI: 10.1016/j.bmcl.2010.11.103 BindingDB Entry DOI: 10.7270/Q2H995J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034259 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Antagonistic activity against human Endothelin A receptor expressed in LtK | J Med Chem 38: 1259-63 (1995) BindingDB Entry DOI: 10.7270/Q2WH2P1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM18374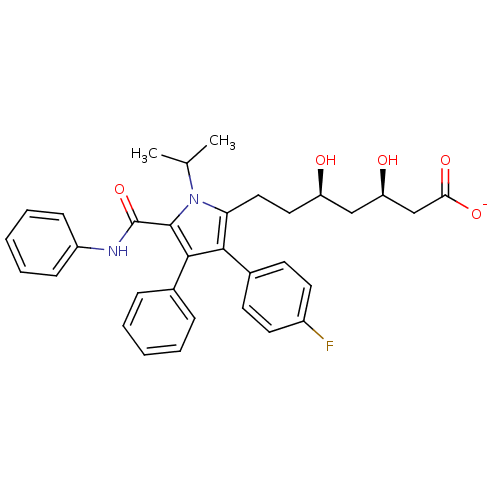 (CHEMBL394937 | Pyrrole-based compound, 30 | sodium...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215706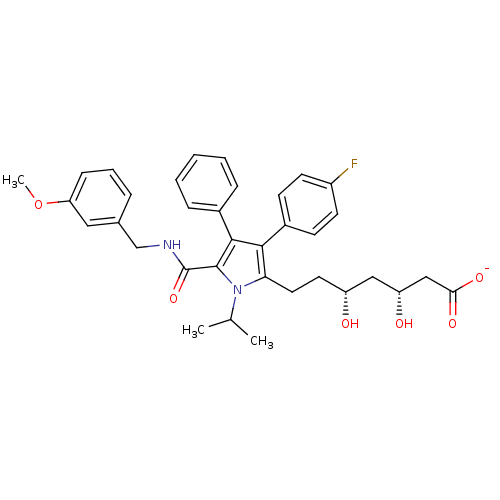 (CHEMBL399315 | sodium (3R,5R)-7-(5-((3-methoxybenz...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077947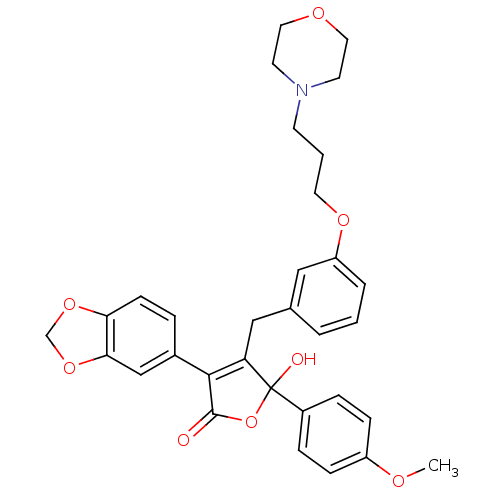 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077938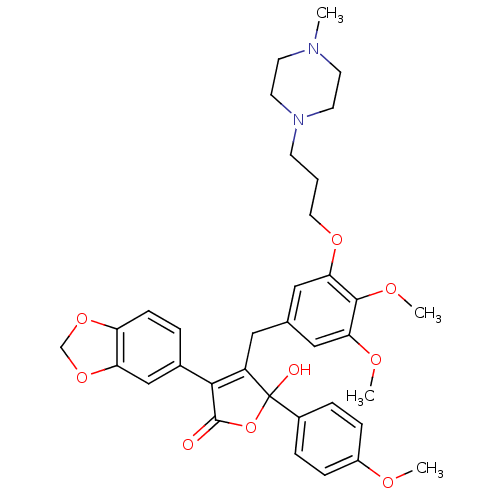 (3-Benzo[1,3]dioxol-5-yl-4-{3,4-dimethoxy-5-[3-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215686 (CHEMBL401293 | sodium (3R,5R)-7-(5-((4-carboxybenz...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034269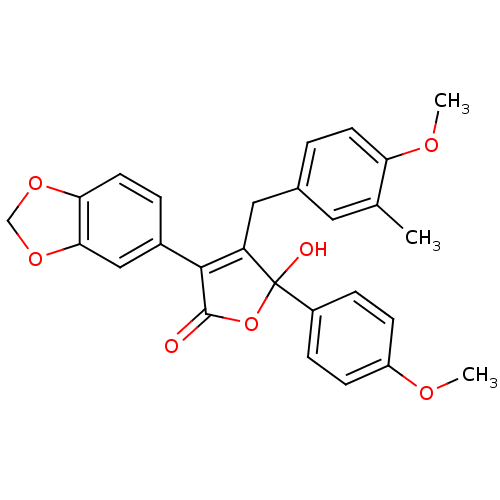 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-4-(4-methoxy-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Antagonistic activity against human Endothelin A receptor expressed in LtK | J Med Chem 38: 1259-63 (1995) BindingDB Entry DOI: 10.7270/Q2WH2P1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50215696 (CHEMBL400560 | sodium (3R,5R)-7-(3-(4-fluorophenyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Inhibition of HMG-CoA reductase | Bioorg Med Chem Lett 17: 4538-44 (2007) Article DOI: 10.1016/j.bmcl.2007.05.096 BindingDB Entry DOI: 10.7270/Q2SJ1K9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28661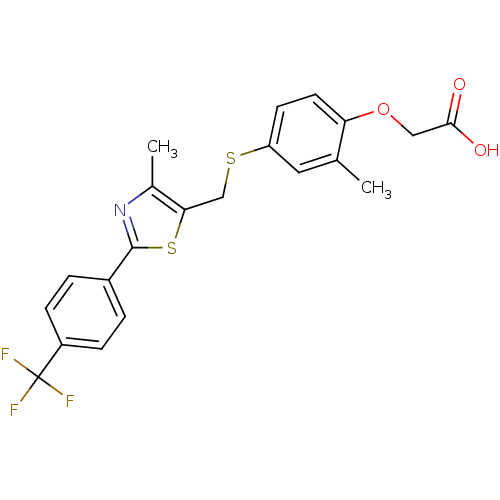 (2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077945 (3-Benzo[1,3]dioxol-5-yl-4-[3-(2-dimethylamino-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50213331 (2-(5-((4-methyl-2-(trifluoromethyl)thiazol-5-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA | Bioorg Med Chem Lett 17: 3630-5 (2007) Article DOI: 10.1016/j.bmcl.2007.04.047 BindingDB Entry DOI: 10.7270/Q2ZK5GBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 329 total ) | Next | Last >> |