

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221787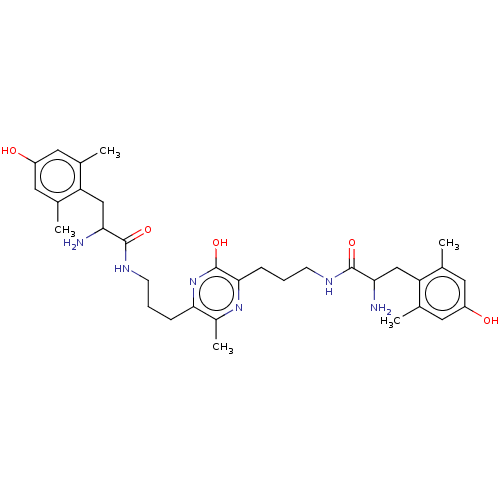 (CHEMBL3216418) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221778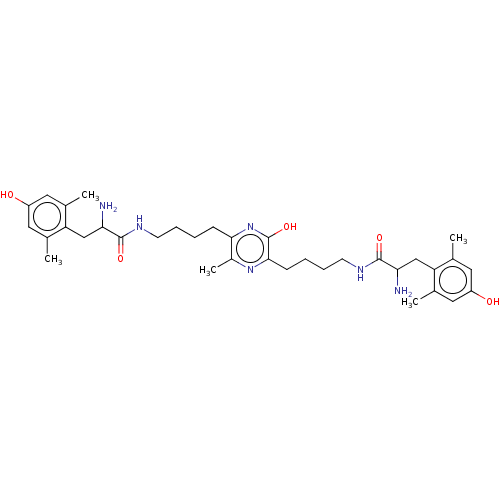 (CHEMBL3216392) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221781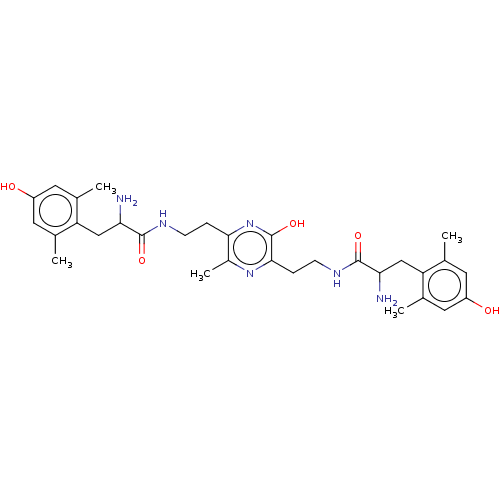 (CHEMBL3215968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221780 (CHEMBL3217089) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221781 (CHEMBL3215968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards opioid receptor delta 1 by displacing [3H]DPDPE radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221787 (CHEMBL3216418) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description pA2 is the negative log of the molar concentration required to double the agonism of opioid receptor delta 1 | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221780 (CHEMBL3217089) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards opioid receptor delta 1 by displacing [3H]DPDPE radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221778 (CHEMBL3216392) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221779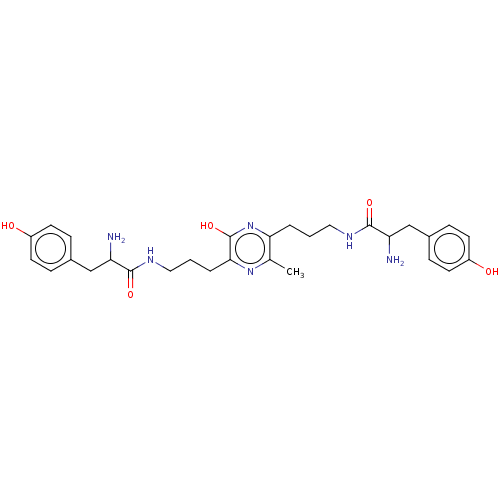 (CHEMBL3215974) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221782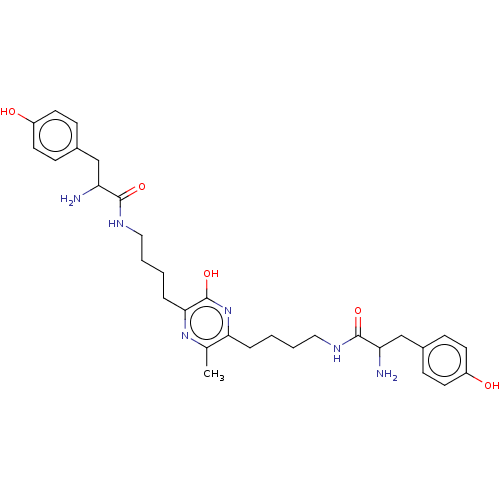 (CHEMBL3215530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50146023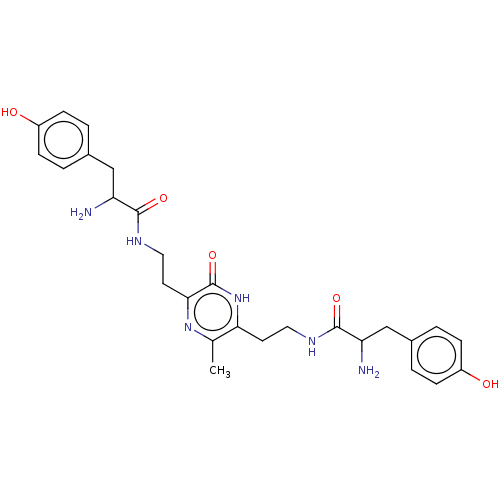 (2-Amino-N-[2-(5-{2-[2-amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221779 (CHEMBL3215974) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards opioid receptor delta 1 by displacing [3H]DPDPE radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50146020 (2-Amino-N-(5-{[2-amino-3-(4-hydroxy-phenyl)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards Opioid receptor mu 1 by displacing [3H]DAGO radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50146020 (2-Amino-N-(5-{[2-amino-3-(4-hydroxy-phenyl)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards opioid receptor delta 1 by displacing [3H]DPDPE radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50221782 (CHEMBL3215530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards opioid receptor delta 1 by displacing [3H]DPDPE radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50146023 (2-Amino-N-[2-(5-{2-[2-amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards opioid receptor delta 1 by displacing [3H]DPDPE radioligand in rat brain P2 synaptosomes membranes. | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062810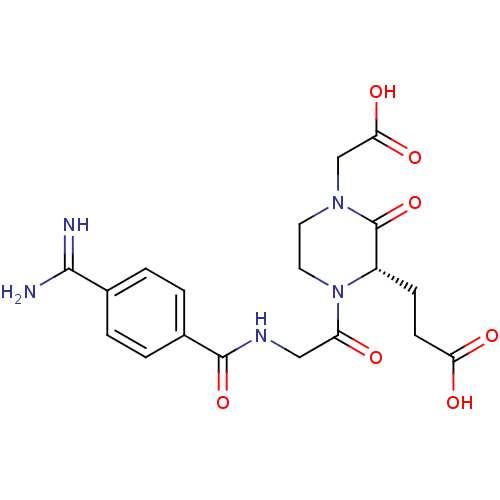 (3-{(S)-1-[2-(4-Carbamimidoyl-benzoylamino)-acetyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062797 (3-{(S)-1-[2-(4-Carbamimidoyl-benzoylamino)-acetyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062785 (CHEMBL147976 | {4-[2-(4-Carbamimidoyl-benzoylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067612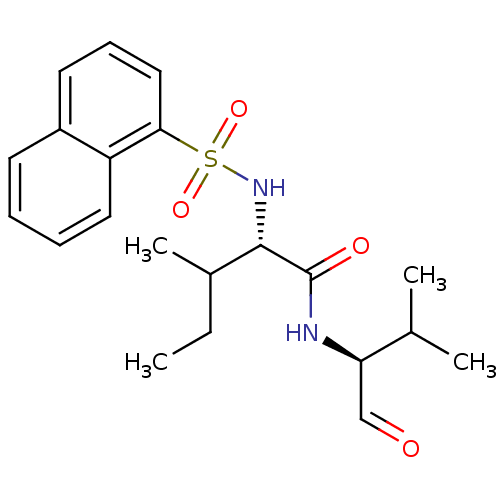 ((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062803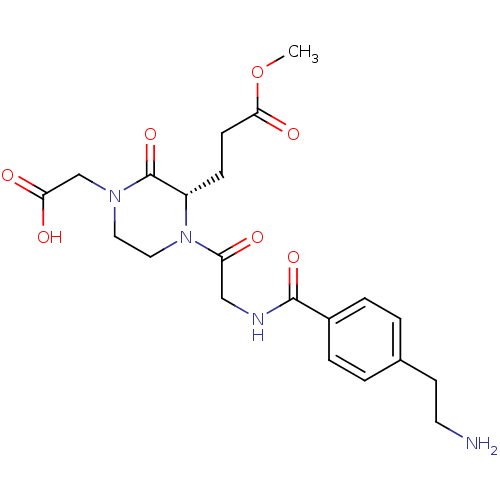 (3-((S)-1-{2-[4-(2-Amino-ethyl)-benzoylamino]-acety...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062789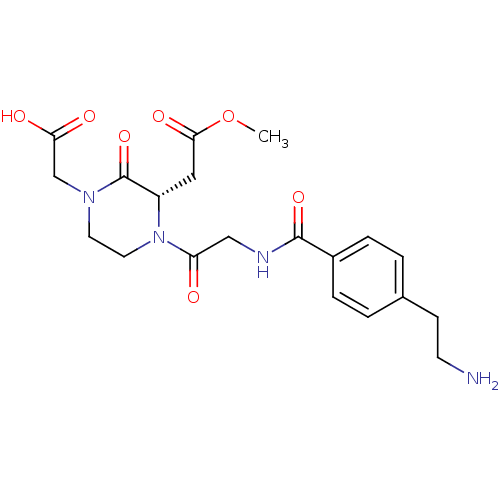 (((S)-4-{2-[4-(2-Amino-ethyl)-benzoylamino]-acetyl}...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062798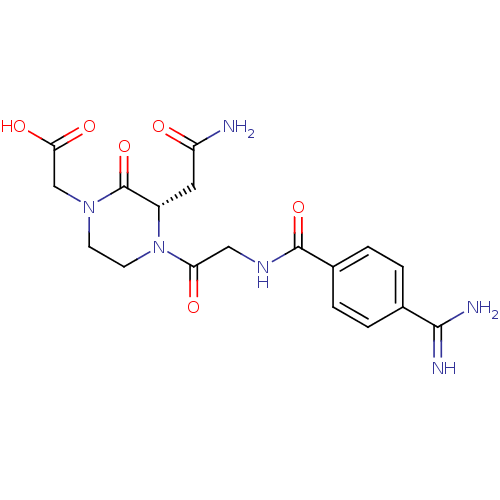 (CHEMBL148010 | {(S)-4-[2-(4-Carbamimidoyl-benzoyla...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067608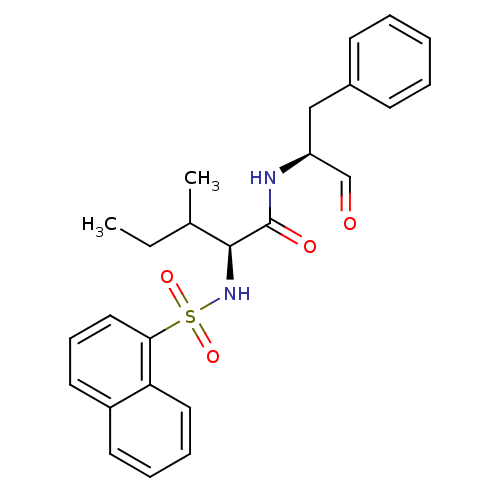 ((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067606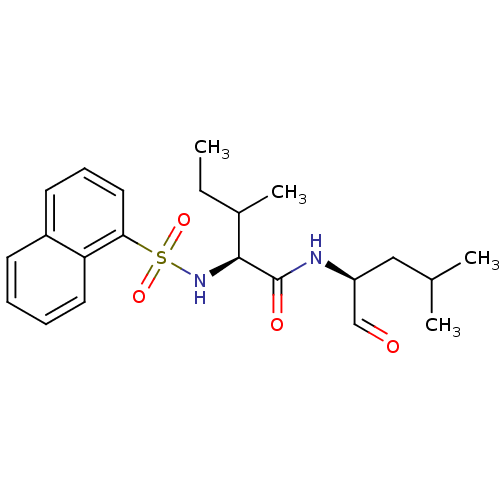 ((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067604 ((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50422277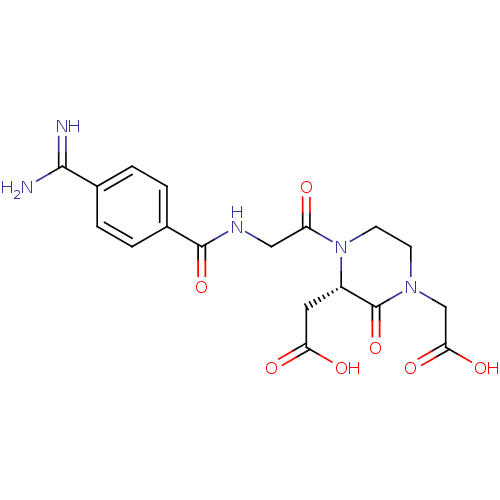 (CHEMBL118402) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062783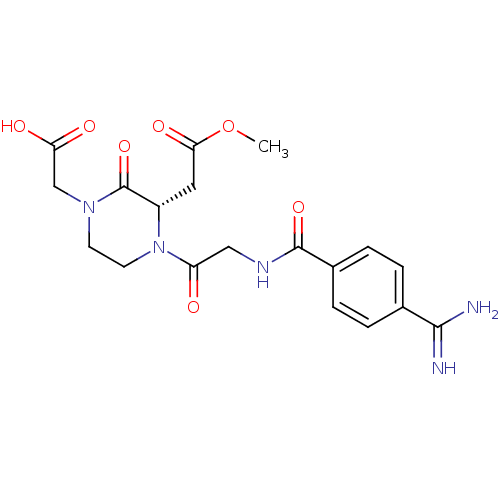 (CHEMBL310081 | TAK-029 | {(S)-1-[2-(4-Carbamimidoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062782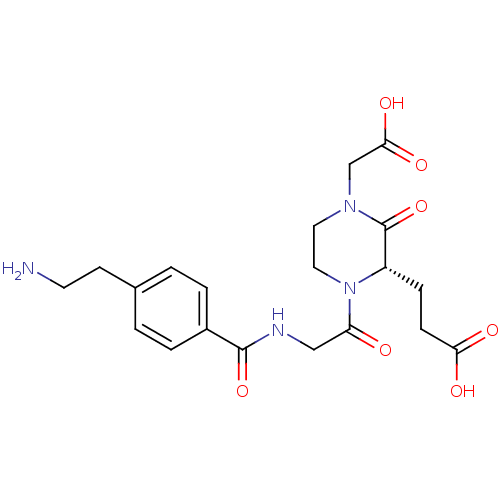 (3-((S)-1-{2-[4-(2-Amino-ethyl)-benzoylamino]-acety...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50221787 (CHEMBL3216418) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067613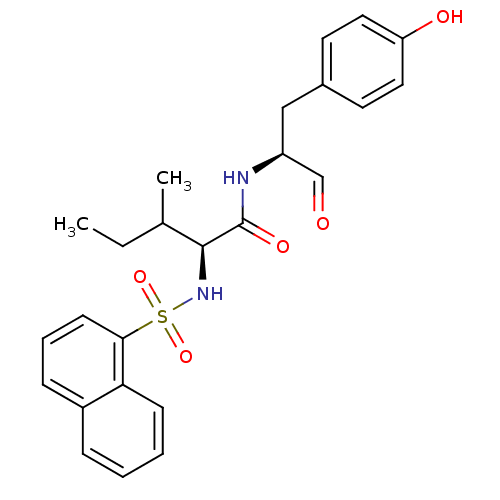 ((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062787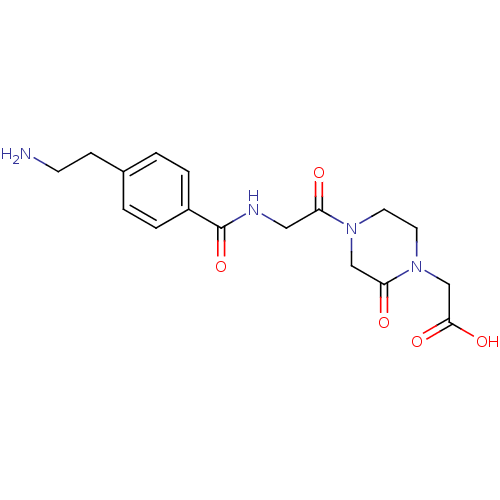 ((4-{2-[4-(2-Amino-ethyl)-benzoylamino]-acetyl}-2-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369397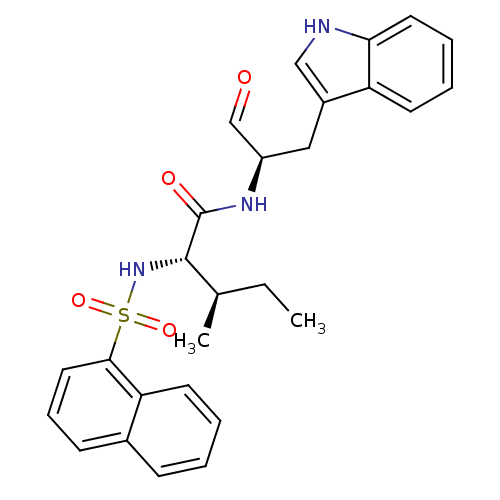 (CHEMBL1790993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50221778 (CHEMBL3216392) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum | J Med Chem 47: 2599-610 (2004) Article DOI: 10.1021/jm0304616 BindingDB Entry DOI: 10.7270/Q2RB755V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369410 (CHEMBL1790989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369405 (CHEMBL1790991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369397 (CHEMBL1790993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Compound was measured for inhibition of collagenolytic of human Cathepsin L | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062791 (CHEMBL146393 | {(S)-3-Benzyl-4-[2-(4-carbamimidoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369402 (CHEMBL1790996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369400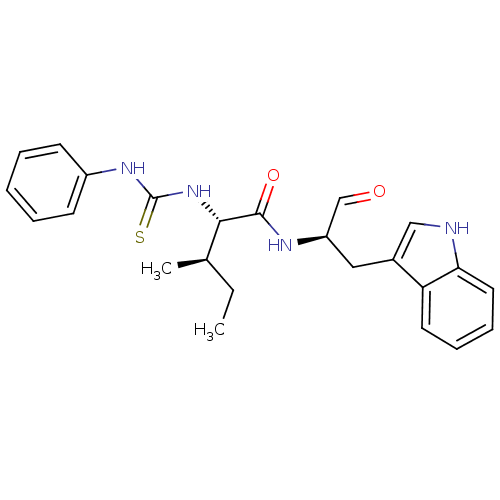 (CHEMBL1791000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067597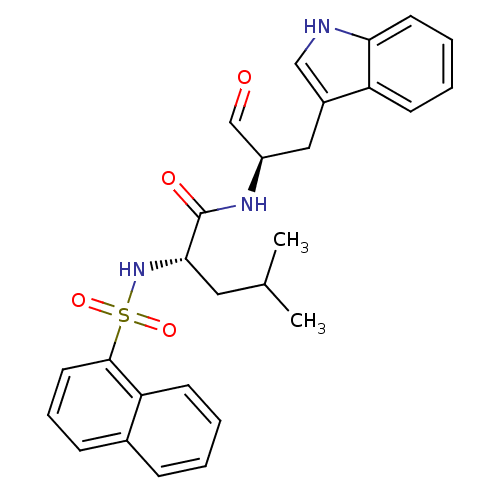 ((S)-4-Methyl-2-(naphthalene-1-sulfonylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369403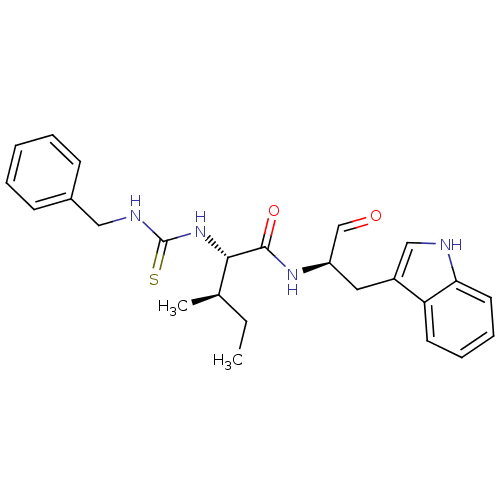 (CHEMBL1790995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369398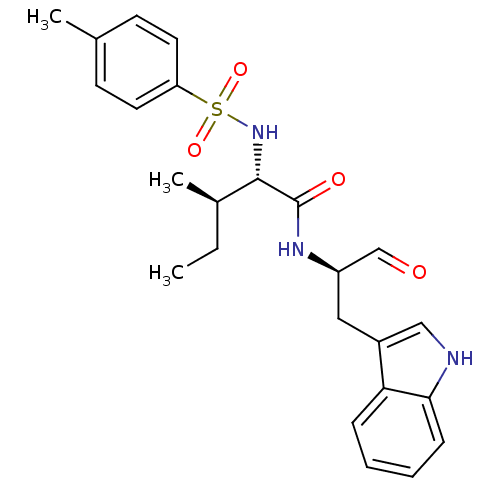 (CHEMBL1790999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50062781 (((S)-1-{2-[4-(2-Amino-ethyl)-benzoylamino]-acetyl}...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit binding of biotin-labeled human fibrinogen to immobilized fibrinogen receptor purified from human erythroleukemia(HEL) cells. | J Med Chem 41: 489-502 (1998) Article DOI: 10.1021/jm970235u BindingDB Entry DOI: 10.7270/Q2571B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Compound was measured for inhibition of collagenolytic of human Cathepsin L | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067594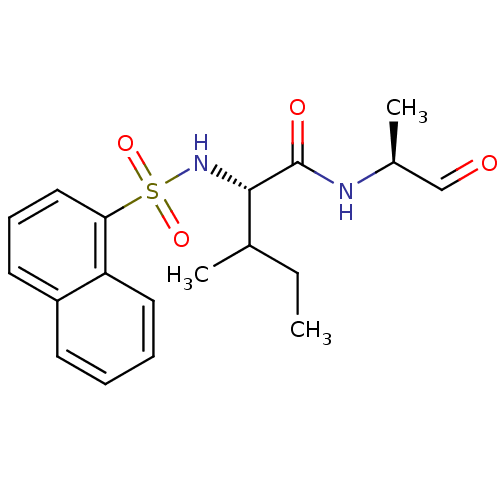 ((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369406 (CHEMBL1791001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369408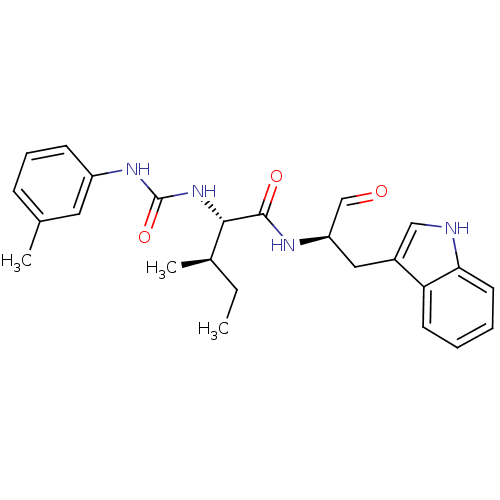 (CHEMBL1790997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50369409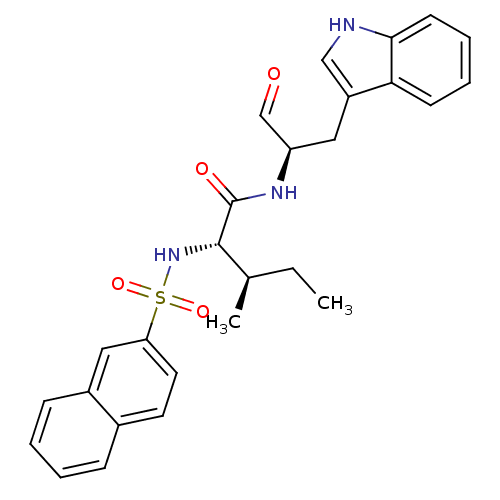 (CHEMBL1791004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50067614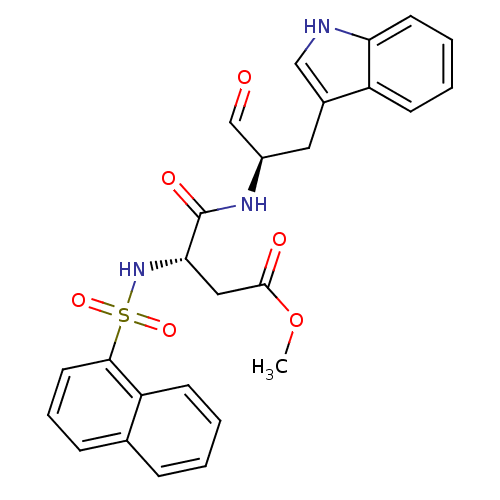 ((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-3-(na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of human cathepsin L. | J Med Chem 41: 4301-8 (1998) Article DOI: 10.1021/jm9803065 BindingDB Entry DOI: 10.7270/Q2VQ33CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 186 total ) | Next | Last >> |