 Found 440 hits with Last Name = 'kitano' and Initial = 'y'
Found 440 hits with Last Name = 'kitano' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium channel subunit beta-2
(Homo sapiens) | BDBM145285
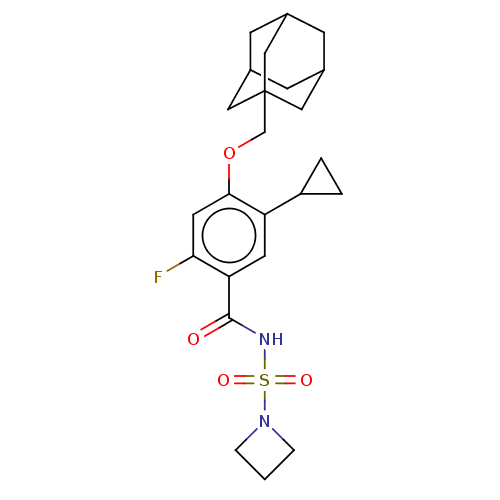
(US8952169, 64 | US9771376, Example 64)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCC1)C1CC1 |TLB:5:6:9:13.12.11,15:6:13:9.10.11,THB:15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H31FN2O4S/c25-21-10-22(31-14-24-11-15-6-16(12-24)8-17(7-15)13-24)19(18-2-3-18)9-20(21)23(28)26-32(29,30)27-4-1-5-27/h9-10,15-18H,1-8,11-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha/subunit beta-1/subunit beta-2
(Mus musculus) | BDBM50257179
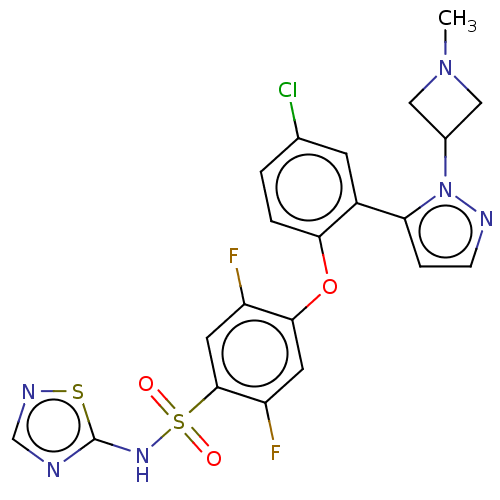
(CHEMBL2325622)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C21H17ClF2N6O3S2/c1-29-9-13(10-29)30-17(4-5-26-30)14-6-12(22)2-3-18(14)33-19-7-16(24)20(8-15(19)23)35(31,32)28-21-25-11-27-34-21/h2-8,11,13H,9-10H2,1H3,(H,25,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM70937
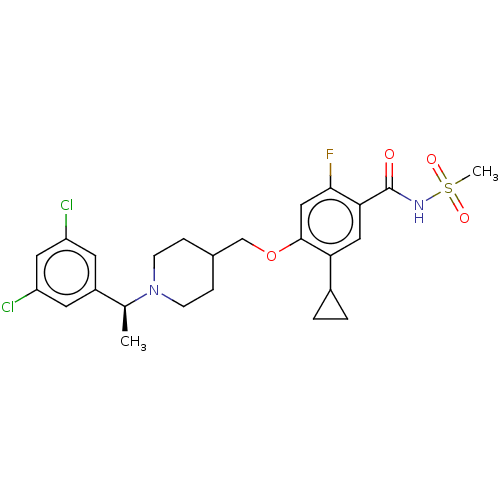
(US9546164, 100 | US9694002, 100)Show SMILES C[C@H](N1CCC(COc2cc(F)c(cc2C2CC2)C(=O)NS(C)(=O)=O)CC1)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C25H29Cl2FN2O4S/c1-15(18-9-19(26)11-20(27)10-18)30-7-5-16(6-8-30)14-34-24-13-23(28)22(12-21(24)17-3-4-17)25(31)29-35(2,32)33/h9-13,15-17H,3-8,14H2,1-2H3,(H,29,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM50240277
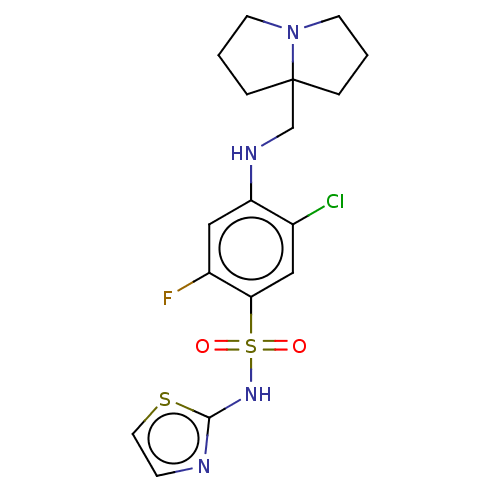
(CHEMBL4061793)Show SMILES Fc1cc(NCC23CCCN2CCC3)c(Cl)cc1S(=O)(=O)Nc1nccs1 Show InChI InChI=1S/C17H20ClFN4O2S2/c18-12-9-15(27(24,25)22-16-20-5-8-26-16)13(19)10-14(12)21-11-17-3-1-6-23(17)7-2-4-17/h5,8-10,21H,1-4,6-7,11H2,(H,20,22) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222422
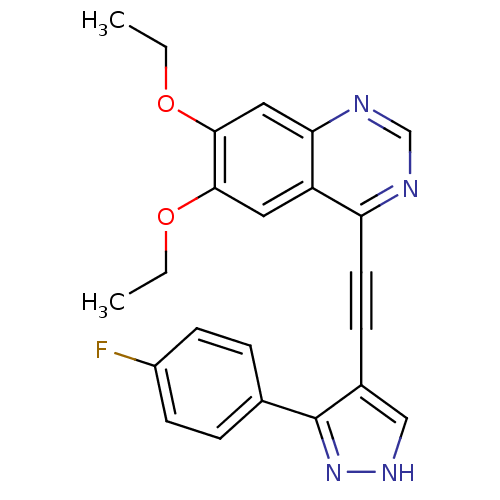
(6,7-diethoxy-4-(2-(3-(4-fluorophenyl)-1H-pyrazol-4...)Show SMILES CCOc1cc2ncnc(C#Cc3c[nH]nc3-c3ccc(F)cc3)c2cc1OCC Show InChI InChI=1S/C23H19FN4O2/c1-3-29-21-11-18-19(25-14-26-20(18)12-22(21)30-4-2)10-7-16-13-27-28-23(16)15-5-8-17(24)9-6-15/h5-6,8-9,11-14H,3-4H2,1-2H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50257179

(CHEMBL2325622)Show SMILES CN1CC(C1)n1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C21H17ClF2N6O3S2/c1-29-9-13(10-29)30-17(4-5-26-30)14-6-12(22)2-3-18(14)33-19-7-16(24)20(8-15(19)23)35(31,32)28-21-25-11-27-34-21/h2-8,11,13H,9-10H2,1H3,(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222428

((R)-4-(6,7-dimethoxyquinazolin-4-yl)-N,N-diethyl-2...)Show SMILES CCN(CC)C(C)(Cc1ccccc1)C#Cc1ncnc2cc(OC)c(OC)cc12 |w:5.5| Show InChI InChI=1S/C25H29N3O2/c1-6-28(7-2)25(3,17-19-11-9-8-10-12-19)14-13-21-20-15-23(29-4)24(30-5)16-22(20)27-18-26-21/h8-12,15-16,18H,6-7,17H2,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50240277

(CHEMBL4061793)Show SMILES Fc1cc(NCC23CCCN2CCC3)c(Cl)cc1S(=O)(=O)Nc1nccs1 Show InChI InChI=1S/C17H20ClFN4O2S2/c18-12-9-15(27(24,25)22-16-20-5-8-26-16)13(19)10-14(12)21-11-17-3-1-6-23(17)7-2-4-17/h5,8-10,21H,1-4,6-7,11H2,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM76858
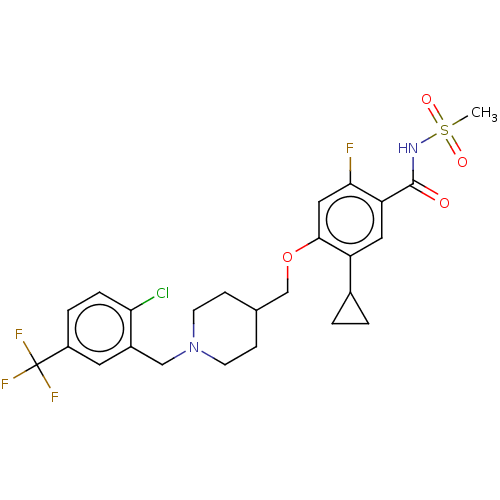
(US9546164, 580 | US9694002, 580)Show SMILES CS(=O)(=O)NC(=O)c1cc(C2CC2)c(OCC2CCN(Cc3cc(ccc3Cl)C(F)(F)F)CC2)cc1F Show InChI InChI=1S/C25H27ClF4N2O4S/c1-37(34,35)31-24(33)20-11-19(16-2-3-16)23(12-22(20)27)36-14-15-6-8-32(9-7-15)13-17-10-18(25(28,29)30)4-5-21(17)26/h4-5,10-12,15-16H,2-3,6-9,13-14H2,1H3,(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217483
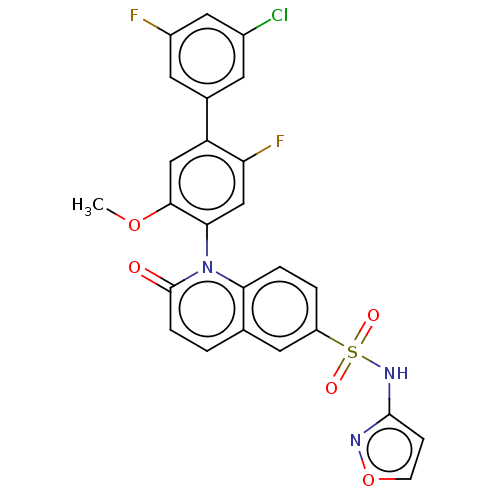
(US9212182, 477 | US9212182, 478)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16ClF2N3O5S/c1-35-23-12-19(15-8-16(26)11-17(27)9-15)20(28)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM50240277

(CHEMBL4061793)Show SMILES Fc1cc(NCC23CCCN2CCC3)c(Cl)cc1S(=O)(=O)Nc1nccs1 Show InChI InChI=1S/C17H20ClFN4O2S2/c18-12-9-15(27(24,25)22-16-20-5-8-26-16)13(19)10-14(12)21-11-17-3-1-6-23(17)7-2-4-17/h5,8-10,21H,1-4,6-7,11H2,(H,20,22) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222430
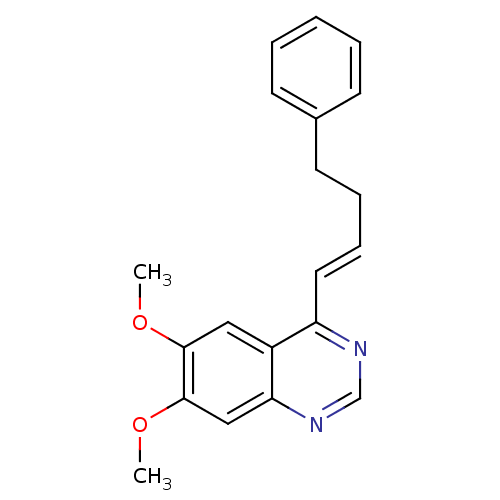
((E)-6,7-dimethoxy-4-(4-phenylbut-1-enyl)quinazolin...)Show InChI InChI=1S/C20H20N2O2/c1-23-19-12-16-17(21-14-22-18(16)13-20(19)24-2)11-7-6-10-15-8-4-3-5-9-15/h3-5,7-9,11-14H,6,10H2,1-2H3/b11-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222431
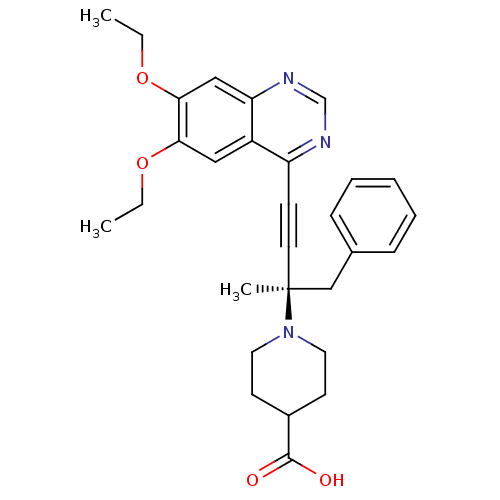
((R)-1-(4-(6,7-diethoxyquinazolin-4-yl)-2-methyl-1-...)Show SMILES CCOc1cc2ncnc(C#C[C@@](C)(Cc3ccccc3)N3CCC(CC3)C(O)=O)c2cc1OCC Show InChI InChI=1S/C29H33N3O4/c1-4-35-26-17-23-24(30-20-31-25(23)18-27(26)36-5-2)11-14-29(3,19-21-9-7-6-8-10-21)32-15-12-22(13-16-32)28(33)34/h6-10,17-18,20,22H,4-5,12-13,15-16,19H2,1-3H3,(H,33,34)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM50240267
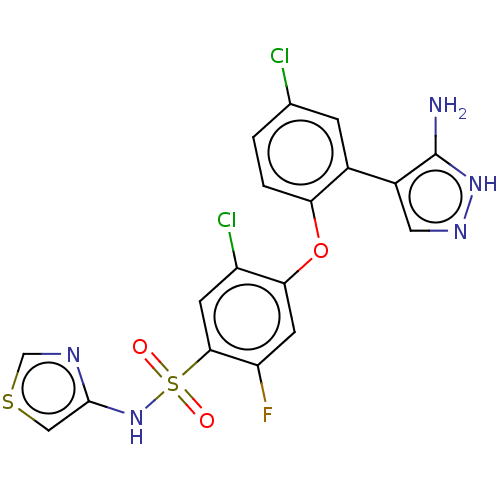
(CHEMBL2325014)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C18H12Cl2FN5O3S2/c19-9-1-2-14(10(3-9)11-6-24-25-18(11)22)29-15-5-13(21)16(4-12(15)20)31(27,28)26-17-7-30-8-23-17/h1-8,26H,(H3,22,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222418

(6,7-diethoxy-4-(4-phenylbut-1-enyl)quinazoline | C...)Show InChI InChI=1S/C22H24N2O2/c1-3-25-21-14-18-19(13-9-8-12-17-10-6-5-7-11-17)23-16-24-20(18)15-22(21)26-4-2/h5-7,9-11,13-16H,3-4,8,12H2,1-2H3/b13-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM50240267

(CHEMBL2325014)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C18H12Cl2FN5O3S2/c19-9-1-2-14(10(3-9)11-6-24-25-18(11)22)29-15-5-13(21)16(4-12(15)20)31(27,28)26-17-7-30-8-23-17/h1-8,26H,(H3,22,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217483

(US9212182, 477 | US9212182, 478)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16ClF2N3O5S/c1-35-23-12-19(15-8-16(26)11-17(27)9-15)20(28)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222434
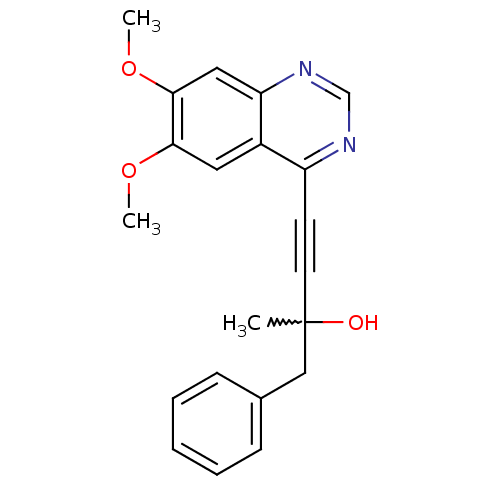
((R)-4-(6,7-dimethoxyquinazolin-4-yl)-2-methyl-1-ph...)Show SMILES COc1cc2ncnc(C#CC(C)(O)Cc3ccccc3)c2cc1OC |w:11.11| Show InChI InChI=1S/C21H20N2O3/c1-21(24,13-15-7-5-4-6-8-15)10-9-17-16-11-19(25-2)20(26-3)12-18(16)23-14-22-17/h4-8,11-12,14,24H,13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222439
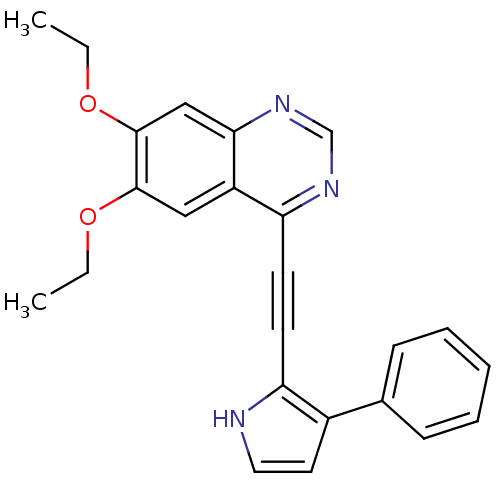
(6,7-diethoxy-4-(2-(3-phenyl-1H-pyrrol-2-yl)ethynyl...)Show SMILES CCOc1cc2ncnc(C#Cc3[nH]ccc3-c3ccccc3)c2cc1OCC Show InChI InChI=1S/C24H21N3O2/c1-3-28-23-14-19-21(26-16-27-22(19)15-24(23)29-4-2)11-10-20-18(12-13-25-20)17-8-6-5-7-9-17/h5-9,12-16,25H,3-4H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM145285

(US8952169, 64 | US9771376, Example 64)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCC1)C1CC1 |TLB:5:6:9:13.12.11,15:6:13:9.10.11,THB:15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H31FN2O4S/c25-21-10-22(31-14-24-11-15-6-16(12-24)8-17(7-15)13-24)19(18-2-3-18)9-20(21)23(28)26-32(29,30)27-4-1-5-27/h9-10,15-18H,1-8,11-14H2,(H,26,28) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222437
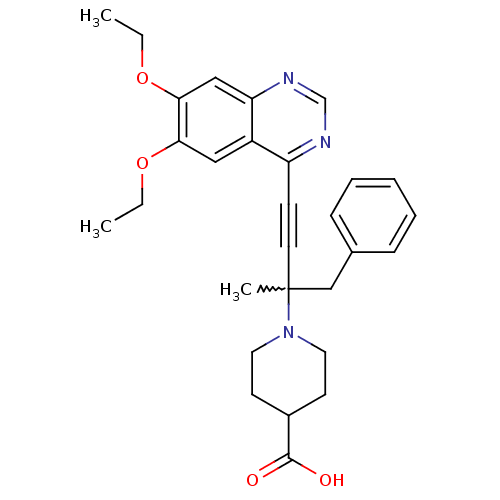
(1-(4-(6,7-diethoxyquinazolin-4-yl)-2-methyl-1-phen...)Show SMILES CCOc1cc2ncnc(C#CC(C)(Cc3ccccc3)N3CCC(CC3)C(O)=O)c2cc1OCC |w:12.12| Show InChI InChI=1S/C29H33N3O4/c1-4-35-26-17-23-24(30-20-31-25(23)18-27(26)36-5-2)11-14-29(3,19-21-9-7-6-8-10-21)32-15-12-22(13-16-32)28(33)34/h6-10,17-18,20,22H,4-5,12-13,15-16,19H2,1-3H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222438
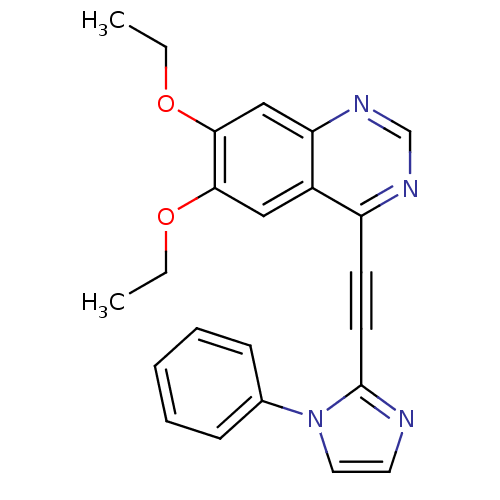
(6,7-diethoxy-4-(2-(1-phenyl-1H-imidazol-2-yl)ethyn...)Show InChI InChI=1S/C23H20N4O2/c1-3-28-21-14-18-19(25-16-26-20(18)15-22(21)29-4-2)10-11-23-24-12-13-27(23)17-8-6-5-7-9-17/h5-9,12-16H,3-4H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM258102
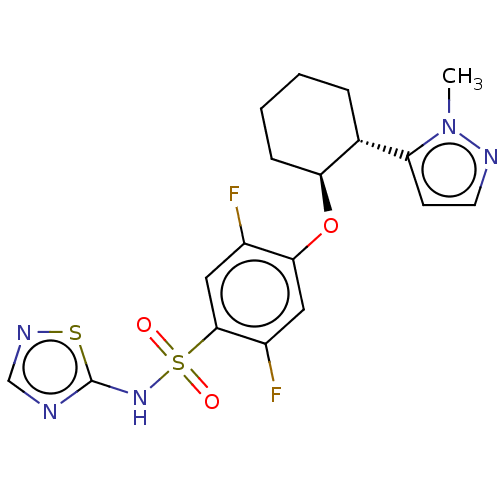
(US9493448, 4 | US9845313, Example 4)Show SMILES Cn1nccc1[C@H]1CCCC[C@@H]1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 |r| Show InChI InChI=1S/C18H19F2N5O3S2/c1-25-14(6-7-22-25)11-4-2-3-5-15(11)28-16-8-13(20)17(9-12(16)19)30(26,27)24-18-21-10-23-29-18/h6-11,15H,2-5H2,1H3,(H,21,23,24)/t11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222417

(4-(3,3-dimethyl-4-phenylbut-1-ynyl)-6,7-dimethoxyq...)Show InChI InChI=1S/C22H22N2O2/c1-22(2,14-16-8-6-5-7-9-16)11-10-18-17-12-20(25-3)21(26-4)13-19(17)24-15-23-18/h5-9,12-13,15H,14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50240267

(CHEMBL2325014)Show SMILES Nc1[nH]ncc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C18H12Cl2FN5O3S2/c19-9-1-2-14(10(3-9)11-6-24-25-18(11)22)29-15-5-13(21)16(4-12(15)20)31(27,28)26-17-7-30-8-23-17/h1-8,26H,(H3,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222435
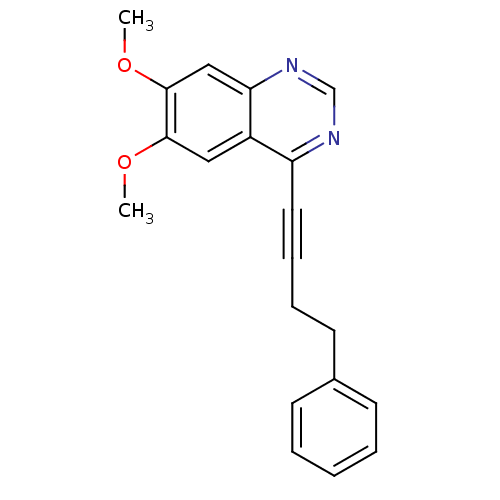
(6,7-dimethoxy-4-(4-phenylbut-1-ynyl)quinazoline | ...)Show InChI InChI=1S/C20H18N2O2/c1-23-19-12-16-17(21-14-22-18(16)13-20(19)24-2)11-7-6-10-15-8-4-3-5-9-15/h3-5,8-9,12-14H,6,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50613335
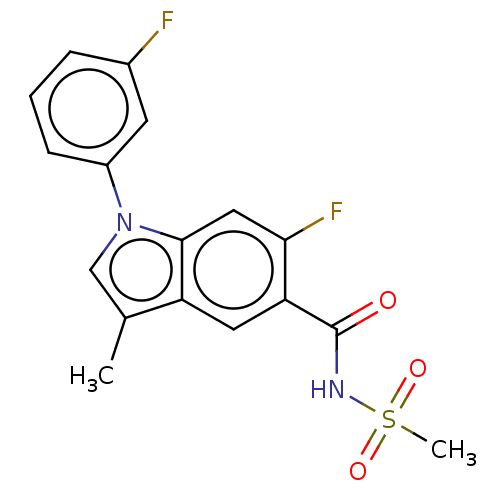
(CHEMBL5288162)Show SMILES Cc1cn(-c2cccc(F)c2)c2cc(F)c(cc12)C(=O)NS(C)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258219
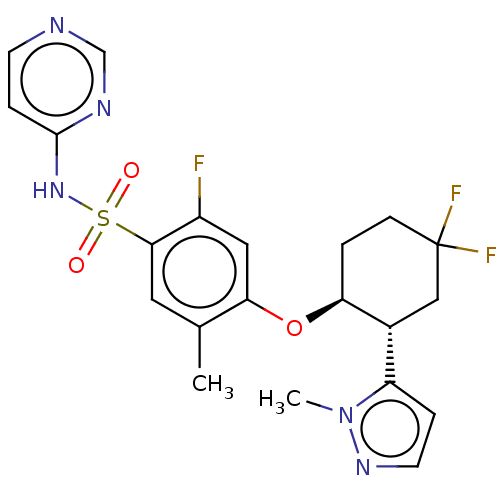
(US9493448, 126 | US9597330, Example 37 | US9845313...)Show SMILES Cc1cc(c(F)cc1O[C@H]1CCC(F)(F)C[C@@H]1c1ccnn1C)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C21H22F3N5O3S/c1-13-9-19(33(30,31)28-20-5-7-25-12-26-20)15(22)10-18(13)32-17-3-6-21(23,24)11-14(17)16-4-8-27-29(16)2/h4-5,7-10,12,14,17H,3,6,11H2,1-2H3,(H,25,26,28)/t14-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222425
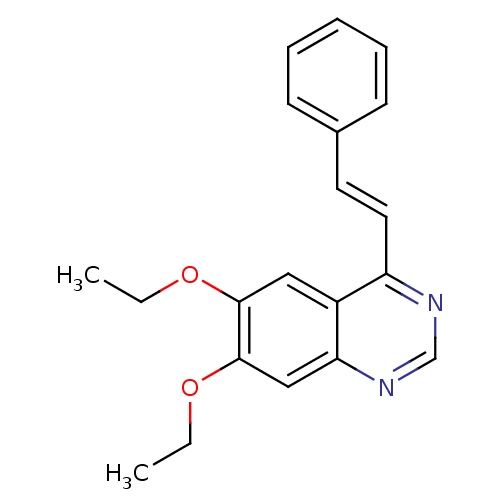
(6,7-diethoxy-4-styrylquinazoline | CHEMBL250925)Show InChI InChI=1S/C20H20N2O2/c1-3-23-19-12-16-17(11-10-15-8-6-5-7-9-15)21-14-22-18(16)13-20(19)24-4-2/h5-14H,3-4H2,1-2H3/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222432
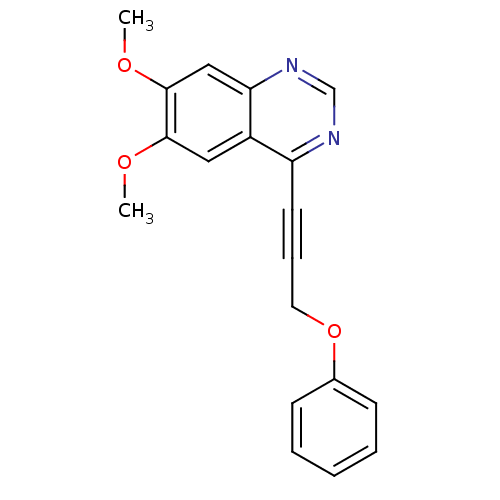
(6,7-dimethoxy-4-(3-phenoxyprop-1-ynyl)quinazoline ...)Show InChI InChI=1S/C19H16N2O3/c1-22-18-11-15-16(20-13-21-17(15)12-19(18)23-2)9-6-10-24-14-7-4-3-5-8-14/h3-5,7-8,11-13H,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of partially purified EGFR tyrosine kinase from human A431 cells |
Bioorg Med Chem Lett 17: 5863-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.020
BindingDB Entry DOI: 10.7270/Q2H70FJ7 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM50613344

(CHEMBL5284082)Show SMILES Cc1cn(-c2cncc(c2)C(F)(F)F)c2cc(F)c(cc12)C(=O)NS(=O)(=O)C1CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50613342
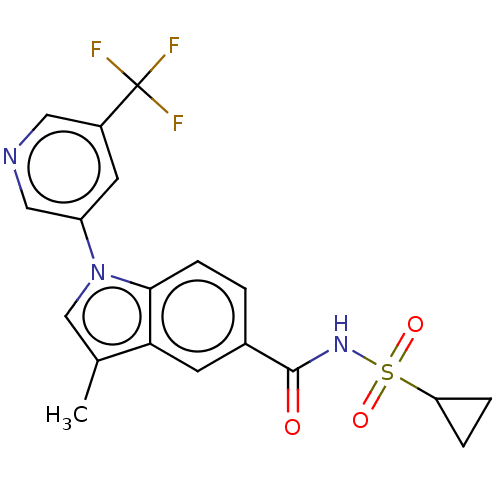
(CHEMBL5286433)Show SMILES Cc1cn(-c2cncc(c2)C(F)(F)F)c2ccc(cc12)C(=O)NS(=O)(=O)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50505257
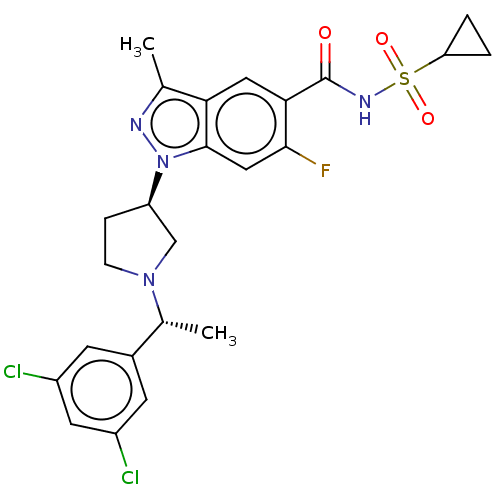
(CHEMBL4471012)Show SMILES C[C@@H](N1CC[C@H](C1)n1nc(C)c2cc(C(=O)NS(=O)(=O)C3CC3)c(F)cc12)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C24H25Cl2FN4O3S/c1-13-20-10-21(24(32)29-35(33,34)19-3-4-19)22(27)11-23(20)31(28-13)18-5-6-30(12-18)14(2)15-7-16(25)9-17(26)8-15/h7-11,14,18-19H,3-6,12H2,1-2H3,(H,29,32)/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM258162

(US9493448, 64 | US9597330, Example 21 | US9845313,...)Show SMILES Cc1cc(c(F)cc1O[C@H]1CCCC[C@@H]1c1ccnn1C)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C21H24FN5O3S/c1-14-11-20(31(28,29)26-21-8-9-23-13-24-21)16(22)12-19(14)30-18-6-4-3-5-15(18)17-7-10-25-27(17)2/h7-13,15,18H,3-6H2,1-2H3,(H,23,24,26)/t15-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258146
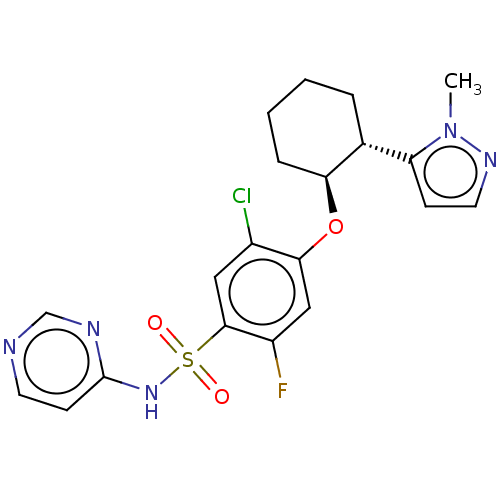
(US9493448, 122 | US9493448, 154 | US9493448, 48 | ...)Show SMILES Cn1nccc1[C@H]1CCCC[C@@H]1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C20H21ClFN5O3S/c1-27-16(6-9-25-27)13-4-2-3-5-17(13)30-18-11-15(22)19(10-14(18)21)31(28,29)26-20-7-8-23-12-24-20/h6-13,17H,2-5H2,1H3,(H,23,24,26)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM50613336
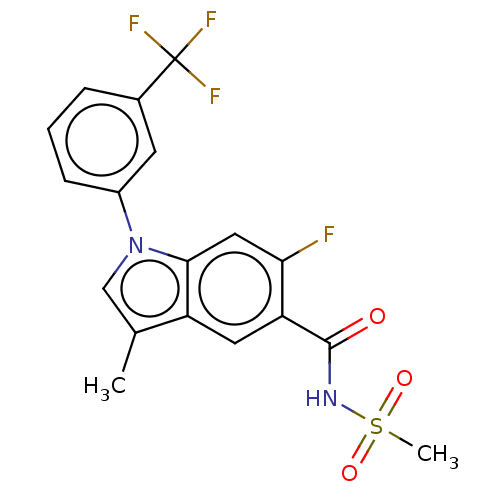
(CHEMBL5276801)Show SMILES Cc1cn(-c2cccc(c2)C(F)(F)F)c2cc(F)c(cc12)C(=O)NS(C)(=O)=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Mus musculus) | BDBM258102

(US9493448, 4 | US9845313, Example 4)Show SMILES Cn1nccc1[C@H]1CCCC[C@@H]1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 |r| Show InChI InChI=1S/C18H19F2N5O3S2/c1-25-14(6-7-22-25)11-4-2-3-5-15(11)28-16-8-13(20)17(9-12(16)19)30(26,27)24-18-21-10-23-29-18/h6-11,15H,2-5H2,1H3,(H,21,23,24)/t11-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM258106

(US9493448, 8 | US9845313, Example 8)Show SMILES Cn1nccc1[C@H]1CCC[C@@H]1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 |r| Show InChI InChI=1S/C17H17F2N5O3S2/c1-24-13(5-6-21-24)10-3-2-4-14(10)27-15-7-12(19)16(8-11(15)18)29(25,26)23-17-20-9-22-28-17/h5-10,14H,2-4H2,1H3,(H,20,22,23)/t10-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM217483

(US9212182, 477 | US9212182, 478)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16ClF2N3O5S/c1-35-23-12-19(15-8-16(26)11-17(27)9-15)20(28)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258244

(US9493448, 152 | US9597330, Example 52 | US9845313...)Show SMILES Fc1cc(O[C@H]2CC(F)(F)CC[C@@H]2c2cn[nH]c2)c(Cl)cc1S(=O)(=O)Nc1ccncn1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258231
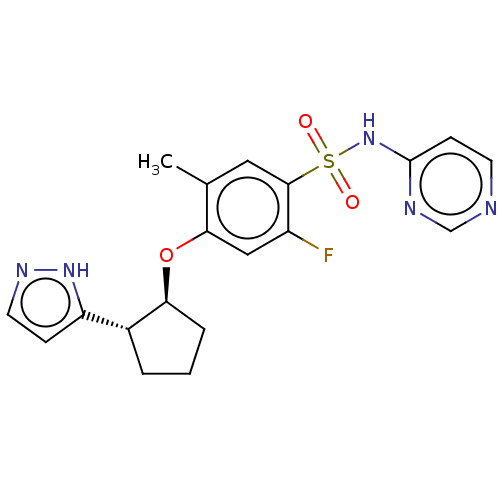
(US9493448, 138 | US9493448, 148 | US9597330, Examp...)Show SMILES Cc1cc(c(F)cc1O[C@H]1CCC[C@@H]1c1ccn[nH]1)S(=O)(=O)Nc1ccncn1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM50613336

(CHEMBL5276801)Show SMILES Cc1cn(-c2cccc(c2)C(F)(F)F)c2cc(F)c(cc12)C(=O)NS(C)(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM217483

(US9212182, 477 | US9212182, 478)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1cc(F)cc(Cl)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-3.44,-1.33,;-1.9,1.33,;-4,.77,;-4,2.31,;-5.25,3.22,;-4.77,4.68,;-3.23,4.68,;-2.76,3.22,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;,-12.32,;2.67,-12.32,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16ClF2N3O5S/c1-35-23-12-19(15-8-16(26)11-17(27)9-15)20(28)13-22(23)31-21-4-3-18(10-14(21)2-5-25(31)32)37(33,34)30-24-6-7-36-29-24/h2-13H,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258146

(US9493448, 122 | US9493448, 154 | US9493448, 48 | ...)Show SMILES Cn1nccc1[C@H]1CCCC[C@@H]1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C20H21ClFN5O3S/c1-27-16(6-9-25-27)13-4-2-3-5-17(13)30-18-11-15(22)19(10-14(18)21)31(28,29)26-20-7-8-23-12-24-20/h6-13,17H,2-5H2,1H3,(H,23,24,26)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258220
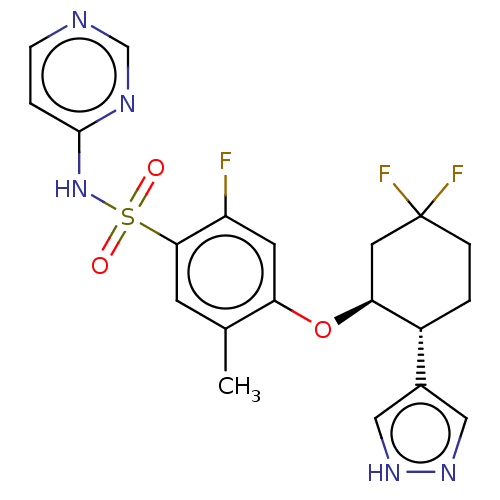
(US9493448, 127 | US9493448, 143 | US9597330, Examp...)Show SMILES Cc1cc(c(F)cc1O[C@H]1CC(F)(F)CC[C@@H]1c1cn[nH]c1)S(=O)(=O)Nc1ccncn1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258233

(US9493448, 140 | US9493448, 150 | US9597330, Examp...)Show SMILES Fc1cc(O[C@H]2CCC[C@@H]2c2ccn[nH]2)c(Cl)cc1S(=O)(=O)Nc1ccncn1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50466964
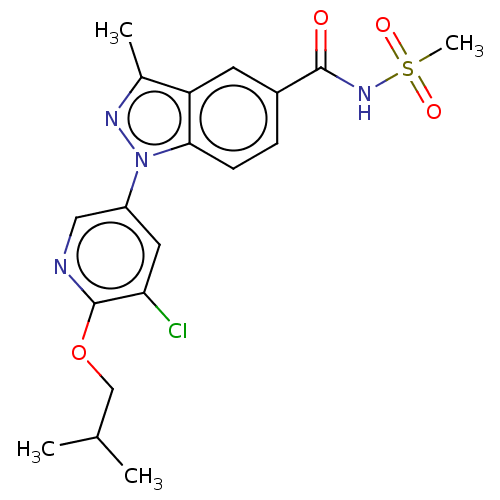
(Pf-05241328)Show SMILES CC(C)COc1ncc(cc1Cl)-n1nc(C)c2cc(ccc12)C(=O)NS(C)(=O)=O Show InChI InChI=1S/C19H21ClN4O4S/c1-11(2)10-28-19-16(20)8-14(9-21-19)24-17-6-5-13(7-15(17)12(3)22-24)18(25)23-29(4,26)27/h5-9,11H,10H2,1-4H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration at which the clotting time was prolonged by twice that of the control by inhibiting thrombin |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258146

(US9493448, 122 | US9493448, 154 | US9493448, 48 | ...)Show SMILES Cn1nccc1[C@H]1CCCC[C@@H]1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C20H21ClFN5O3S/c1-27-16(6-9-25-27)13-4-2-3-5-17(13)30-18-11-15(22)19(10-14(18)21)31(28,29)26-20-7-8-23-12-24-20/h6-13,17H,2-5H2,1H3,(H,23,24,26)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav1.7 expressed in CHO cells with -120 mV holding potential by whole cell manual patch clamp method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM258238
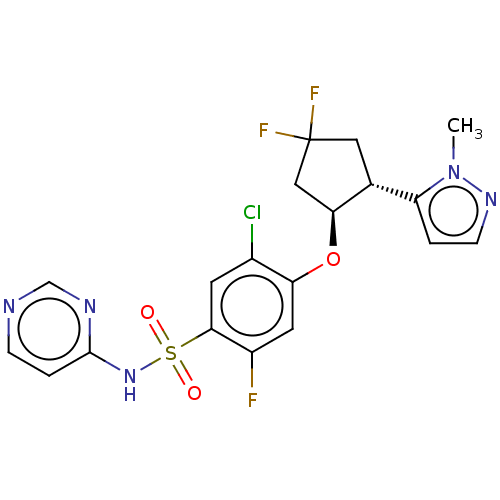
(US9493448, 146 | US9597330, Example 49 | US9845313...)Show SMILES Cn1nccc1[C@H]1CC(F)(F)C[C@@H]1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C19H17ClF3N5O3S/c1-28-14(2-5-26-28)11-8-19(22,23)9-16(11)31-15-7-13(21)17(6-12(15)20)32(29,30)27-18-3-4-24-10-25-18/h2-7,10-11,16H,8-9H2,1H3,(H,24,25,27)/t11-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Company, Limited
US Patent
| Assay Description
Current recording was obtained by an automated patch clamp system IonWorks Quattro (Molecular Devices Corporation) in Population Patch Clamp mode. Th... |
US Patent US9597330 (2017)
BindingDB Entry DOI: 10.7270/Q28054PM |
More data for this
Ligand-Target Pair | |
Sodium channel subunit beta-2
(Homo sapiens) | BDBM258141

(US9493448, 43 | US9597330, Example 10 | US9845313,...)Show SMILES Cc1cc(c(F)cc1O[C@H]1CCC[C@@H]1c1ccnn1C)S(=O)(=O)Nc1ccncn1 |r| Show InChI InChI=1S/C20H22FN5O3S/c1-13-10-19(30(27,28)25-20-7-8-22-12-23-20)15(21)11-18(13)29-17-5-3-4-14(17)16-6-9-24-26(16)2/h6-12,14,17H,3-5H2,1-2H3,(H,22,23,25)/t14-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method |
J Med Chem 63: 10204-10220 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00259
BindingDB Entry DOI: 10.7270/Q2Q52T67 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data