 Found 22826 hits with Last Name = 'lo' and Initial = 'y'
Found 22826 hits with Last Name = 'lo' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266
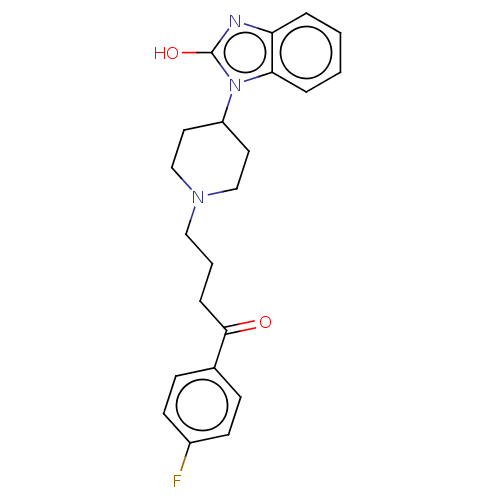
(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D4 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50421510
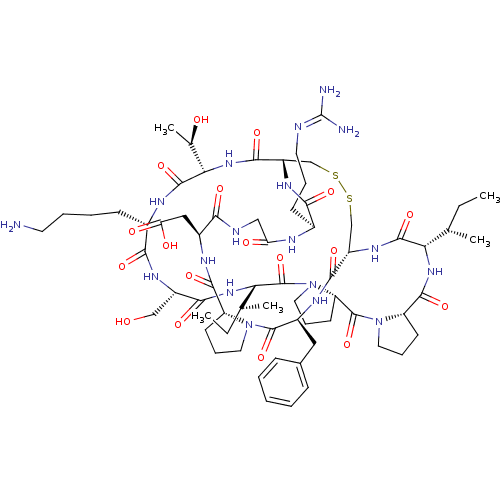
(CHEMBL239127)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-2)-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#6]-[#6] Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46+,47-,48-,51-,52-,53-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Sunflower beta-trypsin |
Bioorg Med Chem Lett 11: 2515-9 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8M0Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568690
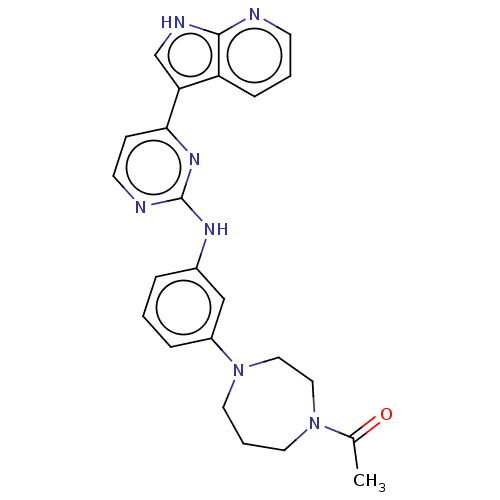
(CHEMBL4870188)Show SMILES CC(=O)N1CCCN(CC1)c1cccc(Nc2nccc(n2)-c2c[nH]c3ncccc23)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568686
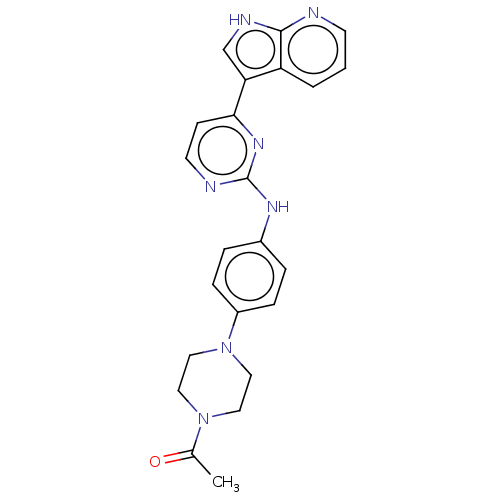
(CHEMBL4864449)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2nccc(n2)-c2c[nH]c3ncccc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041259
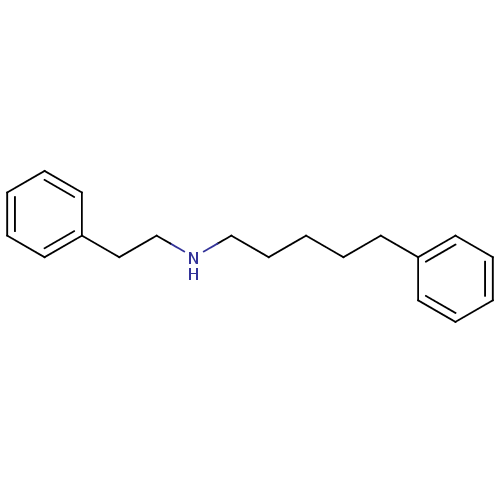
(CHEMBL19355 | Phenethyl-(5-phenyl-pentyl)-amine)Show InChI InChI=1S/C19H25N/c1-4-10-18(11-5-1)12-8-3-9-16-20-17-15-19-13-6-2-7-14-19/h1-2,4-7,10-11,13-14,20H,3,8-9,12,15-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041268

(Benzyl-methyl-(5-phenyl-pentyl)-amine | CHEMBL1931...)Show InChI InChI=1S/C19H25N/c1-20(17-19-14-7-3-8-15-19)16-10-4-9-13-18-11-5-2-6-12-18/h2-3,5-8,11-12,14-15H,4,9-10,13,16-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 5049-52 (2007)
Article DOI: 10.1021/jm070231h
BindingDB Entry DOI: 10.7270/Q29Z935Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568703

(CHEMBL4878159)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2ncc(C#N)c(n2)-c2n[nH]c3ccccc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50397757
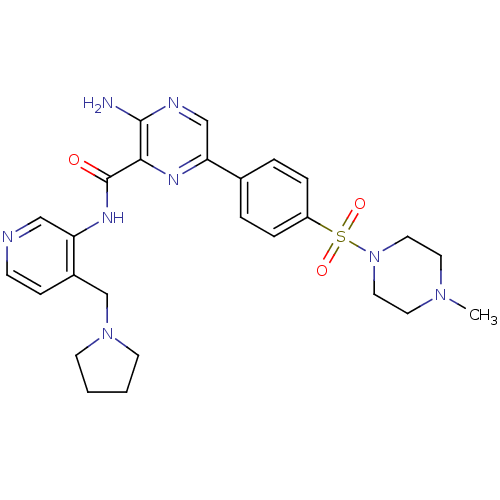
(CHEMBL2177173)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1CN1CCCC1 Show InChI InChI=1S/C26H32N8O3S/c1-32-12-14-34(15-13-32)38(36,37)21-6-4-19(5-7-21)23-17-29-25(27)24(30-23)26(35)31-22-16-28-9-8-20(22)18-33-10-2-3-11-33/h4-9,16-17H,2-3,10-15,18H2,1H3,(H2,27,29)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... |
J Med Chem 55: 9107-19 (2012)
Article DOI: 10.1021/jm201724m
BindingDB Entry DOI: 10.7270/Q2X0685T |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041246

(CHEMBL19544 | Methyl-phenethyl-(5-phenyl-pentyl)-a...)Show InChI InChI=1S/C20H27N/c1-21(18-16-20-14-7-3-8-15-20)17-10-4-9-13-19-11-5-2-6-12-19/h2-3,5-8,11-12,14-15H,4,9-10,13,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041277
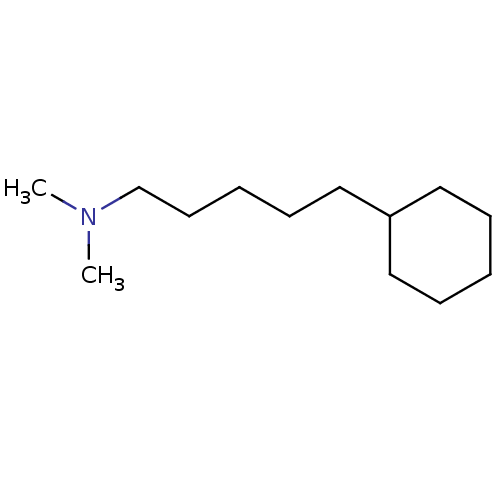
((5-Cyclohexyl-pentyl)-dimethyl-amine | CHEMBL20036)Show InChI InChI=1S/C13H27N/c1-14(2)12-8-4-7-11-13-9-5-3-6-10-13/h13H,3-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041257
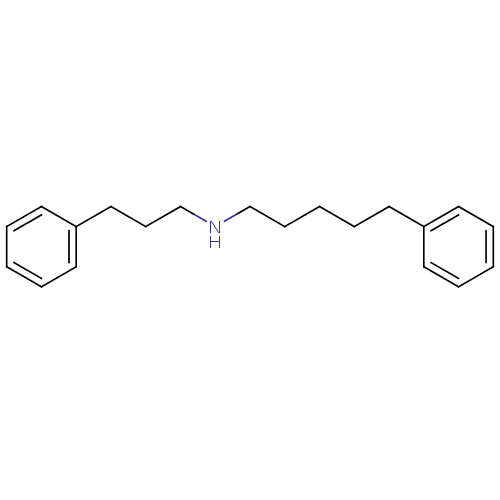
((5-Phenyl-pentyl)-(3-phenyl-propyl)-amine | CHEMBL...)Show InChI InChI=1S/C20H27N/c1-4-11-19(12-5-1)15-8-3-9-17-21-18-10-16-20-13-6-2-7-14-20/h1-2,4-7,11-14,21H,3,8-10,15-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IABN from human recombinant dopamine D3 receptor expressed in HEK293 cell membrane incubated for 60 mins filtration binding as... |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT2A receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041275

(CHEMBL20348 | Methyl-(5-phenyl-pentyl)-propyl-amin...)Show InChI InChI=1S/C15H25N/c1-3-13-16(2)14-9-5-8-12-15-10-6-4-7-11-15/h4,6-7,10-11H,3,5,8-9,12-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50334346
(4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...)Show InChI InChI=1S/C14H12N4O3S/c15-9-10-3-1-2-4-13(10)18-14(19)17-11-5-7-12(8-6-11)22(16,20)21/h1-8H,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of tumor-associated human carbonic anhydrase 9 preincubated for 15 min by CO2 hydration assay |
J Med Chem 54: 1896-902 (2011)
Article DOI: 10.1021/jm101541x
BindingDB Entry DOI: 10.7270/Q2CC110M |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568703

(CHEMBL4878159)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2ncc(C#N)c(n2)-c2n[nH]c3ccccc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 R595_E596insEY mutant (unknown origin) incubated for 120 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50151982

(5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...)Show SMILES NC(=O)c1cc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H27N5O2/c27-16-18-4-6-23-22(13-18)19(17-29-23)3-1-2-8-30-9-11-31(12-10-30)21-5-7-24-20(14-21)15-25(33-24)26(28)32/h4-7,13-15,17,29H,1-3,8-12H2,(H2,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor by liquid scintillation counting |
Eur J Med Chem 53: 124-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.042
BindingDB Entry DOI: 10.7270/Q2PN96NS |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -56.5 | n/a | n/a | 5.70 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 2486-96 (2007)
Article DOI: 10.1021/jm061329j
BindingDB Entry DOI: 10.7270/Q20R9MNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568703

(CHEMBL4878159)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2ncc(C#N)c(n2)-c2n[nH]c3ccccc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 D835Y mutant (unknown origin) incubated for 120 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568703

(CHEMBL4878159)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2ncc(C#N)c(n2)-c2n[nH]c3ccccc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 F594_R595insR mutant (unknown origin) incubated for 120 mins by ADP-glo based luminescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473
(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.309 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A & M University
Curated by ChEMBL
| Assay Description
Cataleptogenic effect against cloned human Dopamine receptor D2 in male Sprague-Dawley rats in a bar test |
Bioorg Med Chem Lett 13: 3779-82 (2003)
BindingDB Entry DOI: 10.7270/Q2BK1BQH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473

(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A & M University
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit the binding of [3H]-spiperone to cloned human Dopamine receptor D2 using apomorphine induced climbing test in male Swiss ... |
Bioorg Med Chem Lett 13: 3779-82 (2003)
BindingDB Entry DOI: 10.7270/Q2BK1BQH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473

(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041243
(Benzyl-(5-phenyl-pentyl)-amine | CHEMBL19315)Show InChI InChI=1S/C18H23N/c1-4-10-17(11-5-1)12-8-3-9-15-19-16-18-13-6-2-7-14-18/h1-2,4-7,10-11,13-14,19H,3,8-9,12,15-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor (unknown origin) |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50000069
(CHEMBL20377 | [2-(3,4-Dichloro-phenyl)-ethyl]-meth...)Show InChI InChI=1S/C15H22Cl2N2/c1-18(10-11-19-7-2-3-8-19)9-6-13-4-5-14(16)15(17)12-13/h4-5,12H,2-3,6-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against [3H]-3-PPP labelled sigma sites |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT2B receptor expressed in cell membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 24: 3464-71 (2016)
Article DOI: 10.1016/j.bmc.2016.05.053
BindingDB Entry DOI: 10.7270/Q2X63PVP |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041255

(CHEMBL19637 | Methyl-(5-phenyl-pentyl)-(3-phenyl-p...)Show InChI InChI=1S/C21H29N/c1-22(19-11-17-21-14-7-3-8-15-21)18-10-4-9-16-20-12-5-2-6-13-20/h2-3,5-8,12-15H,4,9-11,16-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human cloned 5HT2A receptor |
Bioorg Med Chem 16: 7291-301 (2008)
Article DOI: 10.1016/j.bmc.2008.06.030
BindingDB Entry DOI: 10.7270/Q2ZP45XW |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568690

(CHEMBL4870188)Show SMILES CC(=O)N1CCCN(CC1)c1cccc(Nc2nccc(n2)-c2c[nH]c3ncccc23)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type FLT3 (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50397744
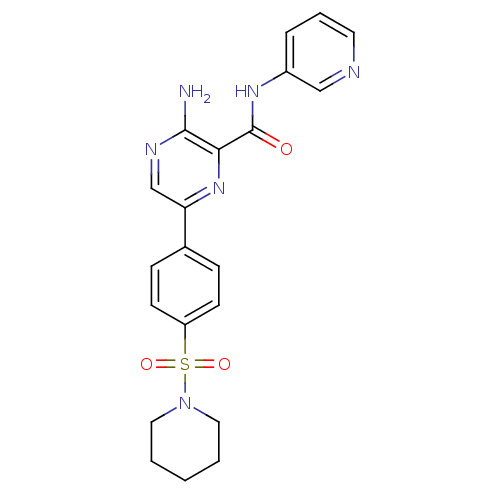
(CHEMBL2177163)Show SMILES Nc1ncc(nc1C(=O)Nc1cccnc1)-c1ccc(cc1)S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C21H22N6O3S/c22-20-19(21(28)25-16-5-4-10-23-13-16)26-18(14-24-20)15-6-8-17(9-7-15)31(29,30)27-11-2-1-3-12-27/h4-10,13-14H,1-3,11-12H2,(H2,22,24)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... |
J Med Chem 55: 9107-19 (2012)
Article DOI: 10.1021/jm201724m
BindingDB Entry DOI: 10.7270/Q2X0685T |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50568698
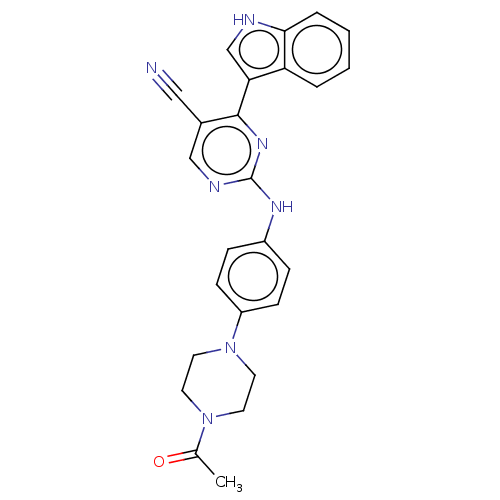
(CHEMBL4855435)Show SMILES CC(=O)N1CCN(CC1)c1ccc(Nc2ncc(C#N)c(n2)-c2c[nH]c3ccccc23)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FLT3 ITD mutant (unknown origin) using Glu/Tyr peptide substrate incubated for 120 mins measured after 40 mins incubation under dark by... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113215
BindingDB Entry DOI: 10.7270/Q22Z198K |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM85093
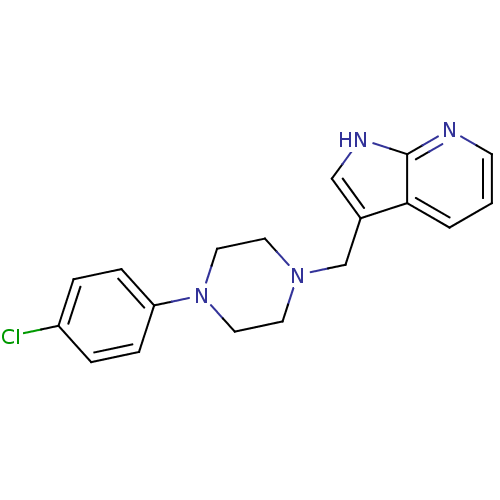
(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by PDSP assay |
Bioorg Med Chem 22: 3105-14 (2014)
Article DOI: 10.1016/j.bmc.2014.04.026
BindingDB Entry DOI: 10.7270/Q25140RW |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093

(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A& M University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine D4 receptor |
Bioorg Med Chem Lett 14: 5739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.046
BindingDB Entry DOI: 10.7270/Q28K79VJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093

(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human dopamine D4 receptor by liquid scintillation counting |
Bioorg Med Chem 17: 1716-23 (2009)
Article DOI: 10.1016/j.bmc.2008.12.054
BindingDB Entry DOI: 10.7270/Q2NG4QGT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50397749
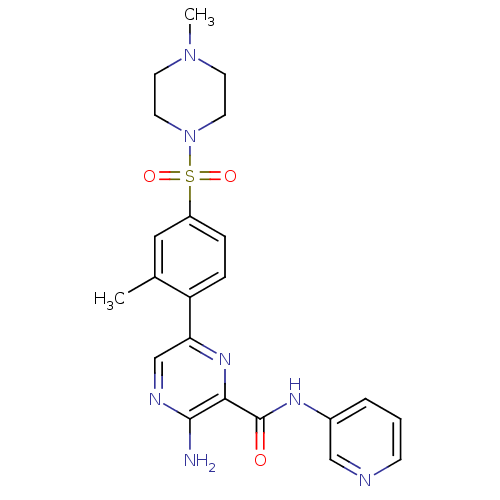
(CHEMBL2182002)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(c(C)c1)-c1cnc(N)c(n1)C(=O)Nc1cccnc1 Show InChI InChI=1S/C22H25N7O3S/c1-15-12-17(33(31,32)29-10-8-28(2)9-11-29)5-6-18(15)19-14-25-21(23)20(27-19)22(30)26-16-4-3-7-24-13-16/h3-7,12-14H,8-11H2,1-2H3,(H2,23,25)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... |
J Med Chem 55: 9107-19 (2012)
Article DOI: 10.1021/jm201724m
BindingDB Entry DOI: 10.7270/Q2X0685T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50199141
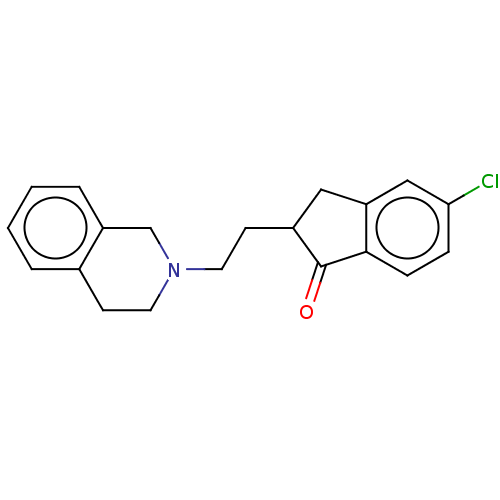
(CHEMBL3898250)Show InChI InChI=1S/C20H20ClNO.ClH/c21-18-5-6-19-17(12-18)11-15(20(19)23)8-10-22-9-7-14-3-1-2-4-16(14)13-22;/h1-6,12,15H,7-11,13H2;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7 receptor by liquid scintillation counting method |
Bioorg Med Chem 24: 5730-5740 (2016)
Article DOI: 10.1016/j.bmc.2016.09.019
BindingDB Entry DOI: 10.7270/Q2639RQF |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM82276
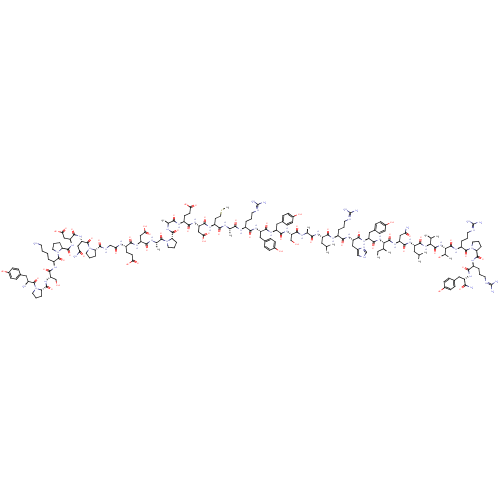
(L31,P34-NPY,human | NPY Leu31, Pro34, human, rat |...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C188H282N54O56S/c1-15-94(8)148(178(292)231-127(81-139(191)251)165(279)223-122(74-92(4)5)168(282)235-147(93(6)7)177(291)237-149(99(13)245)179(293)220-119(31-21-66-206-188(200)201)183(297)241-70-25-35-137(241)174(288)218-114(30-20-65-205-187(198)199)155(269)221-120(150(193)264)76-101-39-49-107(247)50-40-101)236-169(283)125(79-104-45-55-110(250)56-46-104)226-164(278)126(80-105-86-202-90-208-105)227-157(271)113(29-19-64-204-186(196)197)216-161(275)121(73-91(2)3)222-153(267)96(10)210-170(284)132(88-243)233-163(277)124(78-103-43-53-109(249)54-44-103)225-162(276)123(77-102-41-51-108(248)52-42-102)224-156(270)112(28-18-63-203-185(194)195)214-151(265)95(9)209-154(268)117(61-72-299-14)217-166(280)129(84-145(260)261)229-159(273)116(58-60-143(256)257)215-152(266)97(11)211-173(287)135-33-23-67-238(135)180(294)98(12)212-160(274)128(83-144(258)259)228-158(272)115(57-59-142(254)255)213-141(253)87-207-172(286)134-32-22-69-240(134)184(298)131(82-140(192)252)232-167(281)130(85-146(262)263)230-175(289)138-36-26-71-242(138)182(296)118(27-16-17-62-189)219-171(285)133(89-244)234-176(290)136-34-24-68-239(136)181(295)111(190)75-100-37-47-106(246)48-38-100/h37-56,86,90-99,111-138,147-149,243-250H,15-36,57-85,87-89,189-190H2,1-14H3,(H2,191,251)(H2,192,252)(H2,193,264)(H,202,208)(H,207,286)(H,209,268)(H,210,284)(H,211,287)(H,212,274)(H,213,253)(H,214,265)(H,215,266)(H,216,275)(H,217,280)(H,218,288)(H,219,285)(H,220,293)(H,221,269)(H,222,267)(H,223,279)(H,224,270)(H,225,276)(H,226,278)(H,227,271)(H,228,272)(H,229,273)(H,230,289)(H,231,292)(H,232,281)(H,233,277)(H,234,290)(H,235,282)(H,236,283)(H,237,291)(H,254,255)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H4,194,195,203)(H4,196,197,204)(H4,198,199,205)(H4,200,201,206)/t94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,147-,148-,149-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041251
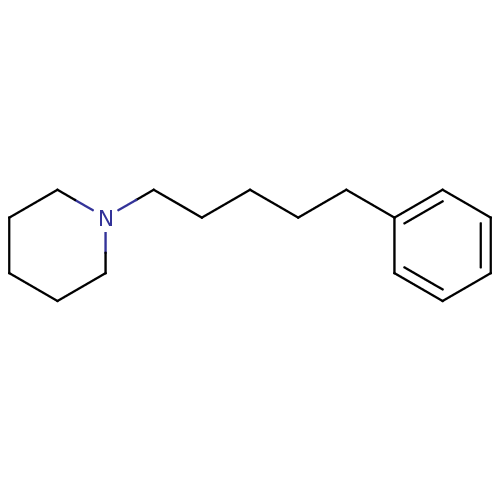
(1-(5-Phenyl-pentyl)-piperidine | CHEMBL20188)Show InChI InChI=1S/C16H25N/c1-4-10-16(11-5-1)12-6-2-7-13-17-14-8-3-9-15-17/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50397756
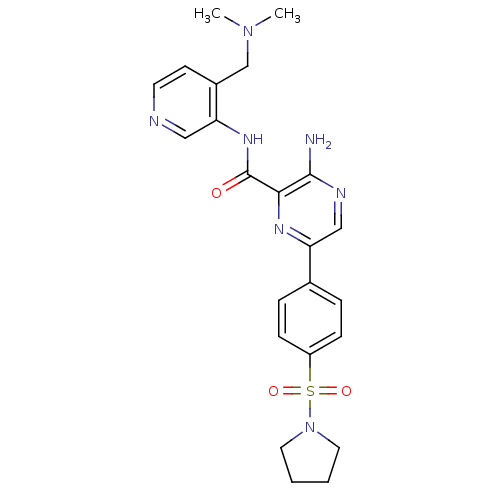
(CHEMBL2177174)Show SMILES CN(C)Cc1ccncc1NC(=O)c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)N1CCCC1 Show InChI InChI=1S/C23H27N7O3S/c1-29(2)15-17-9-10-25-13-19(17)28-23(31)21-22(24)26-14-20(27-21)16-5-7-18(8-6-16)34(32,33)30-11-3-4-12-30/h5-10,13-14H,3-4,11-12,15H2,1-2H3,(H2,24,26)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... |
J Med Chem 55: 9107-19 (2012)
Article DOI: 10.1021/jm201724m
BindingDB Entry DOI: 10.7270/Q2X0685T |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50039200

((4-Phenyl-butyl)-(5-phenyl-pentyl)-amine | CHEMBL2...)Show InChI InChI=1S/C21H29N/c1-4-12-20(13-5-1)16-8-3-10-18-22-19-11-9-17-21-14-6-2-7-15-21/h1-2,4-7,12-15,22H,3,8-11,16-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50091652
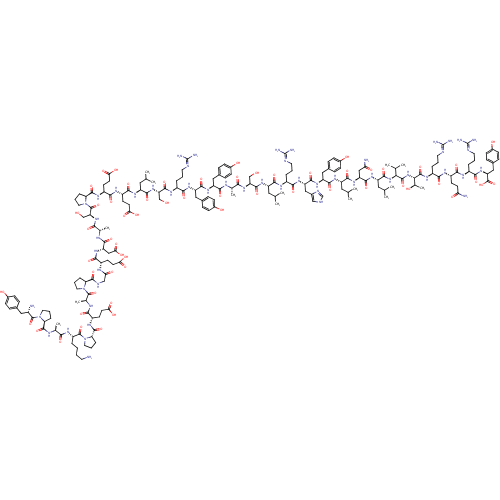
(CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C190H287N53O58/c1-92(2)74-124(166(280)216-114(27-18-66-204-187(195)196)158(272)231-131(83-107-86-203-91-209-107)171(285)230-130(81-105-41-51-111(251)52-42-105)169(283)225-125(75-93(3)4)167(281)232-132(84-143(194)254)172(286)226-127(77-95(7)8)173(287)238-150(96(9)10)180(294)239-151(101(15)247)181(295)222-117(30-21-69-207-190(201)202)156(270)218-119(55-60-142(193)253)161(275)215-116(29-20-68-206-189(199)200)159(273)234-134(186(300)301)82-106-43-53-112(252)54-44-106)227-175(289)135(88-244)235-153(267)97(11)210-164(278)128(79-103-37-47-109(249)48-38-103)229-170(284)129(80-104-39-49-110(250)50-40-104)228-157(271)115(28-19-67-205-188(197)198)217-174(288)136(89-245)236-168(282)126(76-94(5)6)224-163(277)121(58-63-147(260)261)219-162(276)122(59-64-148(262)263)221-179(293)141-34-25-73-243(141)185(299)137(90-246)237-154(268)98(12)211-165(279)133(85-149(264)265)233-160(274)118(56-61-145(256)257)214-144(255)87-208-176(290)138-31-22-70-240(138)182(296)100(14)213-155(269)120(57-62-146(258)259)220-178(292)140-33-24-72-242(140)184(298)123(26-16-17-65-191)223-152(266)99(13)212-177(291)139-32-23-71-241(139)183(297)113(192)78-102-35-45-108(248)46-36-102/h35-54,86,91-101,113-141,150-151,244-252H,16-34,55-85,87-90,191-192H2,1-15H3,(H2,193,253)(H2,194,254)(H,203,209)(H,208,290)(H,210,278)(H,211,279)(H,212,291)(H,213,269)(H,214,255)(H,215,275)(H,216,280)(H,217,288)(H,218,270)(H,219,276)(H,220,292)(H,221,293)(H,222,295)(H,223,266)(H,224,277)(H,225,283)(H,226,286)(H,227,289)(H,228,271)(H,229,284)(H,230,285)(H,231,272)(H,232,281)(H,233,274)(H,234,273)(H,235,267)(H,236,282)(H,237,268)(H,238,287)(H,239,294)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,300,301)(H4,195,196,204)(H4,197,198,205)(H4,199,200,206)(H4,201,202,207)/t97-,98-,99-,100-,101+,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,150-,151-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50015490
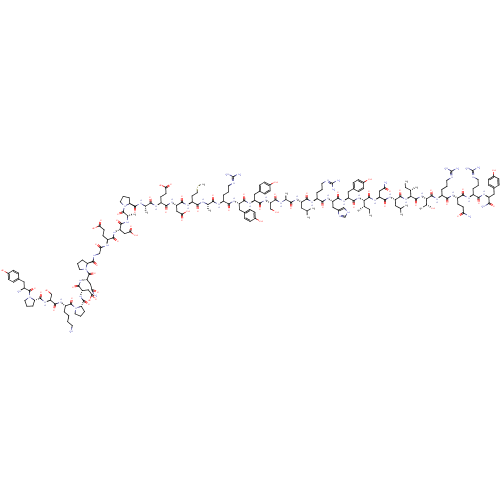
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50015490

(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041266
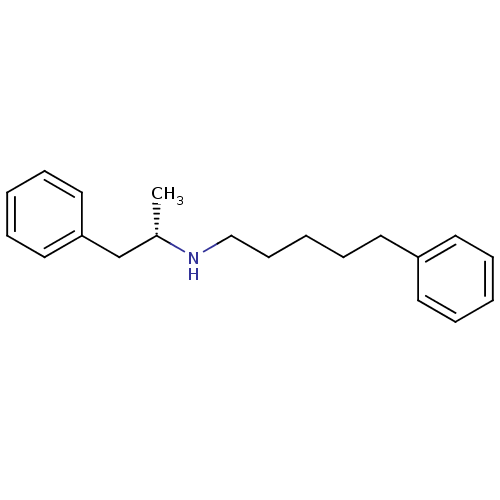
(((S)-1-Methyl-2-phenyl-ethyl)-(5-phenyl-pentyl)-am...)Show InChI InChI=1S/C20H27N/c1-18(17-20-14-7-3-8-15-20)21-16-10-4-9-13-19-11-5-2-6-12-19/h2-3,5-8,11-12,14-15,18,21H,4,9-10,13,16-17H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma-1 receptor of guinea pig brain membranes using [3H]-pentazocine as radioligand |
J Med Chem 37: 1214-9 (1994)
BindingDB Entry DOI: 10.7270/Q29P30QV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50334361
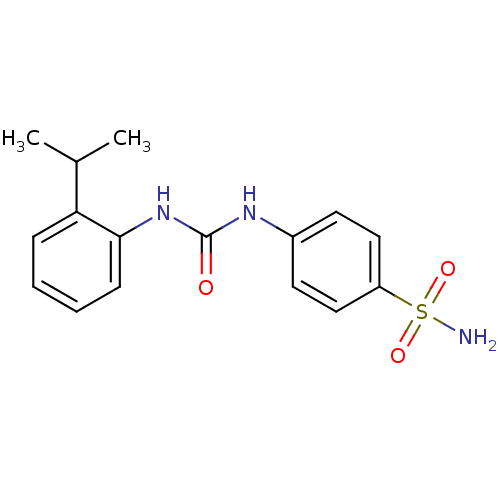
(4-(3-(2-isopropylphenyl)ureido)benzenesulfonamide ...)Show InChI InChI=1S/C16H19N3O3S/c1-11(2)14-5-3-4-6-15(14)19-16(20)18-12-7-9-13(10-8-12)23(17,21)22/h3-11H,1-2H3,(H2,17,21,22)(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of tumor-associated human carbonic anhydrase 9 preincubated for 15 min by CO2 hydration assay |
J Med Chem 54: 1896-902 (2011)
Article DOI: 10.1021/jm101541x
BindingDB Entry DOI: 10.7270/Q2CC110M |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50151982

(5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...)Show SMILES NC(=O)c1cc2cc(ccc2o1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 Show InChI InChI=1S/C26H27N5O2/c27-16-18-4-6-23-22(13-18)19(17-29-23)3-1-2-8-30-9-11-31(12-10-30)21-5-7-24-20(14-21)15-25(33-24)26(28)32/h4-7,13-15,17,29H,1-3,8-12H2,(H2,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citalopram from human SERT by liquid scintillation counting |
Eur J Med Chem 53: 124-32 (2012)
Article DOI: 10.1016/j.ejmech.2012.03.042
BindingDB Entry DOI: 10.7270/Q2PN96NS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50199141

(CHEMBL3898250)Show InChI InChI=1S/C20H20ClNO.ClH/c21-18-5-6-19-17(12-18)11-15(20(19)23)8-10-22-9-7-14-3-1-2-4-16(14)13-22;/h1-6,12,15H,7-11,13H2;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7 receptor by liquid scintillation counting method |
Bioorg Med Chem 24: 5730-5740 (2016)
Article DOI: 10.1016/j.bmc.2016.09.019
BindingDB Entry DOI: 10.7270/Q2639RQF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data