 Found 327 hits with Last Name = 'abell' and Initial = 'ad'
Found 327 hits with Last Name = 'abell' and Initial = 'ad' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039257
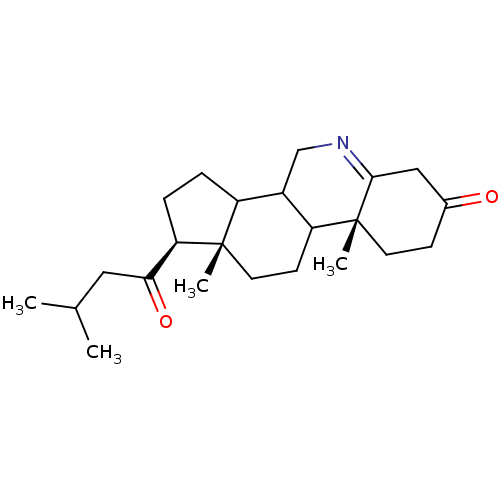
((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604
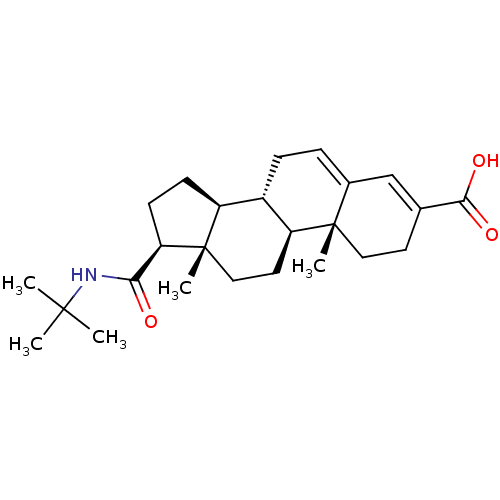
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50299565
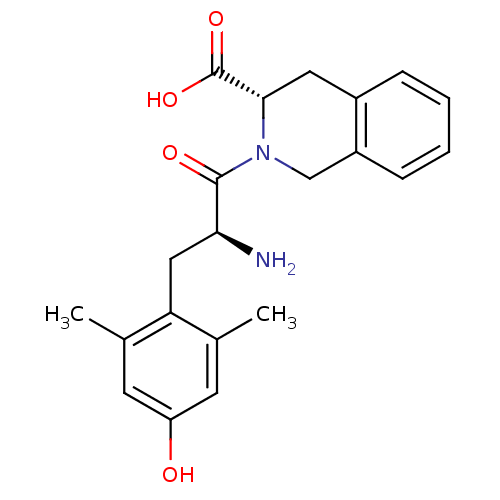
((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H24N2O4/c1-12-7-16(24)8-13(2)17(12)10-18(22)20(25)23-11-15-6-4-3-5-14(15)9-19(23)21(26)27/h3-8,18-19,24H,9-11,22H2,1-2H3,(H,26,27)/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312859
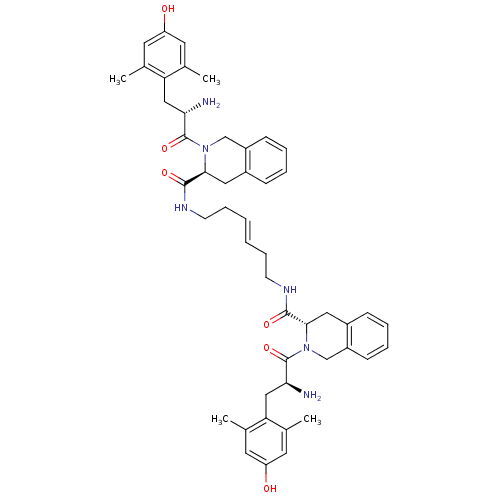
((S,3S,3'S)-N,N'-(hex-3-ene-1,6-diyl)bis(2-((S)-2-a...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCC\C=C\CCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C48H58N6O6/c1-29-19-37(55)20-30(2)39(29)25-41(49)47(59)53-27-35-15-9-7-13-33(35)23-43(53)45(57)51-17-11-5-6-12-18-52-46(58)44-24-34-14-8-10-16-36(34)28-54(44)48(60)42(50)26-40-31(3)21-38(56)22-32(40)4/h5-10,13-16,19-22,41-44,55-56H,11-12,17-18,23-28,49-50H2,1-4H3,(H,51,57)(H,52,58)/b6-5+/t41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403606
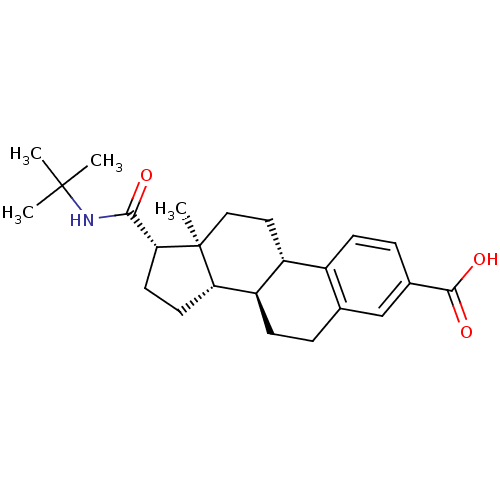
(CHEMBL1627951)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C24H33NO3/c1-23(2,3)25-21(26)20-10-9-19-18-8-5-14-13-15(22(27)28)6-7-16(14)17(18)11-12-24(19,20)4/h6-7,13,17-20H,5,8-12H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403324
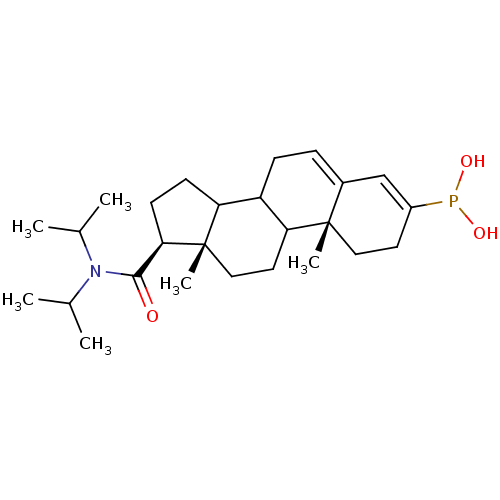
(CHEMBL78060)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)P(O)O |c:17,t:15| Show InChI InChI=1S/C26H42NO3P/c1-16(2)27(17(3)4)24(28)23-10-9-21-20-8-7-18-15-19(31(29)30)11-13-25(18,5)22(20)12-14-26(21,23)6/h7,15-17,20-23,29-30H,8-14H2,1-6H3/t20?,21?,22?,23-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039285
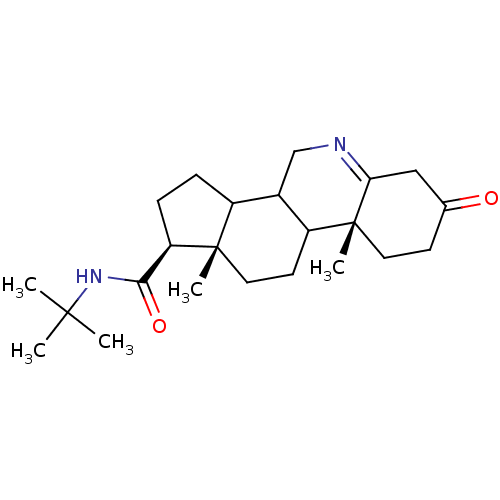
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:13| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)18-7-6-16-15-13-24-19-12-14(26)8-10-23(19,5)17(15)9-11-22(16,18)4/h15-18H,6-13H2,1-5H3,(H,25,27)/t15?,16?,17?,18-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312854

((S,3S,3'S)-N,N'-(ethane-1,2-diyl)bis(2-((S)-2-amin...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C44H52N6O6/c1-25-15-33(51)16-26(2)35(25)21-37(45)43(55)49-23-31-11-7-5-9-29(31)19-39(49)41(53)47-13-14-48-42(54)40-20-30-10-6-8-12-32(30)24-50(40)44(56)38(46)22-36-27(3)17-34(52)18-28(36)4/h5-12,15-18,37-40,51-52H,13-14,19-24,45-46H2,1-4H3,(H,47,53)(H,48,54)/t37-,38-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50403610
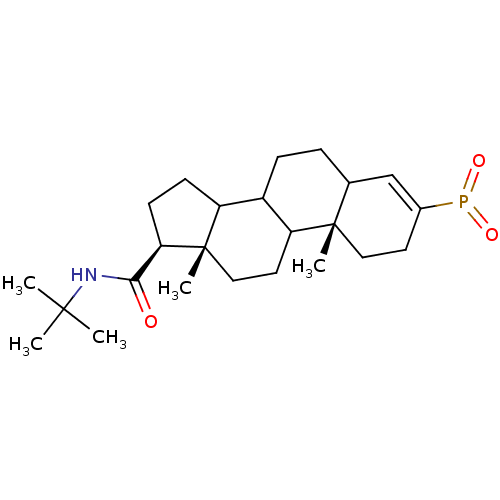
(CHEMBL143220)Show SMILES CC(C)(C)NC(=O)[C@H]1CCC2C3CCC4C=C(CC[C@]4(C)C3CC[C@]12C)P(=O)=O |c:15| Show InChI InChI=1S/C24H38NO3P/c1-22(2,3)25-21(26)20-9-8-18-17-7-6-15-14-16(29(27)28)10-12-23(15,4)19(17)11-13-24(18,20)5/h14-15,17-20H,6-13H2,1-5H3,(H,25,26)/t15?,17?,18?,19?,20-,23+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312859

((S,3S,3'S)-N,N'-(hex-3-ene-1,6-diyl)bis(2-((S)-2-a...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCC\C=C\CCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C48H58N6O6/c1-29-19-37(55)20-30(2)39(29)25-41(49)47(59)53-27-35-15-9-7-13-33(35)23-43(53)45(57)51-17-11-5-6-12-18-52-46(58)44-24-34-14-8-10-16-36(34)28-54(44)48(60)42(50)26-40-31(3)21-38(56)22-32(40)4/h5-10,13-16,19-22,41-44,55-56H,11-12,17-18,23-28,49-50H2,1-4H3,(H,51,57)(H,52,58)/b6-5+/t41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type 2 |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50213061
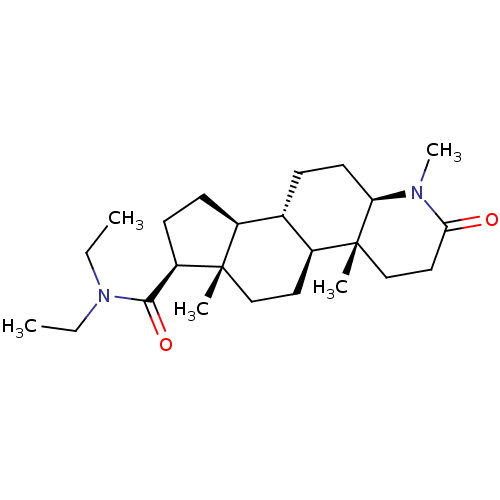
(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50213061

(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50299565

((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H24N2O4/c1-12-7-16(24)8-13(2)17(12)10-18(22)20(25)23-11-15-6-4-3-5-14(15)9-19(23)21(26)27/h3-8,18-19,24H,9-11,22H2,1-2H3,(H,26,27)/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312860

((S,S,3S,3'S,3''S)-N,N',N''-(2,2',2''-nitrilotris(e...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCCN(CCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)CCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C69H84N10O9/c1-40-25-52(80)26-41(2)55(40)34-58(70)67(86)77-37-49-16-10-7-13-46(49)31-61(77)64(83)73-19-22-76(23-20-74-65(84)62-32-47-14-8-11-17-50(47)38-78(62)68(87)59(71)35-56-42(3)27-53(81)28-43(56)4)24-21-75-66(85)63-33-48-15-9-12-18-51(48)39-79(63)69(88)60(72)36-57-44(5)29-54(82)30-45(57)6/h7-18,25-30,58-63,80-82H,19-24,31-39,70-72H2,1-6H3,(H,73,83)(H,74,84)(H,75,85)/t58-,59-,60-,61-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin 2 from delta opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50368782
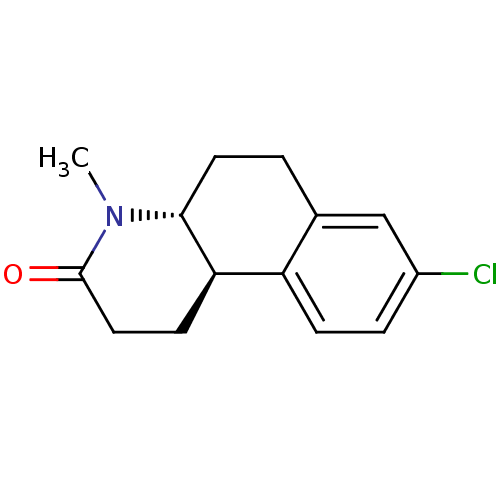
(Bexlosteride | CHEMBL24955 | LY-191704)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50368782

(Bexlosteride | CHEMBL24955 | LY-191704)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50180894
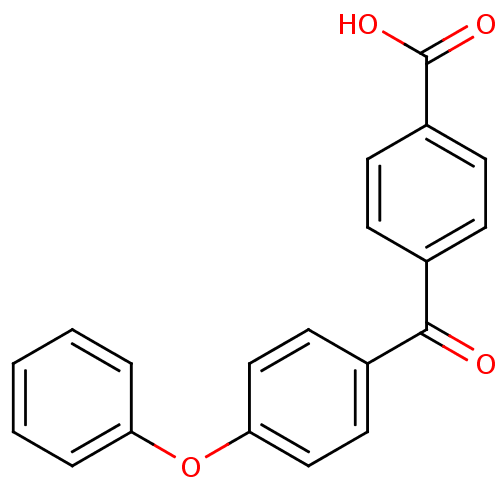
(4-(4-Phenoxy-benzoyl)-benzoic acid | 4-(4-phenoxyb...)Show InChI InChI=1S/C20H14O4/c21-19(14-6-8-16(9-7-14)20(22)23)15-10-12-18(13-11-15)24-17-4-2-1-3-5-17/h1-13H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312860

((S,S,3S,3'S,3''S)-N,N',N''-(2,2',2''-nitrilotris(e...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCCN(CCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)CCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C69H84N10O9/c1-40-25-52(80)26-41(2)55(40)34-58(70)67(86)77-37-49-16-10-7-13-46(49)31-61(77)64(83)73-19-22-76(23-20-74-65(84)62-32-47-14-8-11-17-50(47)38-78(62)68(87)59(71)35-56-42(3)27-53(81)28-43(56)4)24-21-75-66(85)63-33-48-15-9-12-18-51(48)39-79(63)69(88)60(72)36-57-44(5)29-54(82)30-45(57)6/h7-18,25-30,58-63,80-82H,19-24,31-39,70-72H2,1-6H3,(H,73,83)(H,74,84)(H,75,85)/t58-,59-,60-,61-,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312861

((2S,2'S,2''S)-N,N',N''-((4S,4'S,4''S)-2,2',2''-(2,...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1Cc2ccccc2CN(CC(=O)NCCN(CCNC(=O)CN2Cc3ccccc3C[C@H](NC(=O)[C@@H](N)Cc3c(C)cc(O)cc3C)C2=O)CCNC(=O)CN2Cc3ccccc3C[C@H](NC(=O)[C@@H](N)Cc3c(C)cc(O)cc3C)C2=O)C1=O |r| Show InChI InChI=1S/C75H93N13O12/c1-43-25-55(89)26-44(2)58(43)34-61(76)70(95)82-64-31-49-13-7-10-16-52(49)37-86(73(64)98)40-67(92)79-19-22-85(23-20-80-68(93)41-87-38-53-17-11-8-14-50(53)32-65(74(87)99)83-71(96)62(77)35-59-45(3)27-56(90)28-46(59)4)24-21-81-69(94)42-88-39-54-18-12-9-15-51(54)33-66(75(88)100)84-72(97)63(78)36-60-47(5)29-57(91)30-48(60)6/h7-18,25-30,61-66,89-91H,19-24,31-42,76-78H2,1-6H3,(H,79,92)(H,80,93)(H,81,94)(H,82,95)(H,83,96)(H,84,97)/t61-,62-,63-,64-,65-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50039257

((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407305
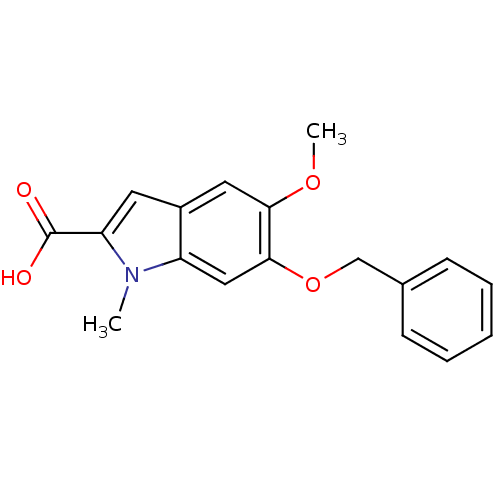
(CHEMBL36772)Show InChI InChI=1S/C18H17NO4/c1-19-14-10-17(23-11-12-6-4-3-5-7-12)16(22-2)9-13(14)8-15(19)18(20)21/h3-10H,11H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312854

((S,3S,3'S)-N,N'-(ethane-1,2-diyl)bis(2-((S)-2-amin...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NCCNC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C44H52N6O6/c1-25-15-33(51)16-26(2)35(25)21-37(45)43(55)49-23-31-11-7-5-9-29(31)19-39(49)41(53)47-13-14-48-42(54)40-20-30-10-6-8-12-32(30)24-50(40)44(56)38(46)22-36-27(3)17-34(52)18-28(36)4/h5-12,15-18,37-40,51-52H,13-14,19-24,45-46H2,1-4H3,(H,47,53)(H,48,54)/t37-,38-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50009101
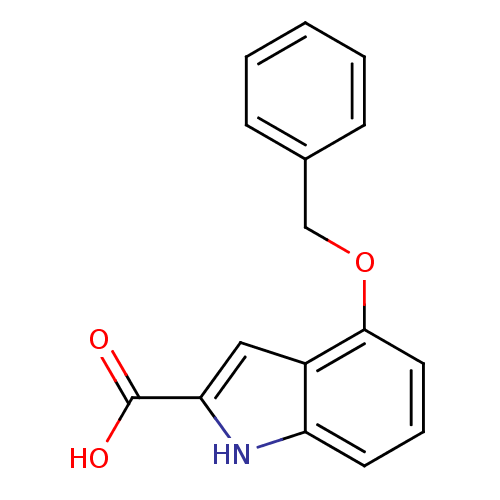
(4-Benzyloxy-1H-indole-2-carboxylic acid | CHEMBL23...)Show InChI InChI=1S/C16H13NO3/c18-16(19)14-9-12-13(17-14)7-4-8-15(12)20-10-11-5-2-1-3-6-11/h1-9,17H,10H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428854
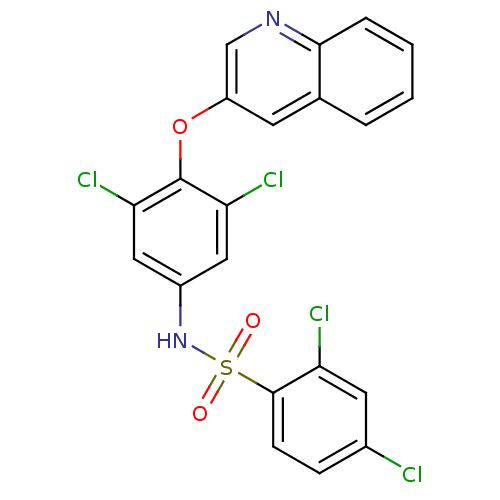
(CHEMBL1236924)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cnc3ccccc3c2)c(Cl)c1 Show InChI InChI=1S/C21H12Cl4N2O3S/c22-13-5-6-20(16(23)8-13)31(28,29)27-14-9-17(24)21(18(25)10-14)30-15-7-12-3-1-2-4-19(12)26-11-15/h1-11,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from human PPARgamma LBD expressed in HEK293 cells by filtration assay |
J Med Chem 60: 4584-4593 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01727
BindingDB Entry DOI: 10.7270/Q23J3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057467
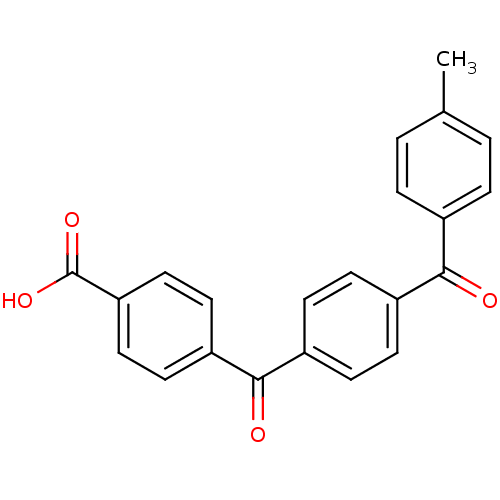
(4-[4-(4-Methyl-benzoyl)-benzoyl]-benzoic acid | CH...)Show SMILES Cc1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H16O4/c1-14-2-4-15(5-3-14)20(23)16-6-8-17(9-7-16)21(24)18-10-12-19(13-11-18)22(25)26/h2-13H,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044885
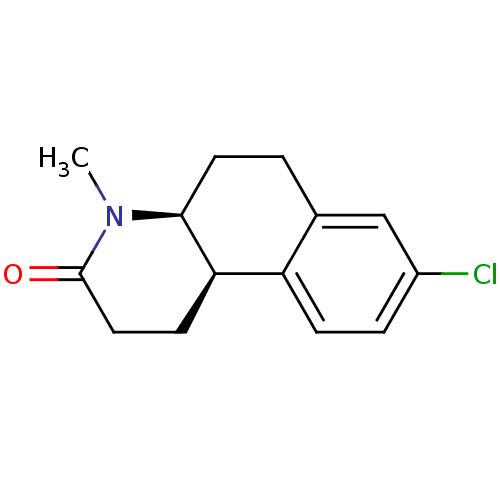
((4aS,10bR)-8-Chloro-4-methyl-1,4,4a,5,6,10b-hexahy...)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407304
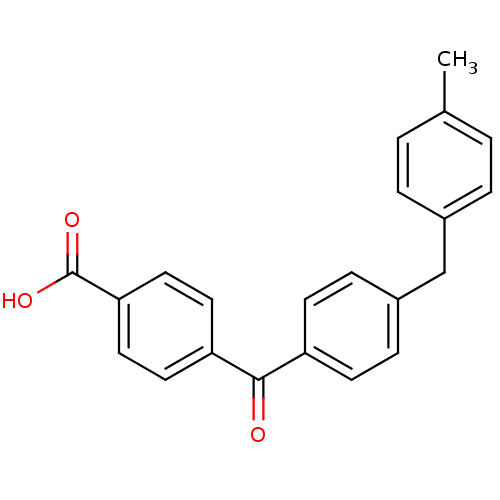
(CHEMBL289822)Show SMILES Cc1ccc(Cc2ccc(cc2)C(=O)c2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C22H18O3/c1-15-2-4-16(5-3-15)14-17-6-8-18(9-7-17)21(23)19-10-12-20(13-11-19)22(24)25/h2-13H,14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044885

((4aS,10bR)-8-Chloro-4-methyl-1,4,4a,5,6,10b-hexahy...)Show InChI InChI=1S/C14H16ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8,12-13H,2,5-7H2,1H3/t12-,13+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50312855

((2S,2'S)-N,N'-((4S,4'S)-2,2'-(2,2'-(ethane-1,2-diy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H]1Cc2ccccc2CN(CC(=O)NCCNC(=O)CN2Cc3ccccc3C[C@H](NC(=O)[C@@H](N)Cc3c(C)cc(O)cc3C)C2=O)C1=O |r| Show InChI InChI=1S/C48H58N8O8/c1-27-15-35(57)16-28(2)37(27)21-39(49)45(61)53-41-19-31-9-5-7-11-33(31)23-55(47(41)63)25-43(59)51-13-14-52-44(60)26-56-24-34-12-8-6-10-32(34)20-42(48(56)64)54-46(62)40(50)22-38-29(3)17-36(58)18-30(38)4/h5-12,15-18,39-42,57-58H,13-14,19-26,49-50H2,1-4H3,(H,51,59)(H,52,60)(H,53,61)(H,54,62)/t39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes |
Bioorg Med Chem Lett 20: 1610-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.055
BindingDB Entry DOI: 10.7270/Q2H995BB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044881
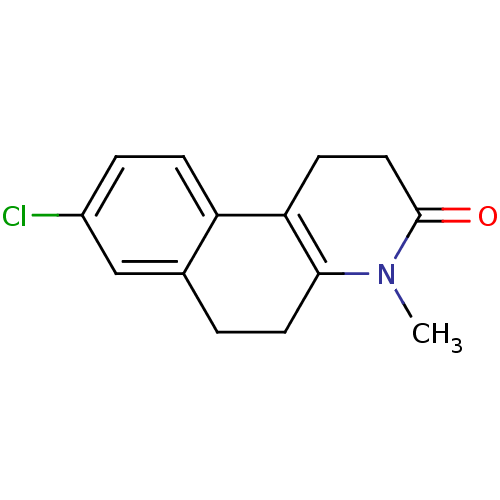
(8-Chloro-4-methyl-1,4,5,6-tetrahydro-2H-benzo[f]qu...)Show InChI InChI=1S/C14H14ClNO/c1-16-13-6-2-9-8-10(15)3-4-11(9)12(13)5-7-14(16)17/h3-4,8H,2,5-7H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of recombinant human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 1365-1368 (1994)
Article DOI: 10.1016/S0960-894X(01)80363-2
BindingDB Entry DOI: 10.7270/Q26M381K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407307
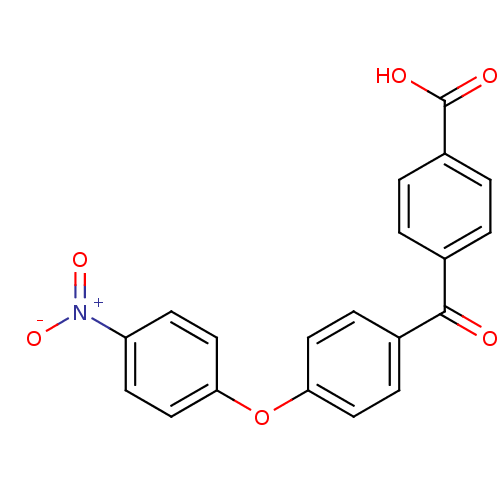
(CHEMBL285651)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(Oc2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C20H13NO6/c22-19(13-1-3-15(4-2-13)20(23)24)14-5-9-17(10-6-14)27-18-11-7-16(8-12-18)21(25)26/h1-12H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50044879

((4aR,10bR)-8-Chloro-4,10b-dimethyl-1,4,4a,5,6,10b-...)Show InChI InChI=1S/C15H18ClNO/c1-15-8-7-14(18)17(2)13(15)6-3-10-9-11(16)4-5-12(10)15/h4-5,9,13H,3,6-8H2,1-2H3/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibition constant towards human Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 4: 2327-2330 (1994)
Article DOI: 10.1016/0960-894X(94)85034-8
BindingDB Entry DOI: 10.7270/Q25Q4X8P |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407316
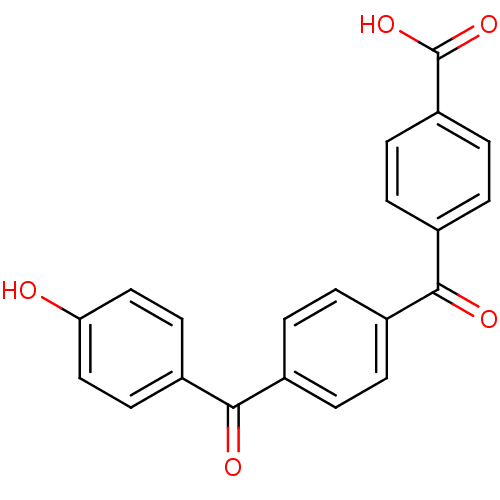
(CHEMBL286372)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(O)cc1 Show InChI InChI=1S/C21H14O5/c22-18-11-9-16(10-12-18)20(24)14-3-1-13(2-4-14)19(23)15-5-7-17(8-6-15)21(25)26/h1-12,22H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057479
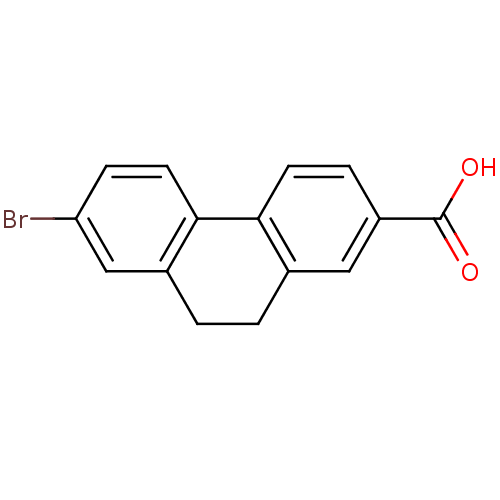
(7-Bromo-9,10-dihydro-phenanthrene-2-carboxylic aci...)Show InChI InChI=1S/C15H11BrO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-1 human steroid 5-alpha-reductase |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057479

(7-Bromo-9,10-dihydro-phenanthrene-2-carboxylic aci...)Show InChI InChI=1S/C15H11BrO2/c16-12-4-6-14-10(8-12)2-1-9-7-11(15(17)18)3-5-13(9)14/h3-8H,1-2H2,(H,17,18) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 10: 1909-11 (2001)
BindingDB Entry DOI: 10.7270/Q2M044PB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391268

(CHEMBL110001)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)[N+]([O-])=O |c:17| Show InChI InChI=1S/C26H42N2O3/c1-16(2)27(17(3)4)24(29)23-10-9-21-20-8-7-18-15-19(28(30)31)11-13-25(18,5)22(20)12-14-26(21,23)6/h15-18,20-23H,7-14H2,1-6H3/t18-,20?,21?,22?,23+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of type-2 human steroid 5-alpha-reductase. |
Bioorg Med Chem Lett 6: 481-484 (1996)
Article DOI: 10.1016/0960-894X(96)00054-6
BindingDB Entry DOI: 10.7270/Q2BZ661K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
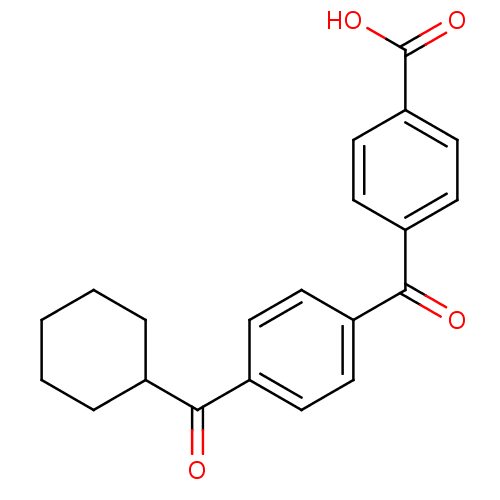
(Homo sapiens (Human)) | BDBM50407311
(CHEMBL34847)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)C1CCCCC1 Show InChI InChI=1S/C21H20O4/c22-19(14-4-2-1-3-5-14)15-6-8-16(9-7-15)20(23)17-10-12-18(13-11-17)21(24)25/h6-14H,1-5H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407308

(CHEMBL35176)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H13F3O4/c23-22(24,25)18-11-9-16(10-12-18)20(27)14-3-1-13(2-4-14)19(26)15-5-7-17(8-6-15)21(28)29/h1-12H,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
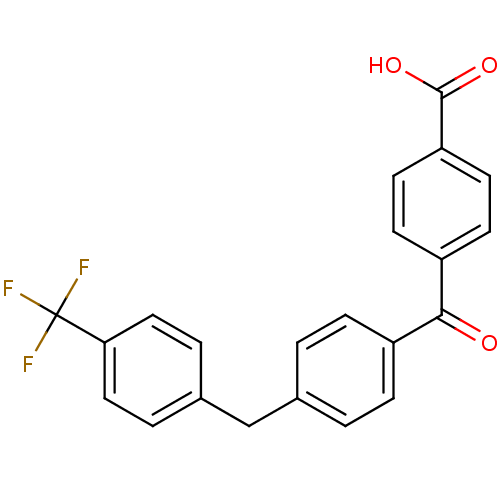
(Homo sapiens (Human)) | BDBM50407303
(CHEMBL36688)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(Cc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H15F3O3/c23-22(24,25)19-11-3-15(4-12-19)13-14-1-5-16(6-2-14)20(26)17-7-9-18(10-8-17)21(27)28/h1-12H,13H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
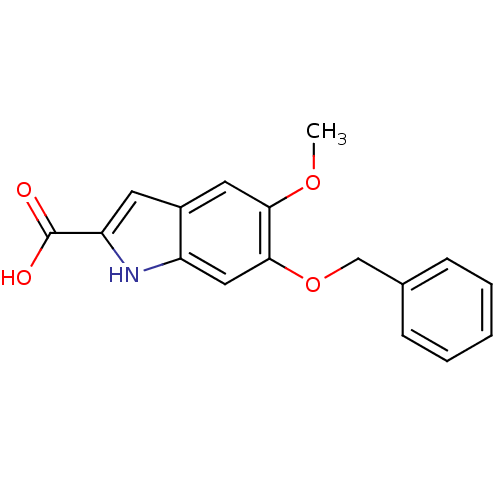
(Homo sapiens (Human)) | BDBM50407310
(CHEMBL37880)Show InChI InChI=1S/C17H15NO4/c1-21-15-8-12-7-14(17(19)20)18-13(12)9-16(15)22-10-11-5-3-2-4-6-11/h2-9,18H,10H2,1H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407313
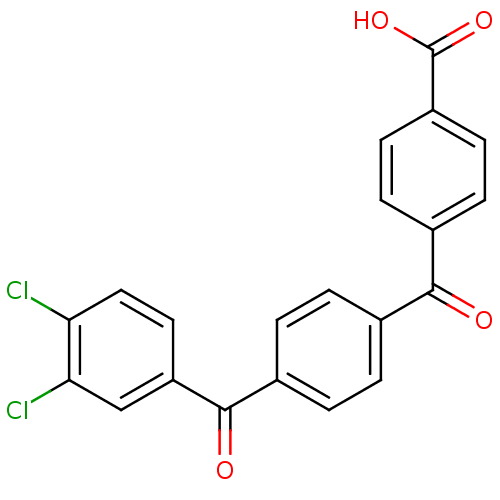
(CHEMBL34924)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H12Cl2O4/c22-17-10-9-16(11-18(17)23)20(25)14-3-1-12(2-4-14)19(24)13-5-7-15(8-6-13)21(26)27/h1-11H,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407320
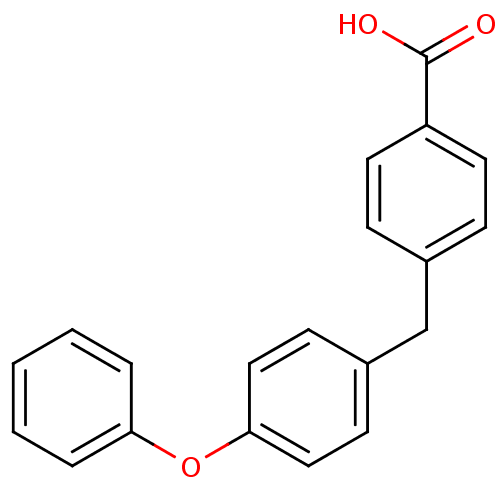
(CHEMBL35884)Show InChI InChI=1S/C20H16O3/c21-20(22)17-10-6-15(7-11-17)14-16-8-12-19(13-9-16)23-18-4-2-1-3-5-18/h1-13H,14H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50407323
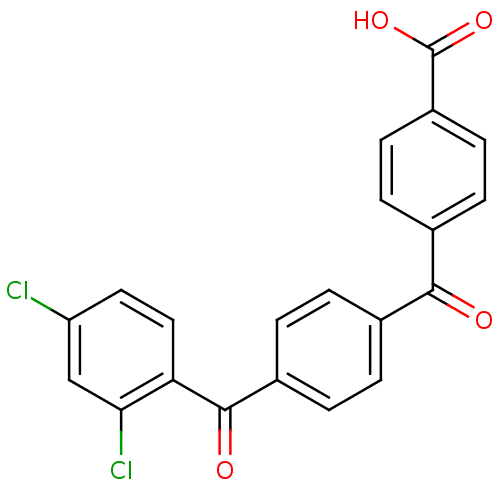
(CHEMBL37091)Show SMILES OC(=O)c1ccc(cc1)C(=O)c1ccc(cc1)C(=O)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C21H12Cl2O4/c22-16-9-10-17(18(23)11-16)20(25)14-3-1-12(2-4-14)19(24)13-5-7-15(8-6-13)21(26)27/h1-11H,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition constant against recombinant human Steroid 5-alpha-reductase type 2 expressed in CHO cells |
J Med Chem 38: 13-5 (1995)
BindingDB Entry DOI: 10.7270/Q2D50P5K |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Rattus norvegicus) | BDBM50289143
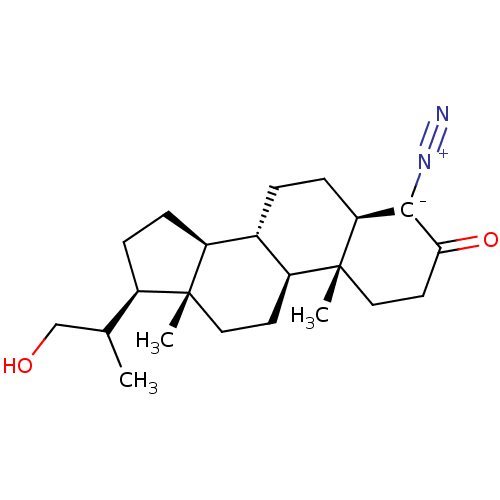
((5R,10R,14S)-17-(2-Hydroxy-1-methyl-ethyl)-10-meth...)Show SMILES [H][C@@]12CC[C@H](C(C)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(=[N+]=[N-])C(=O)CC[C@]12C Show InChI InChI=1S/C22H34N2O2/c1-13(12-25)15-6-7-16-14-4-5-18-20(24-23)19(26)9-11-22(18,3)17(14)8-10-21(15,16)2/h13-18,25H,4-12H2,1-3H3/t13?,14-,15+,16-,17-,18-,21+,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory activity of the compound was determined against rat prostatic Steroid 5-alpha-reductase type I |
Bioorg Med Chem Lett 6: 883-884 (1996)
Article DOI: 10.1016/0960-894X(96)00136-9
BindingDB Entry DOI: 10.7270/Q24B319W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data