 Found 2419 hits with Last Name = 'achab' and Initial = 'a'
Found 2419 hits with Last Name = 'achab' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
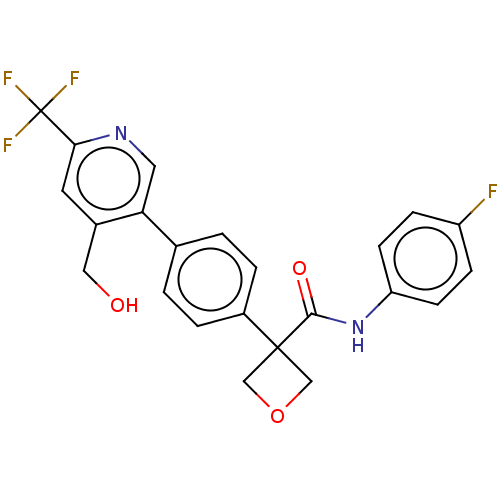
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569664
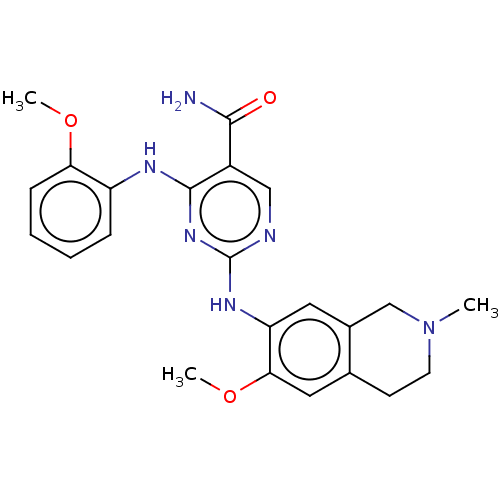
(CHEMBL4859451)Show SMILES COc1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569663
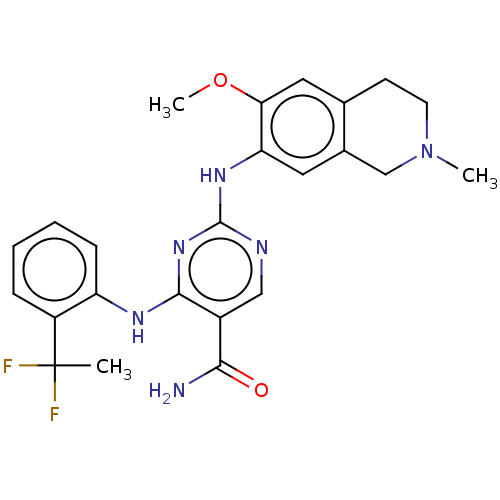
(CHEMBL4870155)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C(C)(F)F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569665
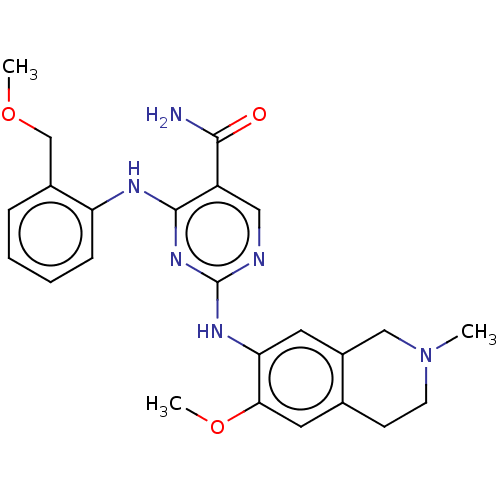
(CHEMBL4864568)Show SMILES COCc1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569667
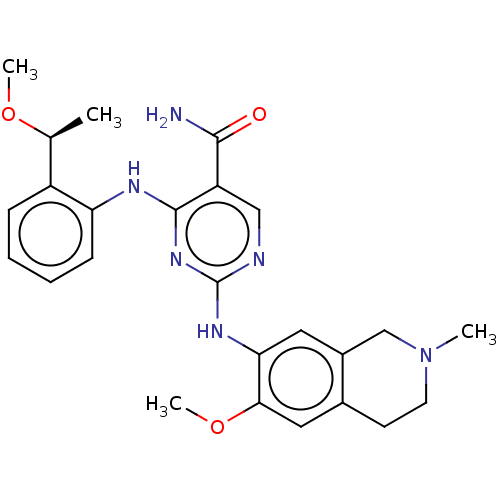
(CHEMBL4848845)Show SMILES CO[C@@H](C)c1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569669
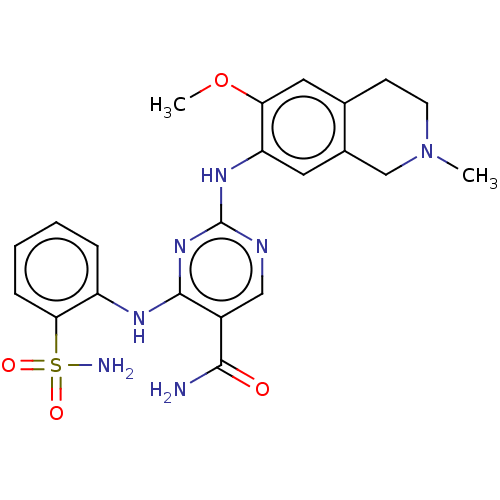
(CHEMBL4866139)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2S(N)(=O)=O)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569672
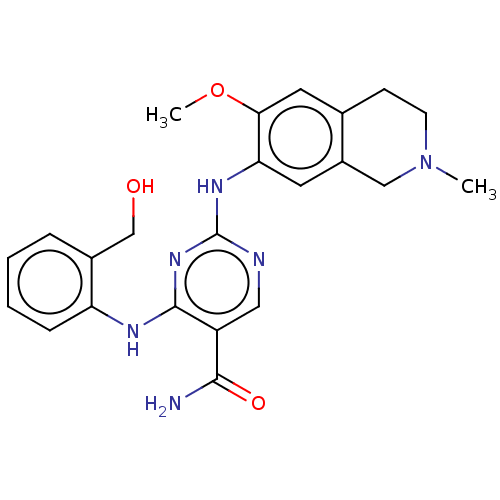
(CHEMBL4865305)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2CO)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569666
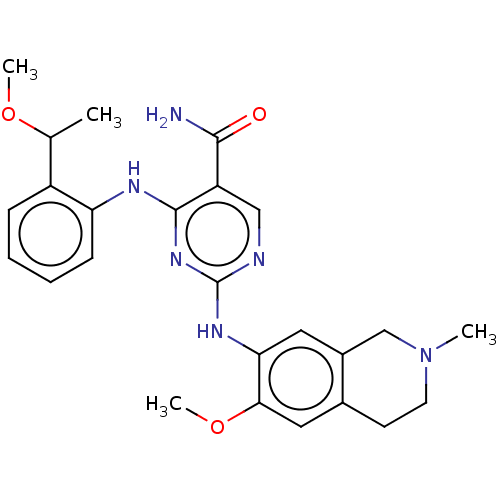
(CHEMBL4855158)Show SMILES COC(C)c1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
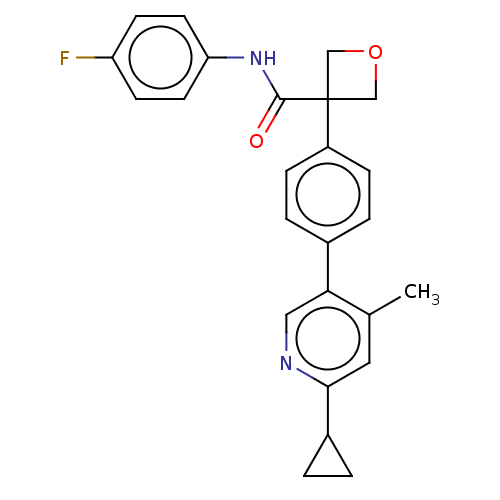
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
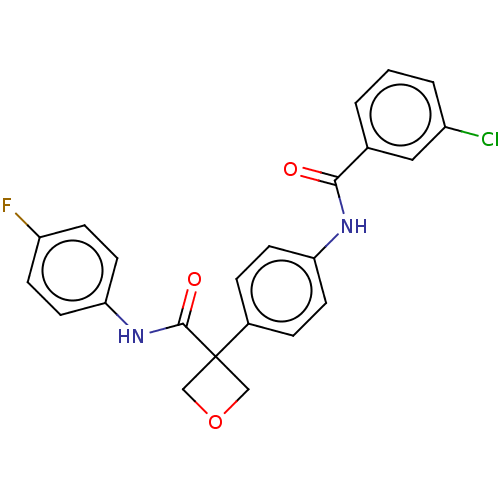
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569657
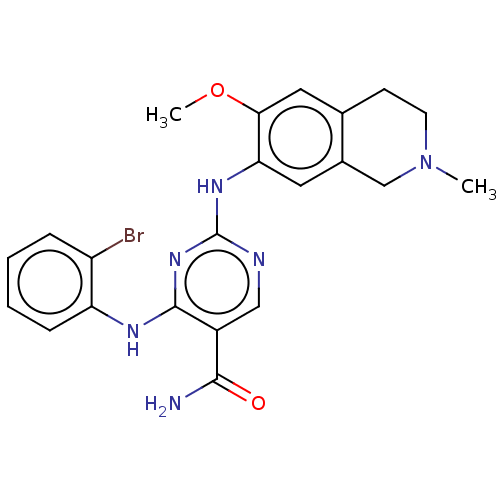
(CHEMBL4863492)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2Br)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569661
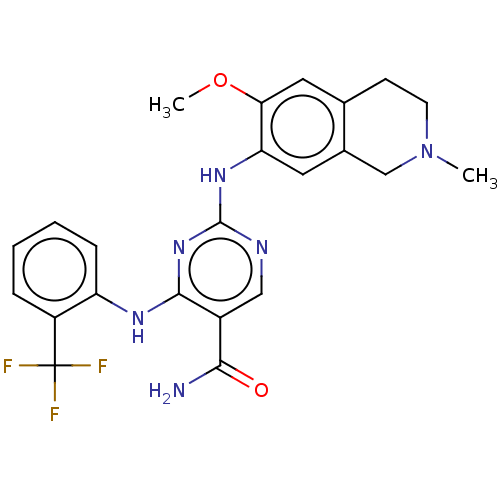
(CHEMBL4872743)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C(F)(F)F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569659
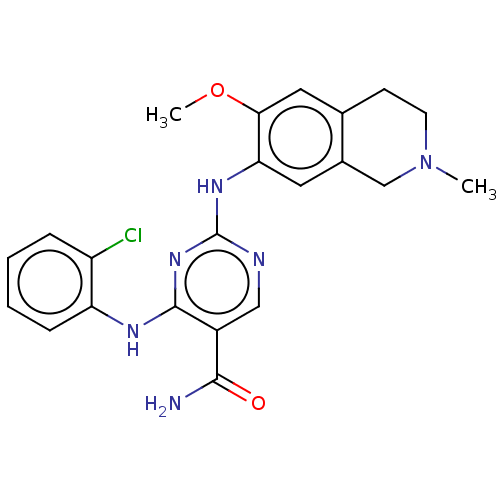
(CHEMBL4866616)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2Cl)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM552514
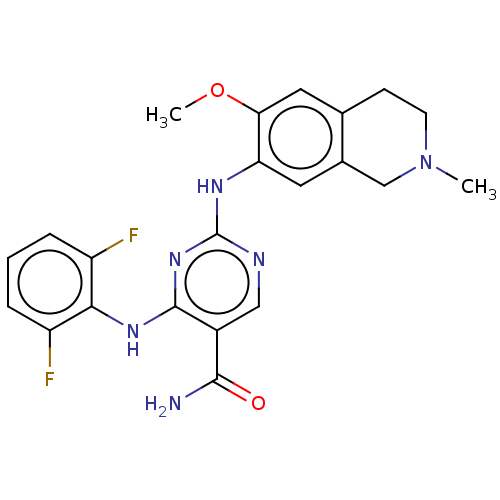
(2-((2-Methoxy-4-(4-methylpiperazine-1-carbonyl)phe...)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(F)cccc2F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569671
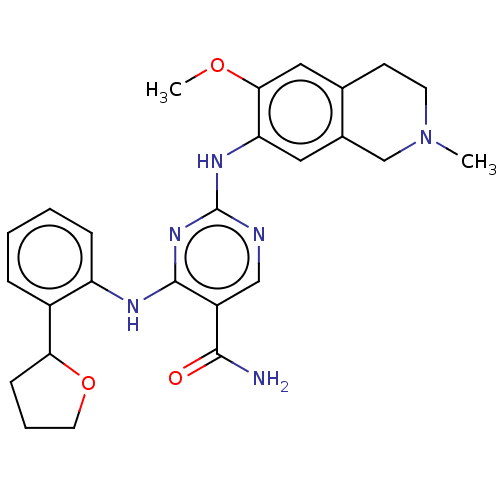
(CHEMBL4860645)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C2CCCO2)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50575884
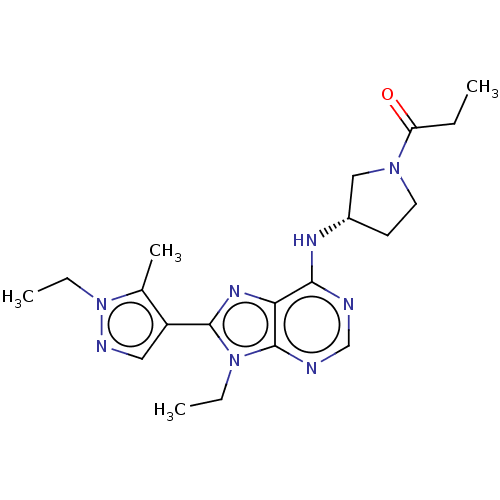
(CHEMBL4849371)Show SMILES CCC(=O)N1CC[C@@H](C1)Nc1ncnc2n(CC)c(nc12)-c1cnn(CC)c1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00237
BindingDB Entry DOI: 10.7270/Q28919NT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569660
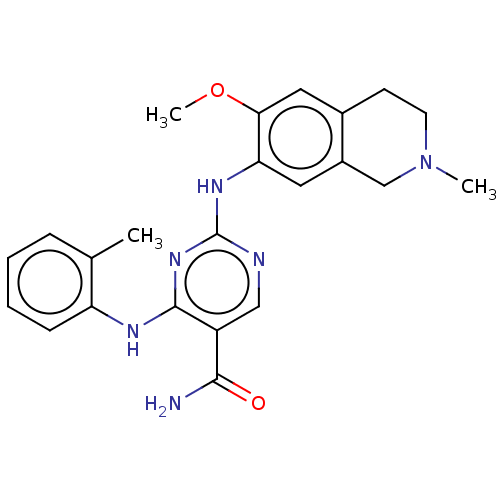
(CHEMBL4849567)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569668
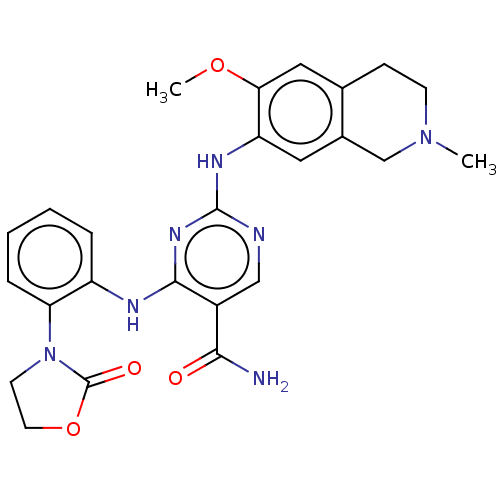
(CHEMBL4857184)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2N2CCOC2=O)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50369748
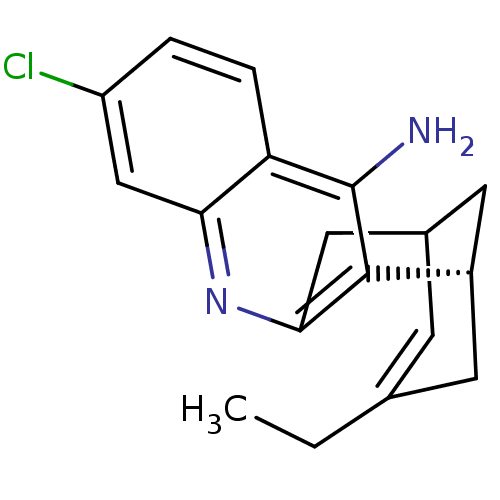
(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50369748

(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM552521
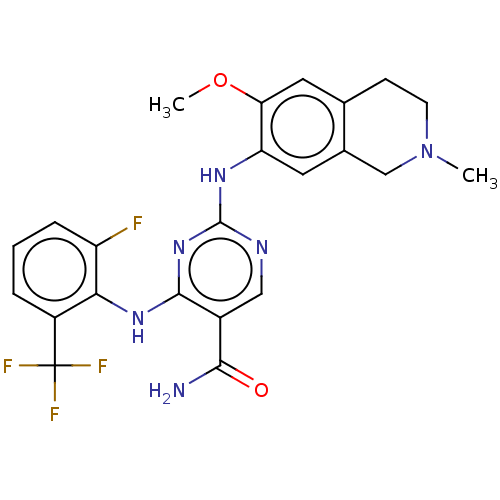
(WO2022098809, Example 4-8)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(F)cccc2C(F)(F)F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569662
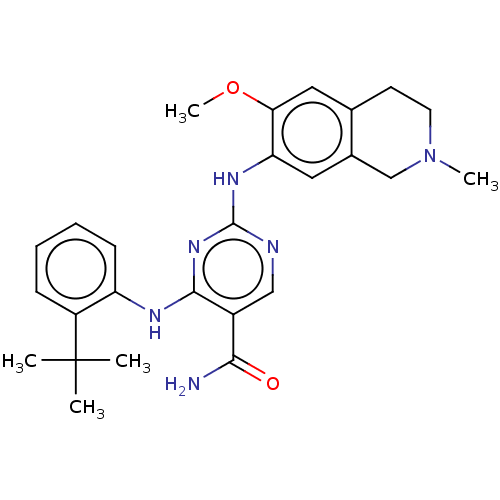
(CHEMBL4874375)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C(C)(C)C)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569656
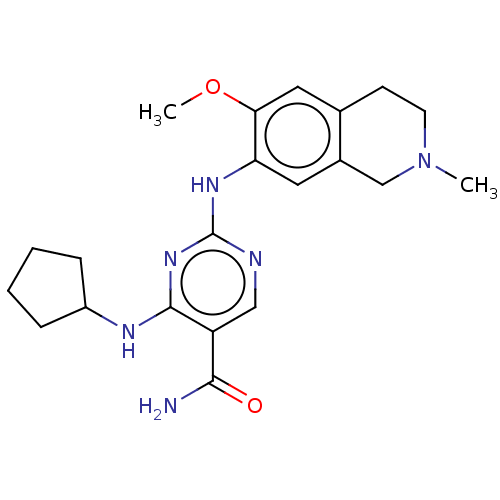
(CHEMBL4871382)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(NC2CCCC2)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM552516
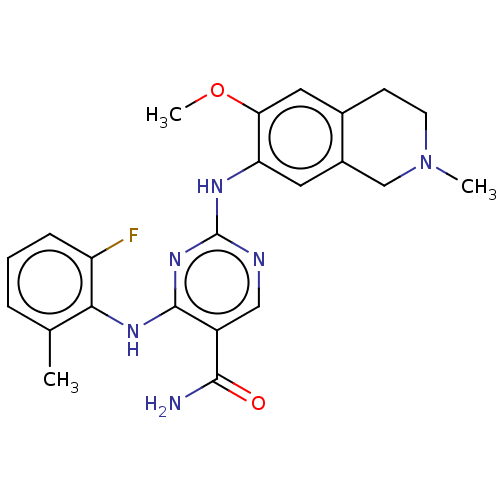
(WO2022098809, Example 4-3 | WO2022098809, Example ...)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(C)cccc2F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase isolated from Human erythrocytes. |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50521218
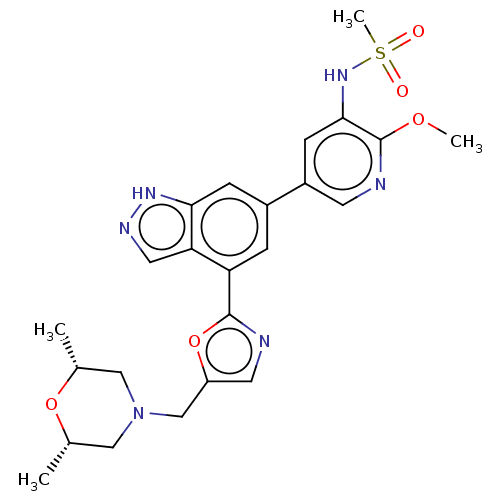
(CHEMBL4434674)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc(-c2ncc(CN3C[C@H](C)O[C@H](C)C3)o2)c2cn[nH]c2c1 |r| Show InChI InChI=1S/C24H28N6O5S/c1-14-11-30(12-15(2)34-14)13-18-9-26-23(35-18)19-5-16(6-21-20(19)10-27-28-21)17-7-22(29-36(4,31)32)24(33-3)25-8-17/h5-10,14-15,29H,11-13H2,1-4H3,(H,27,28)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of PI3KD (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 29: 2575-2580 (2019)
Article DOI: 10.1016/j.bmcl.2019.08.004
BindingDB Entry DOI: 10.7270/Q2PC35RP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50575901
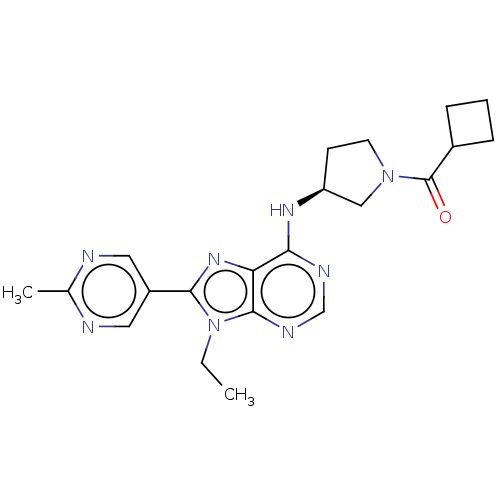
(CHEMBL4878995)Show SMILES CCn1c(nc2c(N[C@H]3CCN(C3)C(=O)C3CCC3)ncnc12)-c1cnc(C)nc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00237
BindingDB Entry DOI: 10.7270/Q28919NT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569675
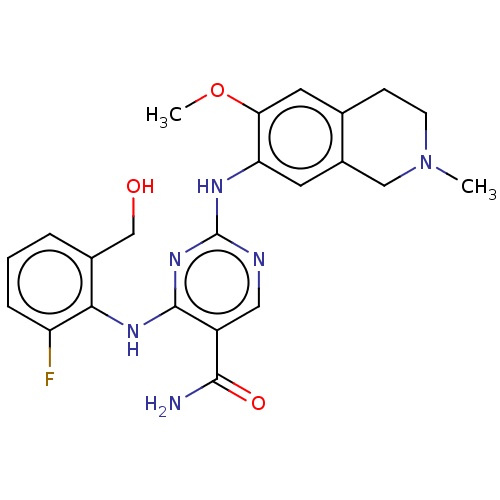
(CHEMBL4859773)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(F)cccc2CO)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM333988

((S)-1-(3-((9-ethyl-8-(6-methoxy- 5-methylpyridin-3...)Show SMILES CCC(=O)N1CCC(C1)Oc1ncnc2n(CC)c(nc12)-c1cnc(OC)c(C)c1 Show InChI InChI=1S/C21H26N6O3/c1-5-16(28)26-8-7-15(11-26)30-21-17-19(23-12-24-21)27(6-2)18(25-17)14-9-13(3)20(29-4)22-10-14/h9-10,12,15H,5-8,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
The PI3-Kinase biochemical assay was optimized using the HTRF kit provided by Upstate (Millipore). The assay kit contains six reagents: 1) 4× Reactio... |
US Patent US9730940 (2017)
BindingDB Entry DOI: 10.7270/Q2BP04WX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50094626
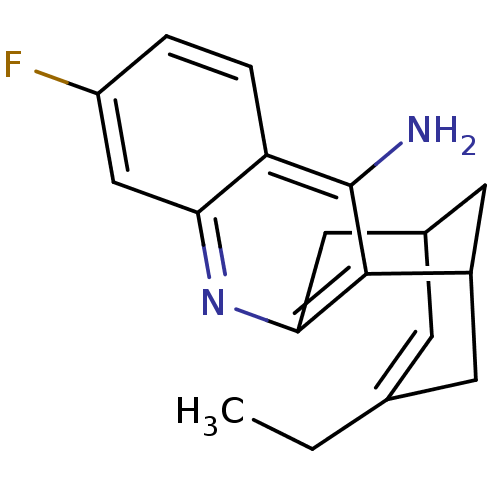
((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:2,TLB:19:8:2.3.7:5| Show InChI InChI=1S/C18H19FN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM333882

(US9730940, Compound 1-35 | tert-butyl (3S)-3-{[8-(...)Show SMILES CCn1c(nc2c(OC3CCN(C3)C(=O)OC(C)(C)C)ncnc12)-c1cnc(s1)C(C)(C)C Show InChI InChI=1S/C23H32N6O3S/c1-8-29-17(15-11-24-20(33-15)22(2,3)4)27-16-18(29)25-13-26-19(16)31-14-9-10-28(12-14)21(30)32-23(5,6)7/h11,13-14H,8-10,12H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
The PI3-Kinase biochemical assay was optimized using the HTRF kit provided by Upstate (Millipore). The assay kit contains six reagents: 1) 4× Reactio... |
US Patent US9730940 (2017)
BindingDB Entry DOI: 10.7270/Q2BP04WX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50570784

(CHEMBL4873442)Show SMILES Oc1cc(F)cc(c1)-c1cc(O[C@@H]2CCN(C2)C(=O)C2CC(F)(F)C2)cc2C(=O)NCc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128046
BindingDB Entry DOI: 10.7270/Q2H998ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50570790
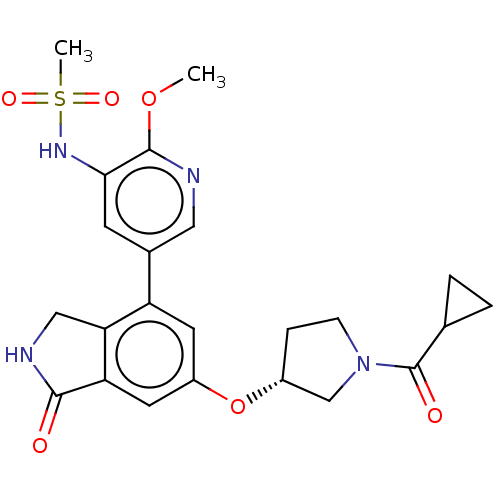
(CHEMBL4847971)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc(O[C@@H]2CCN(C2)C(=O)C2CC2)cc2C(=O)NCc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128046
BindingDB Entry DOI: 10.7270/Q2H998ZN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569658

(CHEMBL4851277)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM333992
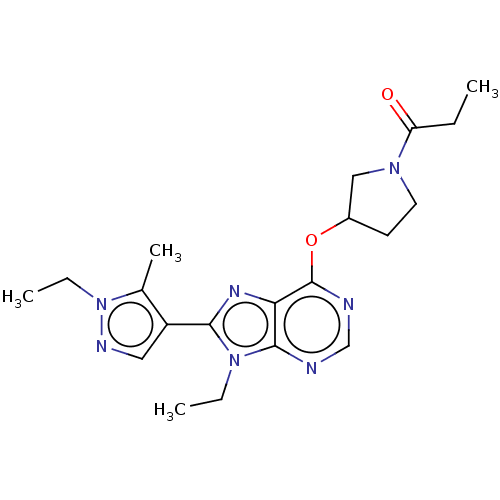
((S)-1-(3-((9-ethyl-8-(1-ethyl-5- methyl-1H-pyrazol...)Show SMILES CCC(=O)N1CCC(C1)Oc1ncnc2n(CC)c(nc12)-c1cnn(CC)c1C Show InChI InChI=1S/C20H27N7O2/c1-5-16(28)25-9-8-14(11-25)29-20-17-19(21-12-22-20)26(6-2)18(24-17)15-10-23-27(7-3)13(15)4/h10,12,14H,5-9,11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
The PI3-Kinase biochemical assay was optimized using the HTRF kit provided by Upstate (Millipore). The assay kit contains six reagents: 1) 4× Reactio... |
US Patent US9730940 (2017)
BindingDB Entry DOI: 10.7270/Q2BP04WX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase activity from bovine erythrocytes after 30 minutes of incubation |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569670
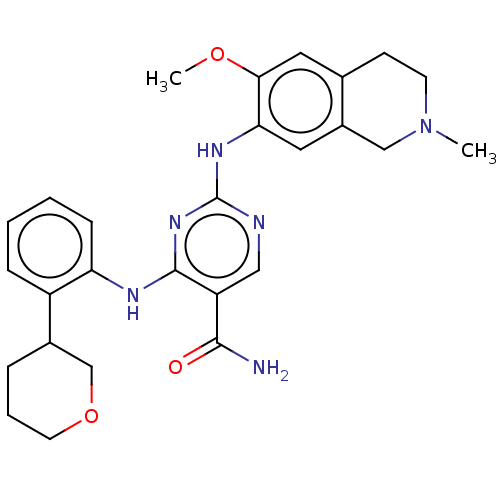
(CHEMBL4868682)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C2CCCOC2)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50575891

(CHEMBL4852541)Show SMILES CCC(=O)N1CC[C@@H](C1)Nc1ncnc2n(C)c(nc12)-c1cnc(s1)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00237
BindingDB Entry DOI: 10.7270/Q28919NT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50522155
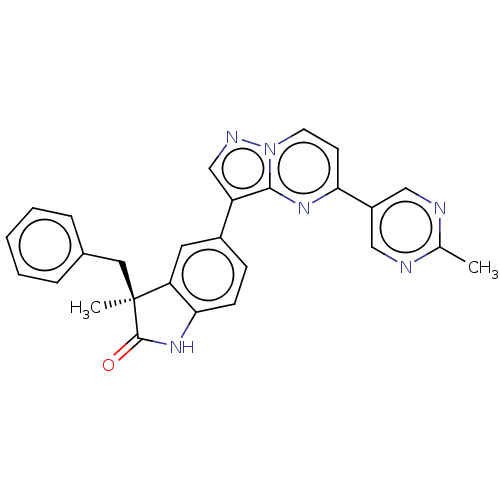
(CHEMBL4452905)Show SMILES Cc1ncc(cn1)-c1ccn2ncc(-c3ccc4NC(=O)[C@@](C)(Cc5ccccc5)c4c3)c2n1 |r| Show InChI InChI=1S/C27H22N6O/c1-17-28-14-20(15-29-17)23-10-11-33-25(31-23)21(16-30-33)19-8-9-24-22(12-19)27(2,26(34)32-24)13-18-6-4-3-5-7-18/h3-12,14-16H,13H2,1-2H3,(H,32,34)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6 tagged recombinant full-length human PI3Kdelta/human recombinant full-length p85alpha expressed in baculovirus infected... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00441
BindingDB Entry DOI: 10.7270/Q2W66QCN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50570788
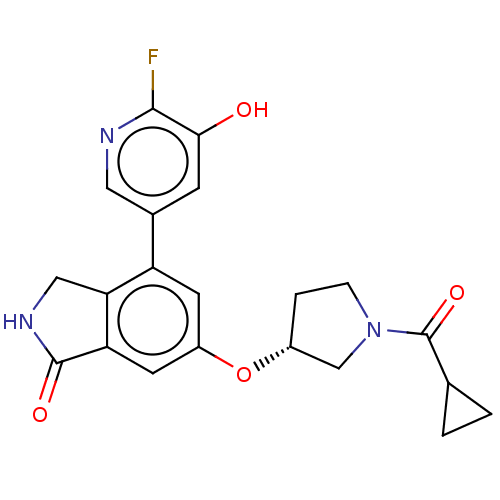
(CHEMBL4877330)Show SMILES Oc1cc(cnc1F)-c1cc(O[C@@H]2CCN(C2)C(=O)C2CC2)cc2C(=O)NCc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128046
BindingDB Entry DOI: 10.7270/Q2H998ZN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50575907
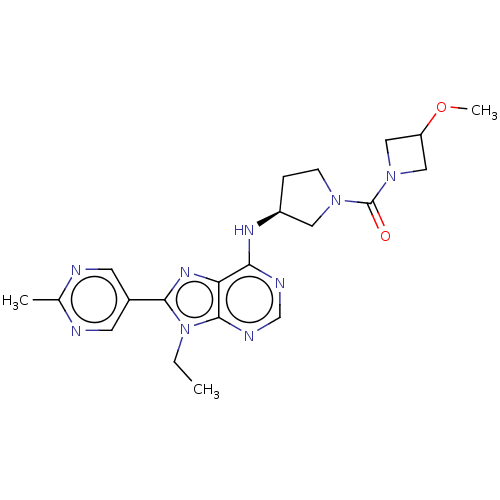
(CHEMBL4878004)Show SMILES CCn1c(nc2c(N[C@H]3CCN(C3)C(=O)N3CC(C3)OC)ncnc12)-c1cnc(C)nc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00237
BindingDB Entry DOI: 10.7270/Q28919NT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM428786
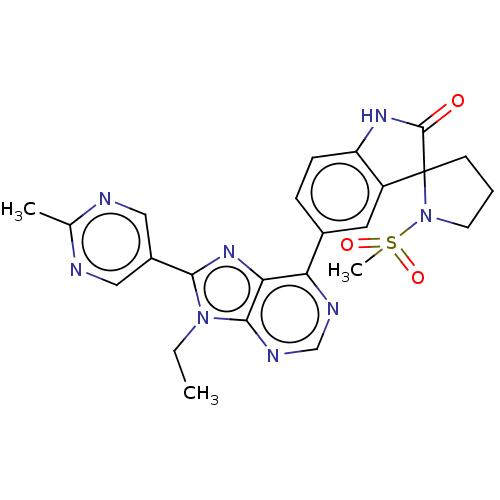
((R or S)-5-(9-ethyl-8-(2- methylpyrimidin-5-yl)-9H...)Show SMILES CCn1c(nc2c(ncnc12)-c1ccc2NC(=O)C3(CCCN3S(C)(=O)=O)c2c1)-c1cnc(C)nc1 Show InChI InChI=1S/C24H24N8O3S/c1-4-31-21(16-11-25-14(2)26-12-16)30-20-19(27-13-28-22(20)31)15-6-7-18-17(10-15)24(23(33)29-18)8-5-9-32(24)36(3,34)35/h6-7,10-13H,4-5,8-9H2,1-3H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The PI3-Kinase biochemical assays were developed to measure the intrinsic potency and compound dependent inhibition of the alpha, beta, delta, and ga... |
US Patent US10544147 (2020)
BindingDB Entry DOI: 10.7270/Q2G1637Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM333881

(US9730940, Compound 1-34 | tert-butyl (3S)-3-{[9-e...)Show SMILES CCn1c(nc2c(OC3CCN(C3)C(=O)OC(C)(C)C)ncnc12)-c1cnc(OC)c(C)c1 Show InChI InChI=1S/C23H30N6O4/c1-7-29-18(15-10-14(2)20(31-6)24-11-15)27-17-19(29)25-13-26-21(17)32-16-8-9-28(12-16)22(30)33-23(3,4)5/h10-11,13,16H,7-9,12H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
The PI3-Kinase biochemical assay was optimized using the HTRF kit provided by Upstate (Millipore). The assay kit contains six reagents: 1) 4× Reactio... |
US Patent US9730940 (2017)
BindingDB Entry DOI: 10.7270/Q2BP04WX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50570783

(CHEMBL4878320)Show SMILES Oc1cc(F)cc(c1)-c1cc(O[C@@H]2CCN(C2)C(=O)C2CCOCC2)cc2C(=O)NCc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128046
BindingDB Entry DOI: 10.7270/Q2H998ZN |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50369748

(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase isolated from Human erythrocytes. |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50369748

(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase isolated from Human erythrocytes. |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase isolated from Human erythrocytes. |
J Med Chem 43: 4657-66 (2001)
BindingDB Entry DOI: 10.7270/Q2Q52Q9B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data