 Found 127 hits with Last Name = 'andreola' and Initial = 'ml'
Found 127 hits with Last Name = 'andreola' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029662

(3-Amino-4-(3,5-dimethyl-phenylsulfanyl)-5-ethyl-6-...)Show InChI InChI=1S/C16H20N2OS/c1-5-13-11(4)18-16(19)14(17)15(13)20-12-7-9(2)6-10(3)8-12/h6-8H,5,17H2,1-4H3,(H,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM25351

(N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydr...)Show SMILES Cc1nnc(o1)C(=O)NC(C)(C)c1nc(C(=O)NCc2ccc(F)cc2)c(O)c(=O)n1C Show InChI InChI=1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10908
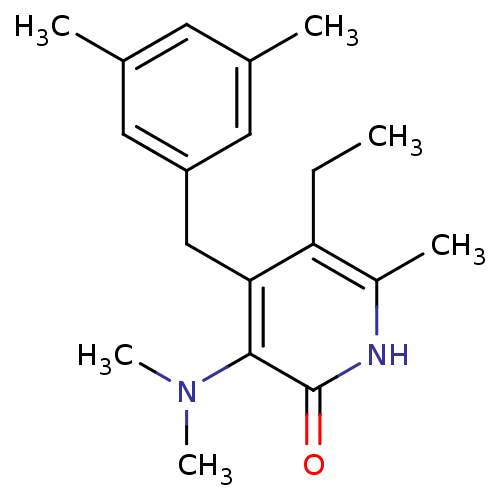
(3-(dimethylamino)-4-[(3,5-dimethylphenyl)methyl]-5...)Show InChI InChI=1S/C19H26N2O/c1-7-16-14(4)20-19(22)18(21(5)6)17(16)11-15-9-12(2)8-13(3)10-15/h8-10H,7,11H2,1-6H3,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10903
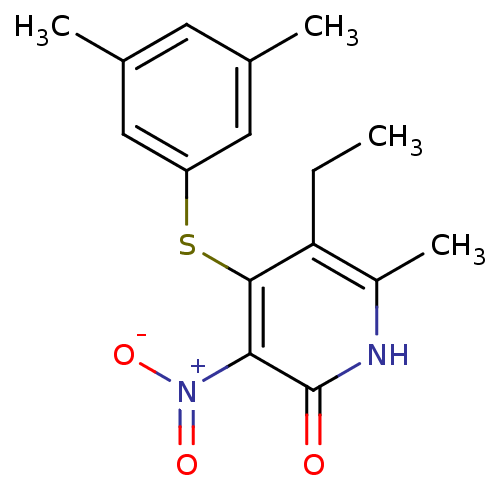
(4-[(3,5-dimethylphenyl)sulfanyl]-5-ethyl-6-methyl-...)Show SMILES CCc1c(C)[nH]c(=O)c(c1Sc1cc(C)cc(C)c1)[N+]([O-])=O Show InChI InChI=1S/C16H18N2O3S/c1-5-13-11(4)17-16(19)14(18(20)21)15(13)22-12-7-9(2)6-10(3)8-12/h6-8H,5H2,1-4H3,(H,17,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029665

(4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-3...)Show SMILES CCc1c(C)[n-]c(=[OH+])c(c1Sc1cc(C)cc(C)c1)[N+]([O-])=O Show InChI InChI=1S/C16H18N2O3S/c1-5-13-11(4)17-16(19)14(18(20)21)15(13)22-12-7-9(2)6-10(3)8-12/h6-8H,5H2,1-4H3,(H,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Integrase
(Human immunodeficiency virus 1) | BDBM50587990
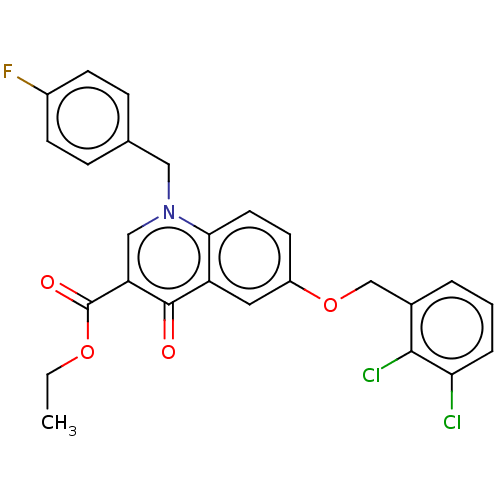
(CHEMBL5182942)Show SMILES CCOC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3cccc(Cl)c3Cl)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10906
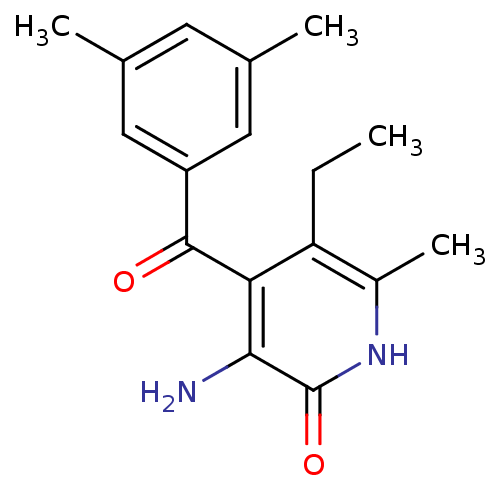
(3-Amino-4-(3,5-dimethylbenzoyl)-5-ethyl-6-methylpy...)Show InChI InChI=1S/C17H20N2O2/c1-5-13-11(4)19-17(21)15(18)14(13)16(20)12-7-9(2)6-10(3)8-12/h6-8H,5,18H2,1-4H3,(H,19,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10905

(3-Amino-4-(3,5-dimethylbenzyl)-5-ethyl-6-methylpyr...)Show InChI InChI=1S/C17H22N2O/c1-5-14-12(4)19-17(20)16(18)15(14)9-13-7-10(2)6-11(3)8-13/h6-8H,5,9,18H2,1-4H3,(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029660

(3-Amino-4-(3,5-dimethyl-phenylsulfanyl)-5-methyl-1...)Show InChI InChI=1S/C14H16N2OS/c1-8-4-9(2)6-11(5-8)18-13-10(3)7-16-14(17)12(13)15/h4-7H,15H2,1-3H3,(H,16,17) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM33411
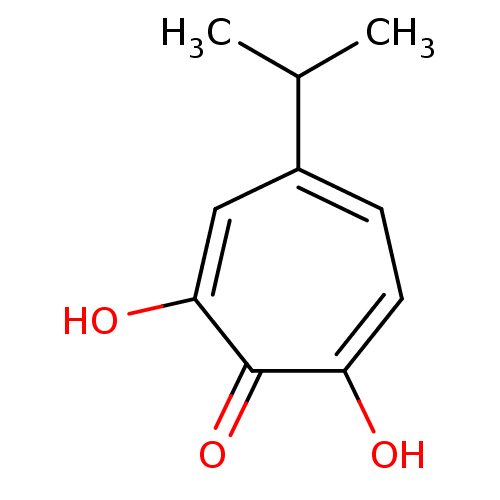
(β-Thujaplicinol | hydroxytropolone, 3)Show InChI InChI=1S/C10H12O3/c1-6(2)7-3-4-8(11)10(13)9(12)5-7/h3-6H,1-2H3,(H2,11,12,13) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1434

(11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...)Show InChI InChI=1S/C15H14N4O/c1-9-6-8-17-14-12(9)18-15(20)11-3-2-7-16-13(11)19(14)10-4-5-10/h2-3,6-8,10H,4-5H2,1H3,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10907
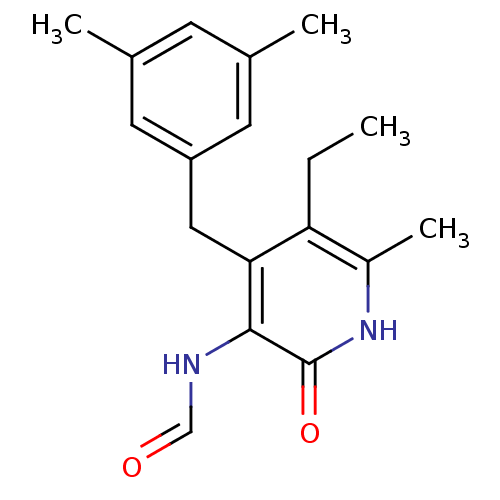
(4-(3,5-Dimethylbenzyl)-5-ethyl-3-formamido-6-methy...)Show InChI InChI=1S/C18H22N2O2/c1-5-15-13(4)20-18(22)17(19-10-21)16(15)9-14-7-11(2)6-12(3)8-14/h6-8,10H,5,9H2,1-4H3,(H,19,21)(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540702
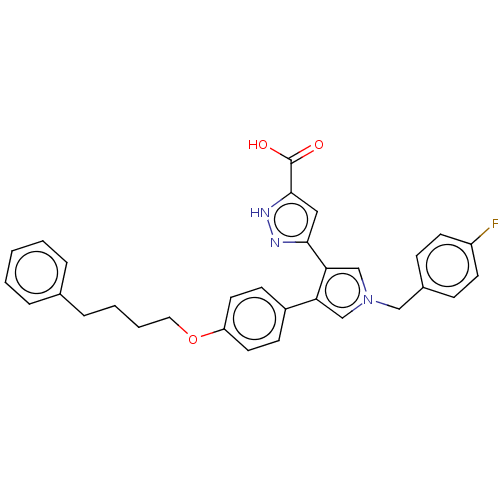
(CHEMBL4641618)Show SMILES OC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C31H28FN3O3/c32-25-13-9-23(10-14-25)19-35-20-27(28(21-35)29-18-30(31(36)37)34-33-29)24-11-15-26(16-12-24)38-17-5-4-8-22-6-2-1-3-7-22/h1-3,6-7,9-16,18,20-21H,4-5,8,17,19H2,(H,33,34)(H,36,37) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM10904
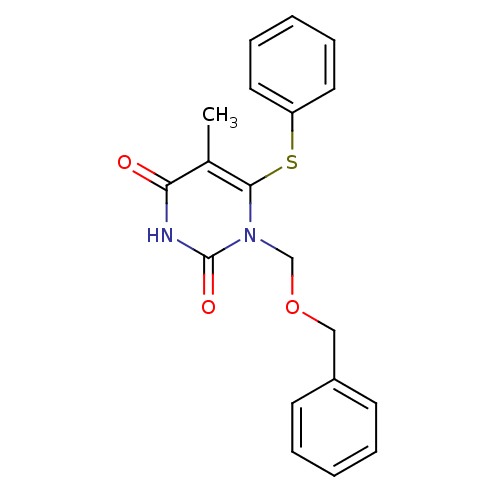
(1-[(benzyloxy)methyl]-5-methyl-6-(phenylsulfanyl)-...)Show InChI InChI=1S/C19H18N2O3S/c1-14-17(22)20-19(23)21(13-24-12-15-8-4-2-5-9-15)18(14)25-16-10-6-3-7-11-16/h2-11H,12-13H2,1H3,(H,20,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 43: 3949-62 (2000)
Article DOI: 10.1021/jm0009437
BindingDB Entry DOI: 10.7270/Q2V12315 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM10904

(1-[(benzyloxy)methyl]-5-methyl-6-(phenylsulfanyl)-...)Show InChI InChI=1S/C19H18N2O3S/c1-14-17(22)20-19(23)21(13-24-12-15-8-4-2-5-9-15)18(14)25-16-10-6-3-7-11-16/h2-11H,12-13H2,1H3,(H,20,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029678
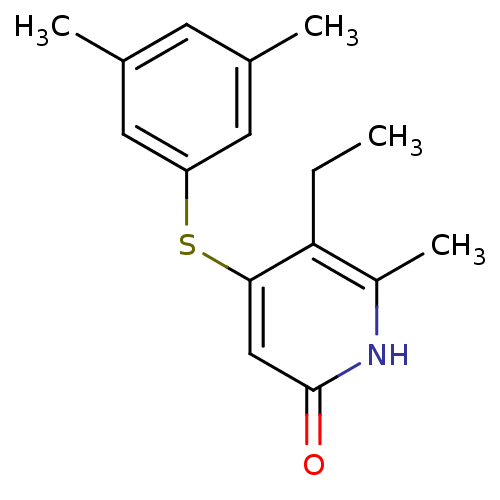
(4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-1...)Show InChI InChI=1S/C16H19NOS/c1-5-14-12(4)17-16(18)9-15(14)19-13-7-10(2)6-11(3)8-13/h6-9H,5H2,1-4H3,(H,17,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50587966
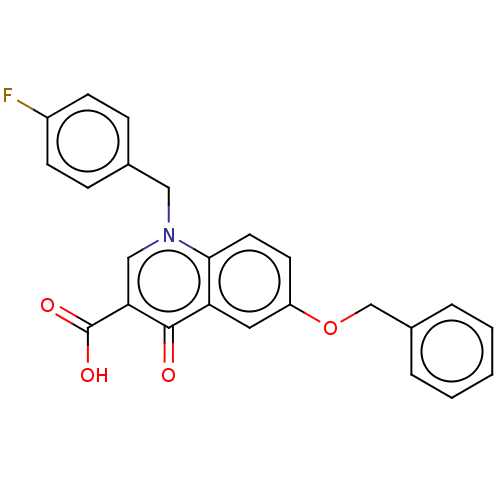
(CHEMBL5188823)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccccc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029663
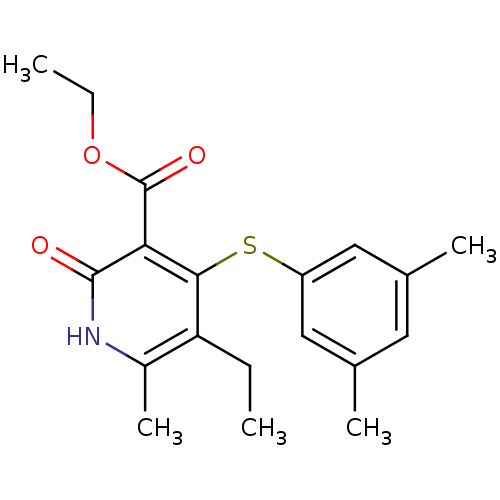
(4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-2...)Show SMILES CCOC(=O)c1c(Sc2cc(C)cc(C)c2)c(CC)c(C)[nH]c1=O Show InChI InChI=1S/C19H23NO3S/c1-6-15-13(5)20-18(21)16(19(22)23-7-2)17(15)24-14-9-11(3)8-12(4)10-14/h8-10H,6-7H2,1-5H3,(H,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587988

(CHEMBL5172754)Show SMILES CCOC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3cccc4ccccc34)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587969
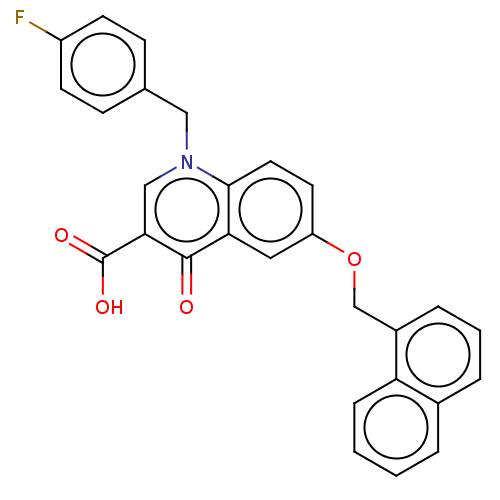
(CHEMBL5173288)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3cccc4ccccc34)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540705
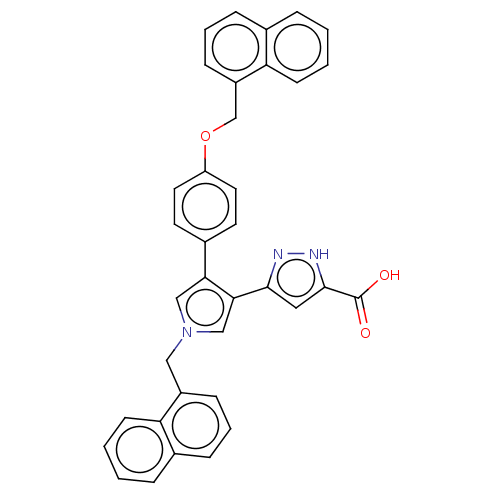
(CHEMBL4641010)Show SMILES OC(=O)c1cc(n[nH]1)-c1cn(Cc2cccc3ccccc23)cc1-c1ccc(OCc2cccc3ccccc23)cc1 Show InChI InChI=1S/C36H27N3O3/c40-36(41)35-19-34(37-38-35)33-22-39(20-27-11-5-9-24-7-1-3-13-30(24)27)21-32(33)26-15-17-29(18-16-26)42-23-28-12-6-10-25-8-2-4-14-31(25)28/h1-19,21-22H,20,23H2,(H,37,38)(H,40,41) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587990

(CHEMBL5182942)Show SMILES CCOC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3cccc(Cl)c3Cl)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587968
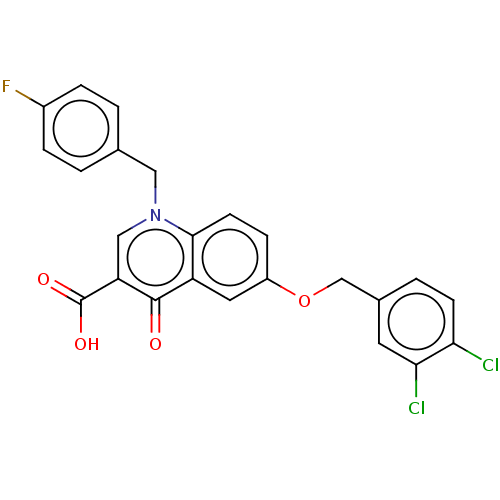
(CHEMBL5198940)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccc(Cl)c(Cl)c3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587974
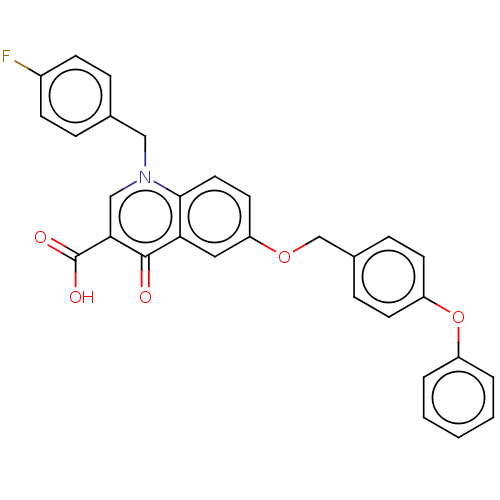
(CHEMBL5185855)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccc(Oc4ccccc4)cc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540703

(CHEMBL4639414)Show SMILES OC(=O)c1cc(n[nH]1)-c1cn(Cc2cccc3ccccc23)cc1-c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C35H31N3O3/c39-35(40)34-21-33(36-37-34)32-24-38(22-28-14-8-13-26-12-4-5-15-30(26)28)23-31(32)27-16-18-29(19-17-27)41-20-7-6-11-25-9-2-1-3-10-25/h1-5,8-10,12-19,21,23-24H,6-7,11,20,22H2,(H,36,37)(H,39,40) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540701
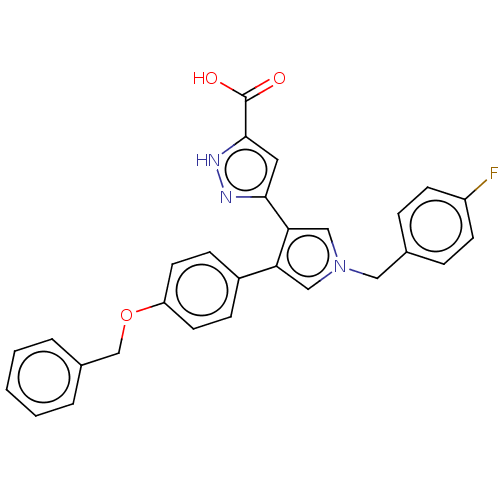
(CHEMBL4648383)Show SMILES OC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C28H22FN3O3/c29-22-10-6-19(7-11-22)15-32-16-24(25(17-32)26-14-27(28(33)34)31-30-26)21-8-12-23(13-9-21)35-18-20-4-2-1-3-5-20/h1-14,16-17H,15,18H2,(H,30,31)(H,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50587968

(CHEMBL5198940)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccc(Cl)c(Cl)c3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50587965
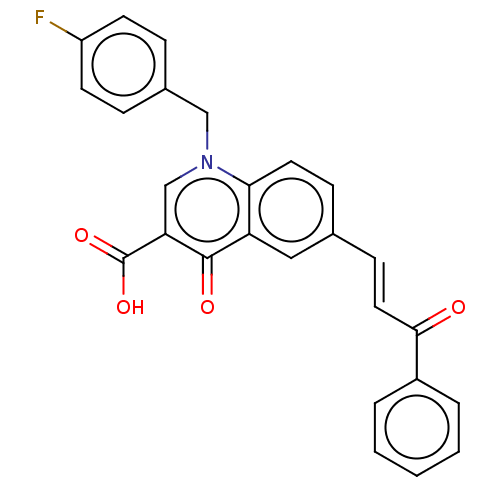
(CHEMBL5176128)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(\C=C\C(=O)c3ccccc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029679
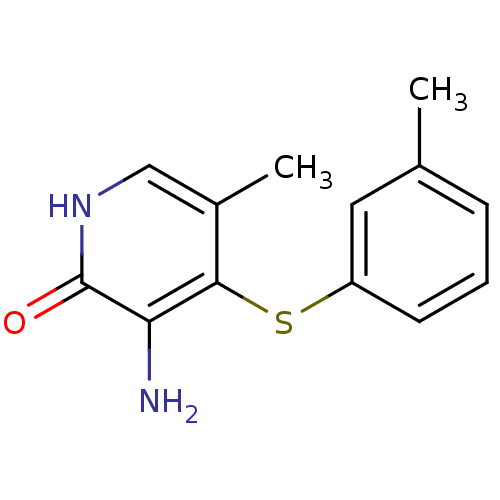
(3-Amino-5-methyl-4-m-tolylsulfanyl-1H-pyridin-2-on...)Show InChI InChI=1S/C13H14N2OS/c1-8-4-3-5-10(6-8)17-12-9(2)7-15-13(16)11(12)14/h3-7H,14H2,1-2H3,(H,15,16) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029671
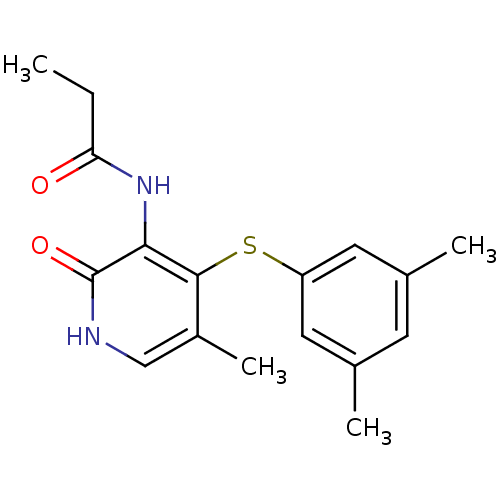
(CHEMBL126931 | N-[4-(3,5-Dimethyl-phenylsulfanyl)-...)Show InChI InChI=1S/C17H20N2O2S/c1-5-14(20)19-15-16(12(4)9-18-17(15)21)22-13-7-10(2)6-11(3)8-13/h6-9H,5H2,1-4H3,(H,18,21)(H,19,20) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50029672
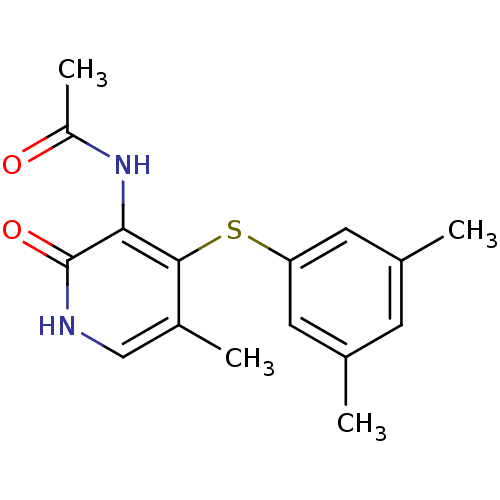
(CHEMBL341880 | N-[4-(3,5-Dimethyl-phenylsulfanyl)-...)Show InChI InChI=1S/C16H18N2O2S/c1-9-5-10(2)7-13(6-9)21-15-11(3)8-17-16(20)14(15)18-12(4)19/h5-8H,1-4H3,(H,17,20)(H,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540696

(CHEMBL4643229)Show SMILES CCOC(=O)c1cc(n[nH]1)-c1cn(Cc2cccc3ccccc23)cc1-c1ccc(OCc2cccc3ccccc23)cc1 Show InChI InChI=1S/C38H31N3O3/c1-2-43-38(42)37-21-36(39-40-37)35-24-41(22-29-13-7-11-26-9-3-5-15-32(26)29)23-34(35)28-17-19-31(20-18-28)44-25-30-14-8-12-27-10-4-6-16-33(27)30/h3-21,23-24H,2,22,25H2,1H3,(H,39,40) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540704
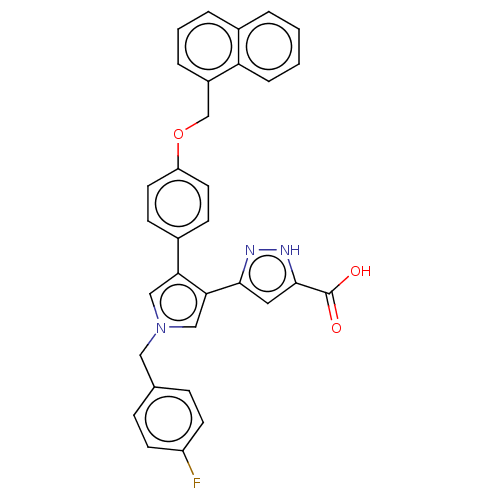
(CHEMBL4634514)Show SMILES OC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCc2cccc3ccccc23)cc1 Show InChI InChI=1S/C32H24FN3O3/c33-25-12-8-21(9-13-25)17-36-18-28(29(19-36)30-16-31(32(37)38)35-34-30)23-10-14-26(15-11-23)39-20-24-6-3-5-22-4-1-2-7-27(22)24/h1-16,18-19H,17,20H2,(H,34,35)(H,37,38) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587971
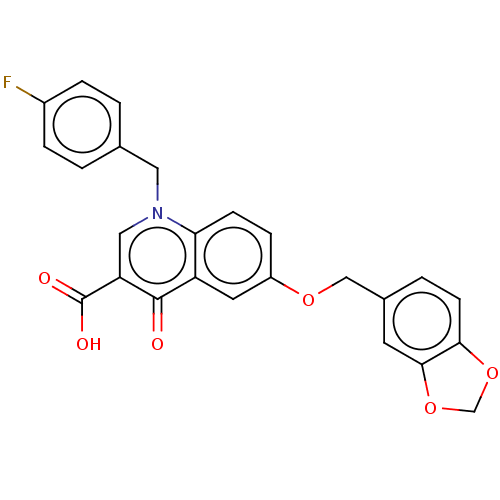
(CHEMBL5183730)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccc4OCOc4c3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540692
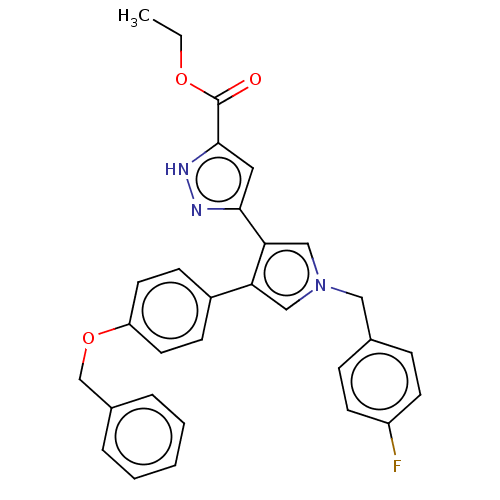
(CHEMBL4649730)Show SMILES CCOC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C30H26FN3O3/c1-2-36-30(35)29-16-28(32-33-29)27-19-34(17-21-8-12-24(31)13-9-21)18-26(27)23-10-14-25(15-11-23)37-20-22-6-4-3-5-7-22/h3-16,18-19H,2,17,20H2,1H3,(H,32,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540695
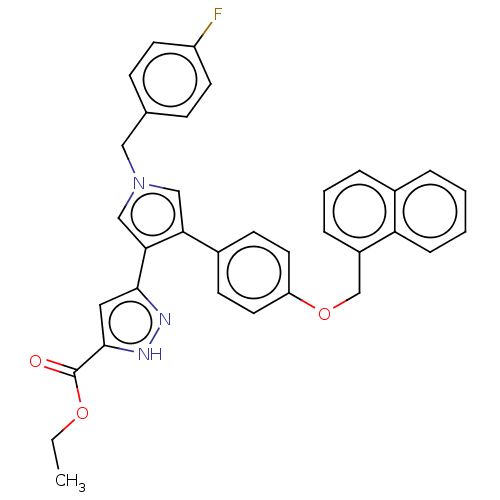
(CHEMBL4638007)Show SMILES CCOC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCc2cccc3ccccc23)cc1 Show InChI InChI=1S/C34H28FN3O3/c1-2-40-34(39)33-18-32(36-37-33)31-21-38(19-23-10-14-27(35)15-11-23)20-30(31)25-12-16-28(17-13-25)41-22-26-8-5-7-24-6-3-4-9-29(24)26/h3-18,20-21H,2,19,22H2,1H3,(H,36,37) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587967

(CHEMBL5197780)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3cccc(Cl)c3Cl)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540708

(CHEMBL4640609)Show SMILES OC(=O)c1cc(n[nH]1)-c1cn(Cc2cccc3ccccc23)cc1-c1ccc(OCC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C33H26N4O4/c38-32(34-25-10-2-1-3-11-25)21-41-26-15-13-23(14-16-26)28-19-37(20-29(28)30-17-31(33(39)40)36-35-30)18-24-9-6-8-22-7-4-5-12-27(22)24/h1-17,19-20H,18,21H2,(H,34,38)(H,35,36)(H,39,40) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587965

(CHEMBL5176128)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(\C=C\C(=O)c3ccccc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50004152
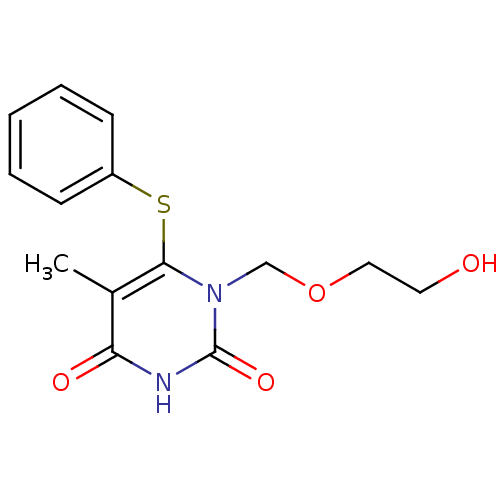
((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...)Show InChI InChI=1S/C14H16N2O4S/c1-10-12(18)15-14(19)16(9-20-8-7-17)13(10)21-11-5-3-2-4-6-11/h2-6,17H,7-9H2,1H3,(H,15,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase(RT) |
J Med Chem 38: 4679-86 (1995)
BindingDB Entry DOI: 10.7270/Q2QF8RWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540698
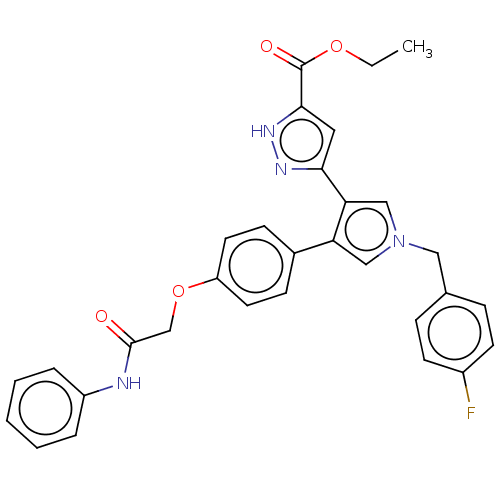
(CHEMBL4644576)Show SMILES CCOC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C31H27FN4O4/c1-2-39-31(38)29-16-28(34-35-29)27-19-36(17-21-8-12-23(32)13-9-21)18-26(27)22-10-14-25(15-11-22)40-20-30(37)33-24-6-4-3-5-7-24/h3-16,18-19H,2,17,20H2,1H3,(H,33,37)(H,34,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540710
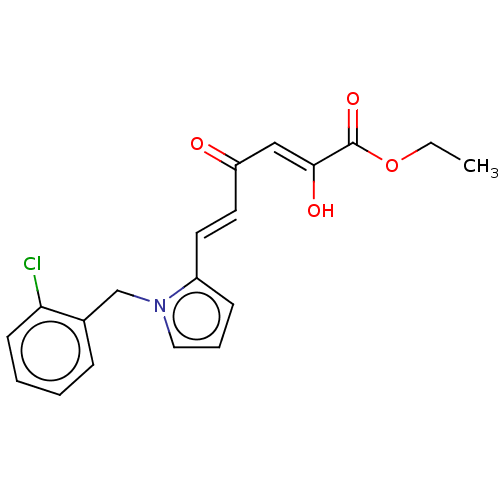
(CHEMBL4643404)Show SMILES CCOC(=O)C(\O)=C\C(=O)\C=C\c1cccn1Cc1ccccc1Cl Show InChI InChI=1S/C19H18ClNO4/c1-2-25-19(24)18(23)12-16(22)10-9-15-7-5-11-21(15)13-14-6-3-4-8-17(14)20/h3-12,23H,2,13H2,1H3/b10-9+,18-12- | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant HIV-1 reverse transcriptase p66/p51 expressed in Escherichia coli M15 using 5'-[gamma32P]ATP-labeled-tC... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587973
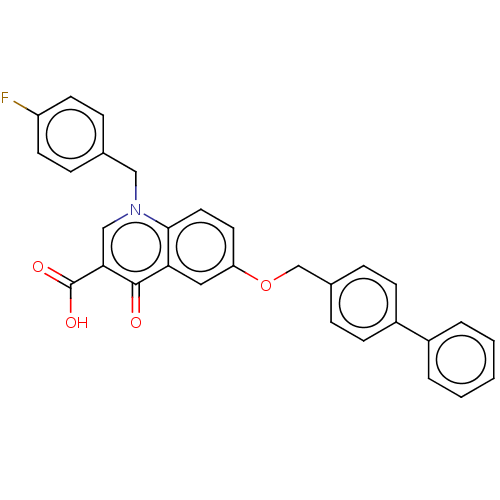
(CHEMBL5198310)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccc(cc3)-c3ccccc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587974

(CHEMBL5185855)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccc(Oc4ccccc4)cc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540699

(CHEMBL4648093)Show SMILES CCOC(=O)c1cc(n[nH]1)-c1cn(Cc2cccc3ccccc23)cc1-c1ccc(OCC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C35H30N4O4/c1-2-42-35(41)33-19-32(37-38-33)31-22-39(20-26-11-8-10-24-9-6-7-14-29(24)26)21-30(31)25-15-17-28(18-16-25)43-23-34(40)36-27-12-4-3-5-13-27/h3-19,21-22H,2,20,23H2,1H3,(H,36,40)(H,37,38) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587991

(CHEMBL5174596)Show SMILES CCOC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3ccccc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50540697

(CHEMBL4633074)Show SMILES CCOC(=O)c1cc(n[nH]1)-c1cn(Cc2ccc(F)cc2)cc1-c1ccc(OCC(=O)Nc2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C32H27FN4O6/c1-2-42-32(41)29-15-28(35-36-29)27-18-37(16-20-3-9-23(33)10-4-20)17-26(27)21-7-13-25(14-8-21)43-19-30(38)34-24-11-5-22(6-12-24)31(39)40/h3-15,17-18H,2,16,19H2,1H3,(H,34,38)(H,35,36)(H,39,40) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of RNase H activity of recombinant His-tagged HIV-1 group M subtype B reverse transcriptase p66/p51 expressed in Escherichia coli M15 usin... |
ACS Med Chem Lett 11: 798-805 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00617
BindingDB Entry DOI: 10.7270/Q2T1576W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587967

(CHEMBL5197780)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCc3cccc(Cl)c3Cl)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50587972

(CHEMBL5202973)Show SMILES OC(=O)c1cn(Cc2ccc(F)cc2)c2ccc(OCCCCc3ccccc3)cc2c1=O | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00535
BindingDB Entry DOI: 10.7270/Q2V98D1X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data