

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50373939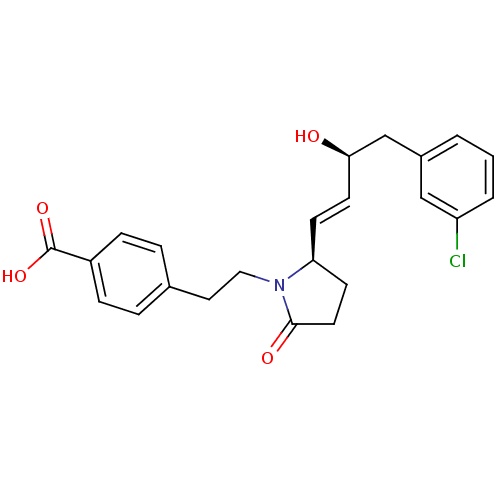 (CHEMBL258332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50373942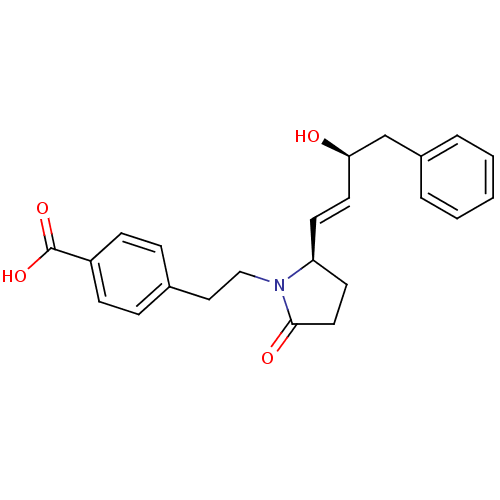 (CHEMBL272276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111741 (CHEMBL19666 | N-(5-Carbamimidoyl-thiophen-2-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111728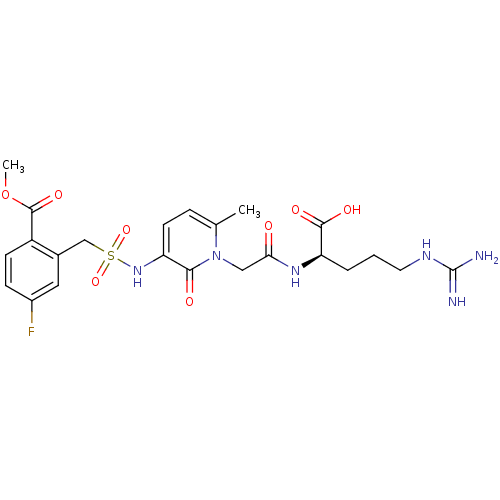 (4-Fluoro-2-({1-[((R)-1-formyl-4-guanidino-butylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111729 (CHEMBL277695 | N-(5-Carbamimidoyl-thiophen-2-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111730 (2-[3-(2-Fluoro-phenylmethanesulfonylamino)-6-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 4323-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.025 BindingDB Entry DOI: 10.7270/Q2RF5VVC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111724 (CHEMBL19731 | N-(4-Carbamimidoyl-benzyl)-2-(6-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111717 (2-[3-(2-Fluoro-phenylmethanesulfonylamino)-2-oxo-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111726 (CHEMBL19359 | N-(4-Carbamimidoyl-benzyl)-2-(6-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796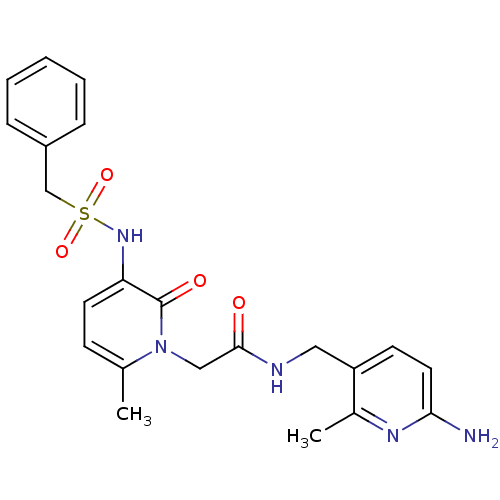 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute Curated by ChEMBL | Assay Description Binding affinity at human prostaglandin EP4 receptor | Bioorg Med Chem Lett 17: 6572-5 (2007) Article DOI: 10.1016/j.bmcl.2007.09.074 BindingDB Entry DOI: 10.7270/Q29887V9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 4323-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.025 BindingDB Entry DOI: 10.7270/Q2RF5VVC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50083623 ((3R,8R)-8-Phenethyl-2-phenyl-3-p-tolyl-8-aza-bicyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]mazindol from cocaine binding site on Dopamine transporter (DAT) | Bioorg Med Chem Lett 9: 3325-8 (2000) BindingDB Entry DOI: 10.7270/Q2RB754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797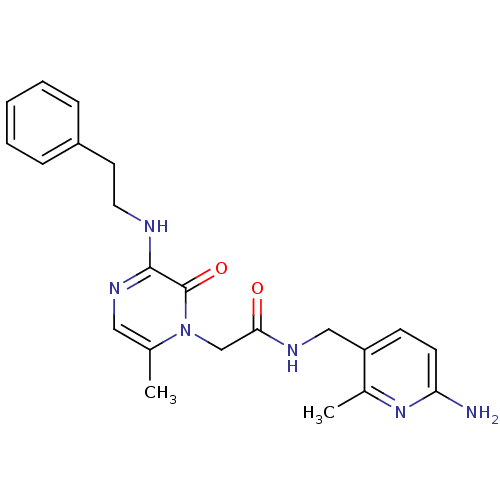 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111739 (CHEMBL19811 | N-((S)-2-Amino-1,4,5,6-tetrahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50373941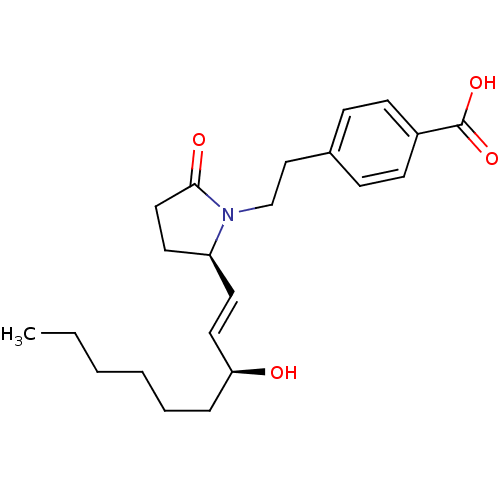 (CHEMBL257217) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50213980 (4-(2-((R)-2-((S)-3-hydroxyhept-1-enyl)-5-oxopyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111738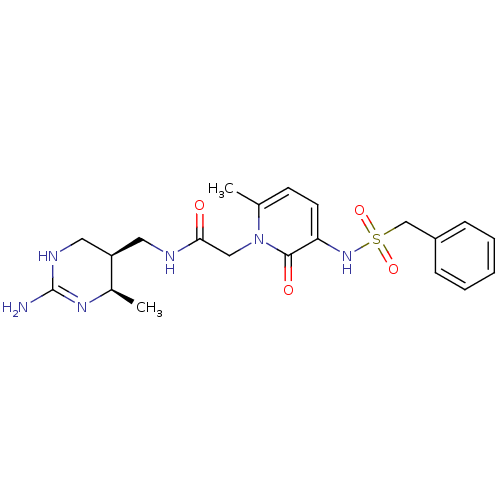 (CHEMBL274968 | N-((4R,5S)-2-Amino-4-methyl-1,4,5,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068477 ((2S,8S)-8-Methyl-2-propyl-3-p-tolyl-8-aza-bicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity for [3H]DA (Dopamine transporter) uptake by striated synaptosomes | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068470 ((2S,8S)-2-Butyl-8-methyl-3-p-tolyl-8-aza-bicyclo[3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity for [3H]DA (Dopamine transporter) uptake by striated synaptosomes | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50083617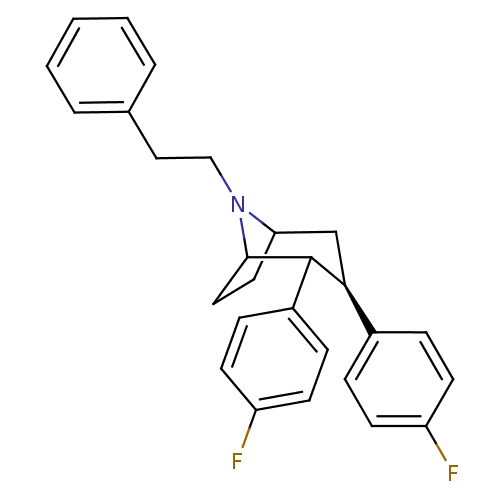 ((3R,8R)-2,3-Bis-(4-fluoro-phenyl)-8-phenethyl-8-az...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibition of [3H]5-HT uptake at striatal nerve endings by serotonin transporter | Bioorg Med Chem Lett 9: 3325-8 (2000) BindingDB Entry DOI: 10.7270/Q2RB754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068477 ((2S,8S)-8-Methyl-2-propyl-3-p-tolyl-8-aza-bicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity of compound on dopamine transporters of rat striated membranes using [3H]- mazindol. | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50083623 ((3R,8R)-8-Phenethyl-2-phenyl-3-p-tolyl-8-aza-bicyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine uptake at striatal nerve endings by dopamine transporter | Bioorg Med Chem Lett 9: 3325-8 (2000) BindingDB Entry DOI: 10.7270/Q2RB754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111714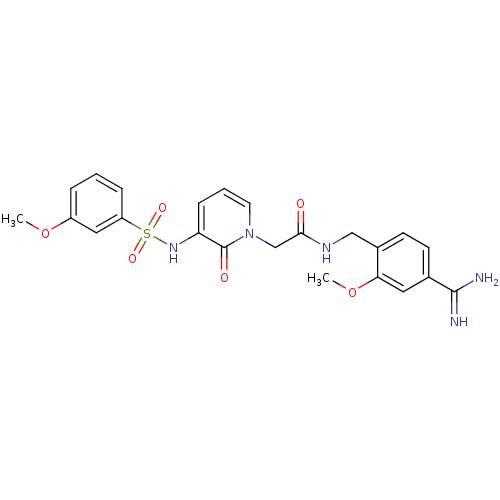 (CHEMBL416912 | N-(4-Carbamimidoyl-2-methoxy-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068470 ((2S,8S)-2-Butyl-8-methyl-3-p-tolyl-8-aza-bicyclo[3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity of compound on dopamine transporters of rat striated membranes using [3H]- mazindol. | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111742 (CHEMBL417635 | N-((S)-2-Amino-1,4,5,6-tetrahydro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50373944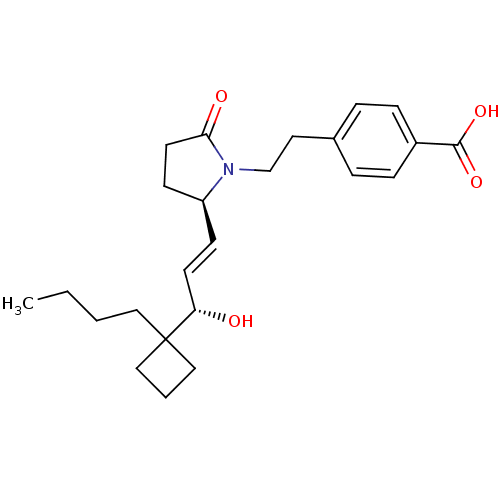 (CHEMBL272277) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068473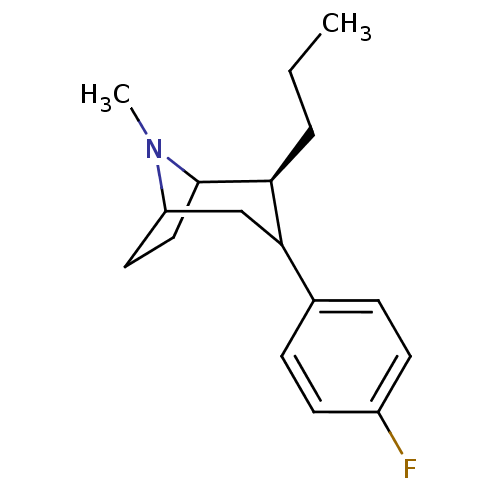 ((2S,8S)-3-(4-Fluoro-phenyl)-8-methyl-2-propyl-8-az...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity for [3H]DA (Dopamine transporter) uptake by striated synaptosomes | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50156547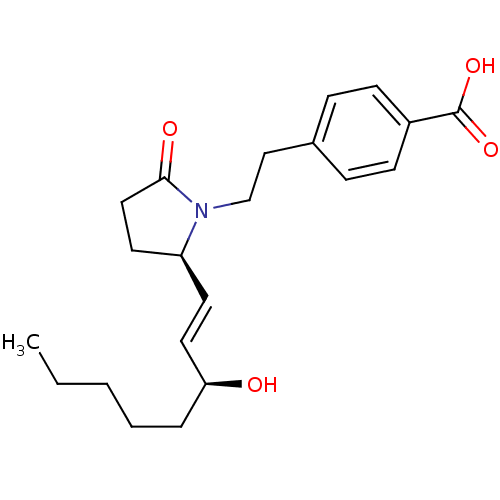 (4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE4 from human EP4 receptor | Bioorg Med Chem Lett 18: 821-4 (2008) Article DOI: 10.1016/j.bmcl.2007.11.020 BindingDB Entry DOI: 10.7270/Q2W096SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50213980 (4-(2-((R)-2-((S)-3-hydroxyhept-1-enyl)-5-oxopyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 4323-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.025 BindingDB Entry DOI: 10.7270/Q2RF5VVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215314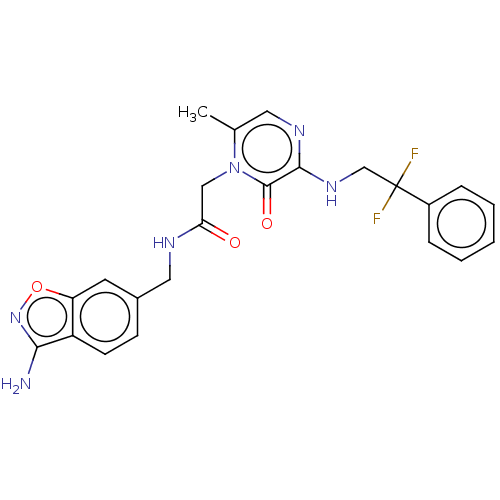 (CHEMBL100049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068471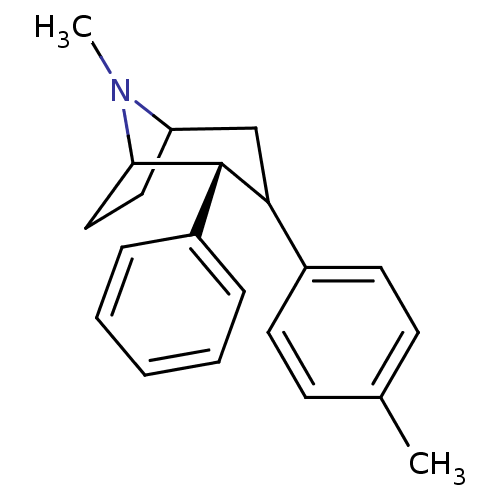 ((2R,8S)-8-Methyl-2-phenyl-3-p-tolyl-8-aza-bicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity of compound on dopamine transporters of rat striated membranes using [3H]- mazindol. | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068471 ((2R,8S)-8-Methyl-2-phenyl-3-p-tolyl-8-aza-bicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity for [3H]DA (Dopamine transporter) uptake by striated synaptosomes | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068479 ((2S,3R,5R)-8-Methyl-2-phenyl-3-p-tolyl-8-aza-bicyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity of compound on dopamine transporters of rat striated membranes using [3H]- mazindol. | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215447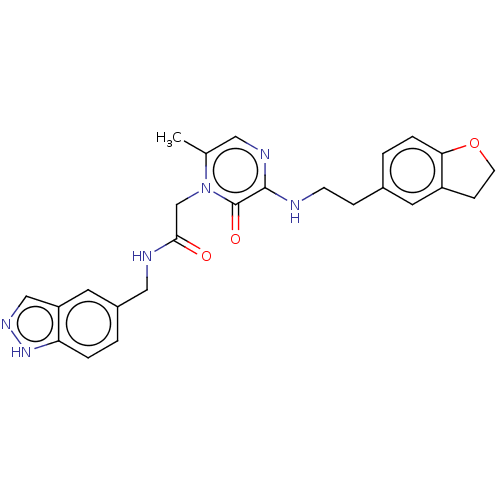 (CHEMBL101867) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50100973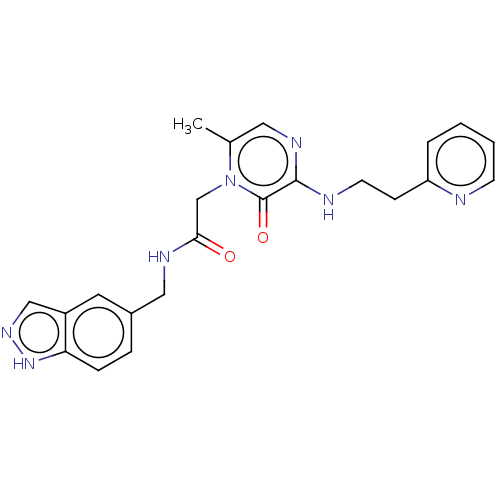 (CHEMBL101605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111736 (CHEMBL19548 | N-((4R,5S)-2-Amino-4-methyl-1,4,5,6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111720 (CHEMBL19619 | N-(4-Carbamimidoyl-3-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against human thrombin (FIIa). | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50215308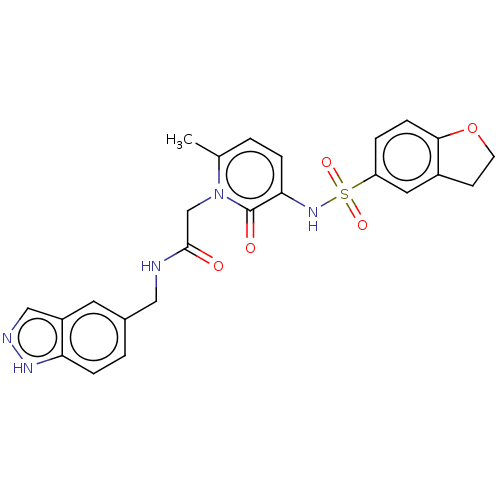 (CHEMBL99185) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50213978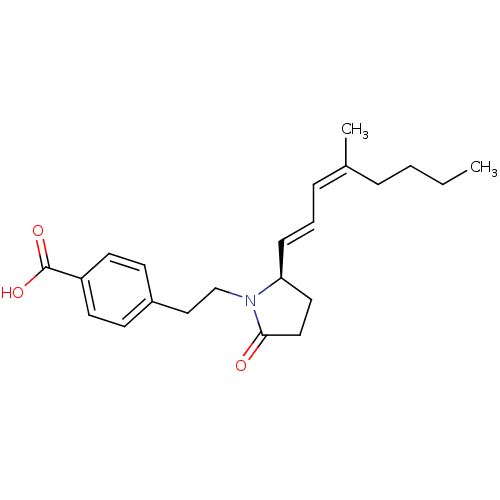 (4-(2-((R)-2-(4-methylocta-1,3-dienyl)-5-oxopyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 4323-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.025 BindingDB Entry DOI: 10.7270/Q2RF5VVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50105480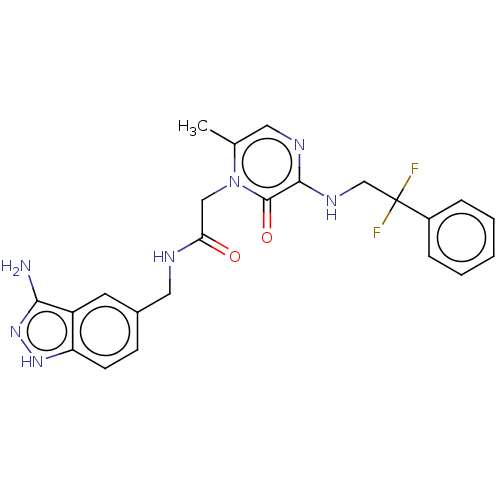 (CHEMBL98194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Thrombin (FIIa) cleavage of the chromogenic substrate | Bioorg Med Chem Lett 12: 2925-30 (2002) BindingDB Entry DOI: 10.7270/Q27946WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068479 ((2S,3R,5R)-8-Methyl-2-phenyl-3-p-tolyl-8-aza-bicyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity for [3H]DA (Dopamine transporter) uptake by striated synaptosomes | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50111729 (CHEMBL277695 | N-(5-Carbamimidoyl-thiophen-2-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory constant against trypsin | Bioorg Med Chem Lett 12: 1203-8 (2002) BindingDB Entry DOI: 10.7270/Q2PK0FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068481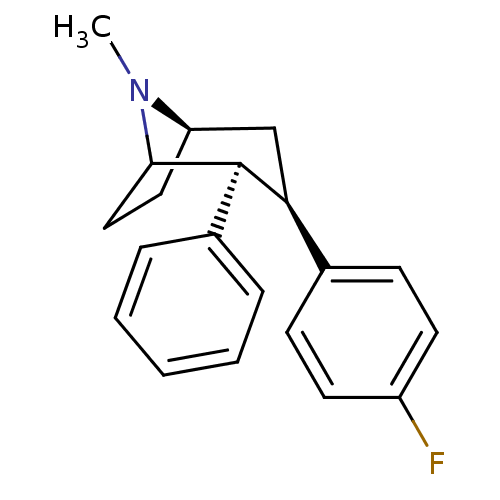 ((2S,3R,5R)-3-(4-Fluoro-phenyl)-8-methyl-2-phenyl-8...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding affinity for [3H]DA (Dopamine transporter) uptake by striated synaptosomes | J Med Chem 41: 4973-82 (1999) Article DOI: 10.1021/jm9802564 BindingDB Entry DOI: 10.7270/Q29S1RQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50068471 ((2R,8S)-8-Methyl-2-phenyl-3-p-tolyl-8-aza-bicyclo[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibition of [3H]dopamine uptake at striatal nerve endings by dopamine transporter | Bioorg Med Chem Lett 9: 3325-8 (2000) BindingDB Entry DOI: 10.7270/Q2RB754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 468 total ) | Next | Last >> |