

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gamma-aminobutyric acid receptor subunit alpha-1 (Rattus norvegicus (Rat)) | BDBM50118406 (2-(3-Bromo-phenyl)-6-methyl-chromen-4-one | 6-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biología Celular Curated by PDSP Ki Database | Biochem Biophys Res Commun 262: 643-6 (1999) Article DOI: 10.1006/bbrc.1999.1273 BindingDB Entry DOI: 10.7270/Q2VT1QMS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Rattus norvegicus (Rat)) | BDBM50118406 (2-(3-Bromo-phenyl)-6-methyl-chromen-4-one | 6-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biología Celular Curated by PDSP Ki Database | Biochem Biophys Res Commun 262: 643-6 (1999) Article DOI: 10.1006/bbrc.1999.1273 BindingDB Entry DOI: 10.7270/Q2VT1QMS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Rattus norvegicus (Rat)) | BDBM50118406 (2-(3-Bromo-phenyl)-6-methyl-chromen-4-one | 6-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biología Celular Curated by PDSP Ki Database | Biochem Biophys Res Commun 262: 643-6 (1999) Article DOI: 10.1006/bbrc.1999.1273 BindingDB Entry DOI: 10.7270/Q2VT1QMS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Rattus norvegicus (Rat)) | BDBM50118406 (2-(3-Bromo-phenyl)-6-methyl-chromen-4-one | 6-Meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Biología Celular Curated by PDSP Ki Database | Biochem Biophys Res Commun 262: 643-6 (1999) Article DOI: 10.1006/bbrc.1999.1273 BindingDB Entry DOI: 10.7270/Q2VT1QMS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23232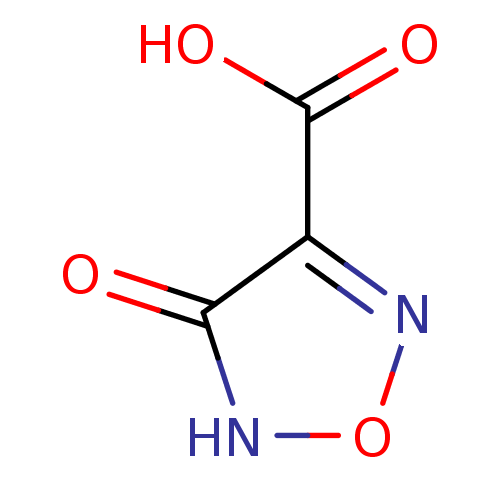 (1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23251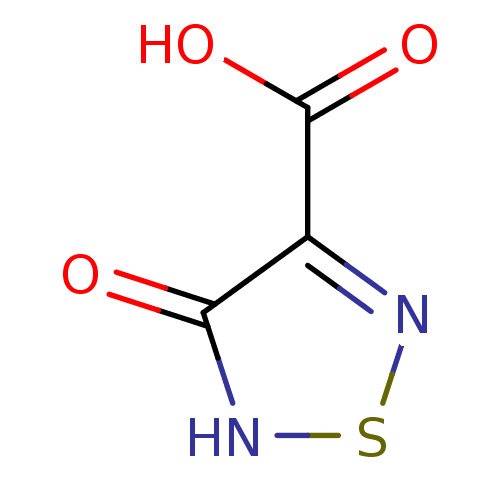 (1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23242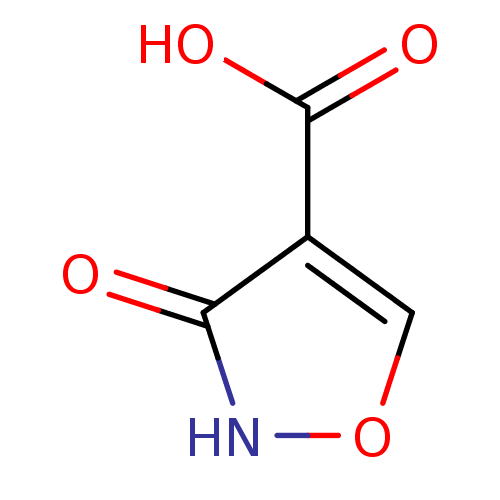 (1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50320887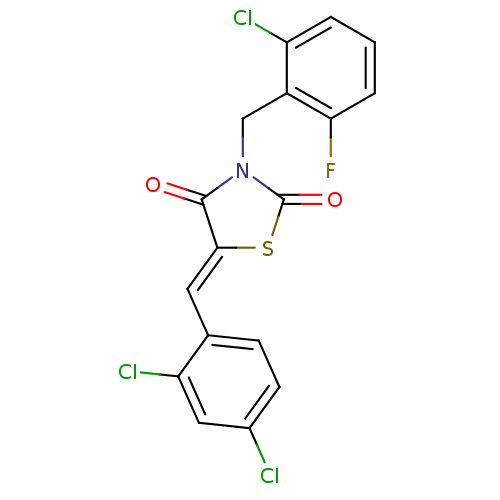 (3-(2-Chloro-6-fluoro-benzyl)-5-(2,4-dichlorobenzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Pernambuco Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPARgamma ligand binding domain expressed in Escherichia coli BL21 after 12 hrs by liquid scintillation ... | Bioorg Med Chem 18: 3805-11 (2010) Article DOI: 10.1016/j.bmc.2010.04.045 BindingDB Entry DOI: 10.7270/Q28S4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50320886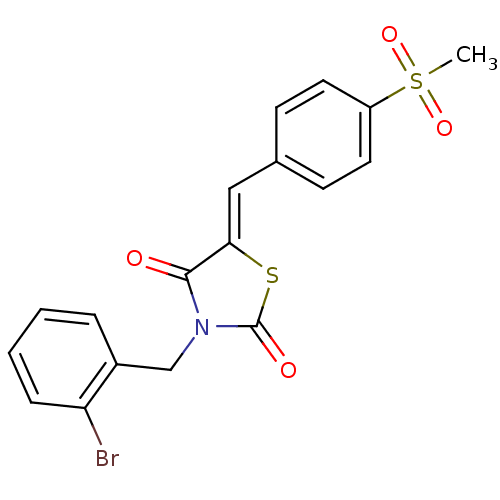 (3-(2-Bromo-benzyl)-5-(4-methanesulfonyl-benzyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Pernambuco Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPARgamma ligand binding domain expressed in Escherichia coli BL21 after 12 hrs by liquid scintillation ... | Bioorg Med Chem 18: 3805-11 (2010) Article DOI: 10.1016/j.bmc.2010.04.045 BindingDB Entry DOI: 10.7270/Q28S4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50320884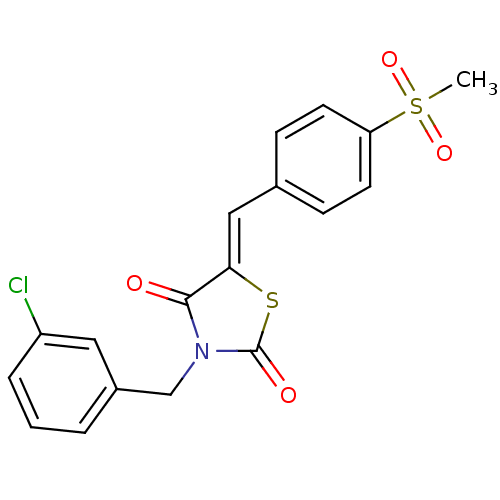 (3-(3-Chloro-benzyl)-5-(4-methanesulfonyl-benzylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Pernambuco Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPARgamma ligand binding domain expressed in Escherichia coli BL21 after 12 hrs by liquid scintillation ... | Bioorg Med Chem 18: 3805-11 (2010) Article DOI: 10.1016/j.bmc.2010.04.045 BindingDB Entry DOI: 10.7270/Q28S4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50320885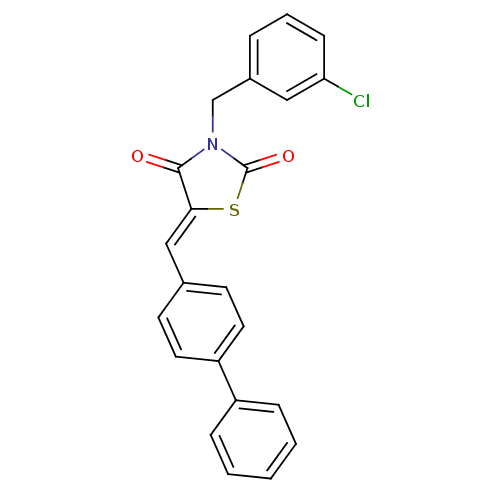 (5-Bipheny-4-ylmethylene-3-(3-chloro-benzyl)-thiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Pernambuco Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPARgamma ligand binding domain expressed in Escherichia coli BL21 after 12 hrs by liquid scintillation ... | Bioorg Med Chem 18: 3805-11 (2010) Article DOI: 10.1016/j.bmc.2010.04.045 BindingDB Entry DOI: 10.7270/Q28S4Q24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8137 (N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8126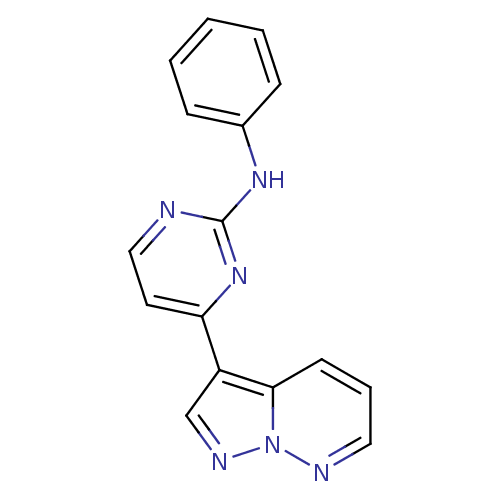 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR assessed as inhibition of 4EBP1 phosphorylation after 30 mins by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8137 (N-(3,4-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50506295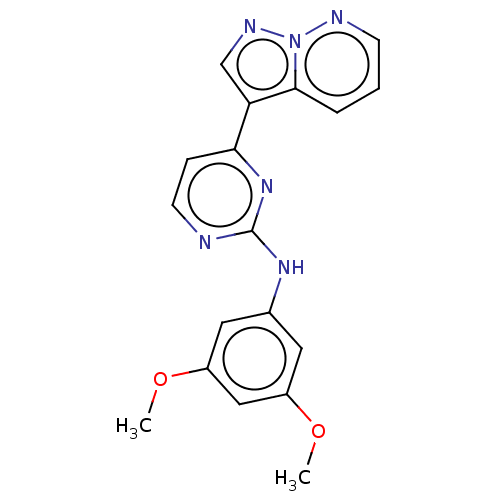 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-delta assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50506295 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50506295 (GW801372X) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8126 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-alpha assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8197 (4-{6-methyl-2-phenylpyrazolo[1,5-a]pyridazin-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241780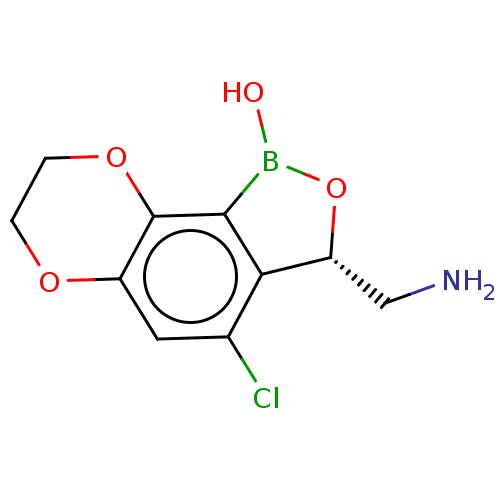 (CHEMBL4105269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8189 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-[3-(trif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8133 (N-(4-tert-butylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8152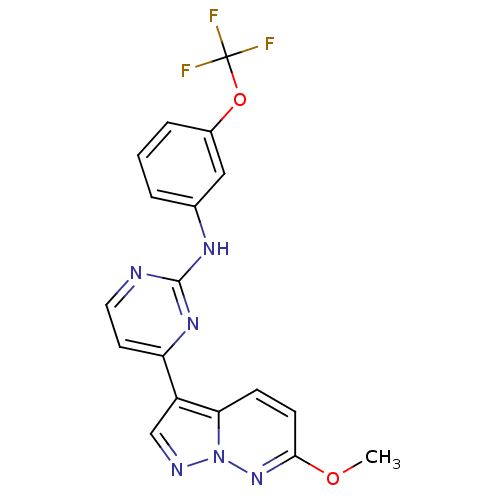 (4-{6-methoxypyrazolo[1,5-a]pyridazin-3-yl}-N-[3-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human GSK3beta using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate af... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241754 (CHEMBL4077894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against sigma receptor from guinea pig brain, using [3H](+)-3-PPP as radioligand. | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8128 (N-(3-methoxyphenyl)-4-{pyrazolo[1,5-a]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241778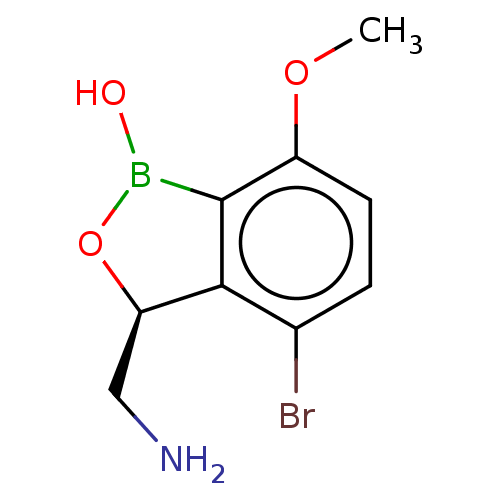 (CHEMBL4086672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241756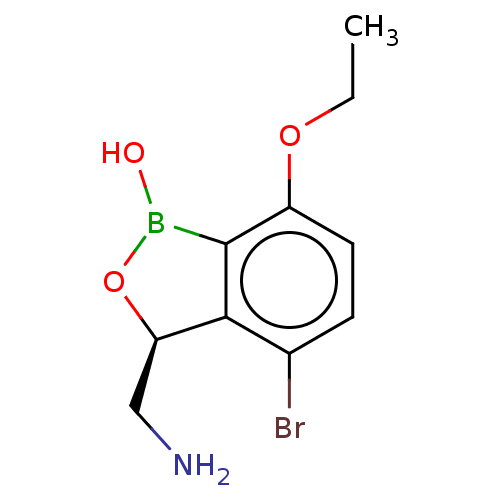 (CHEMBL4075879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241795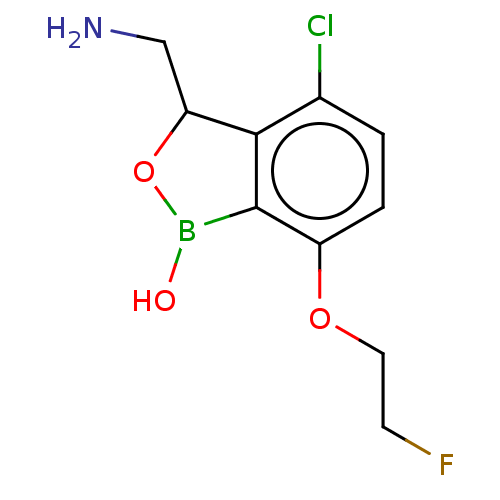 (CHEMBL4104309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241792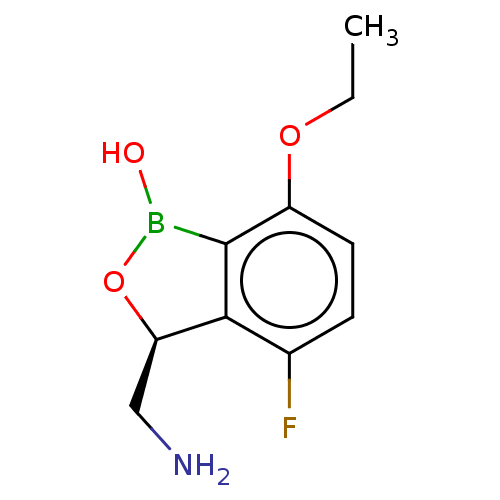 (CHEMBL4102148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241791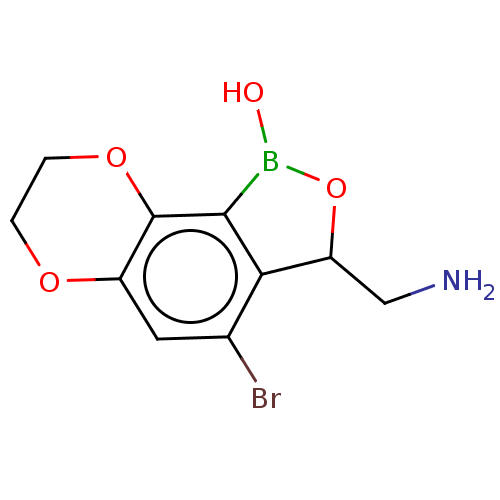 (CHEMBL4082544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM92862 (US9284315, BEZ-235 | mTOR Inhibitor, BEZ235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241782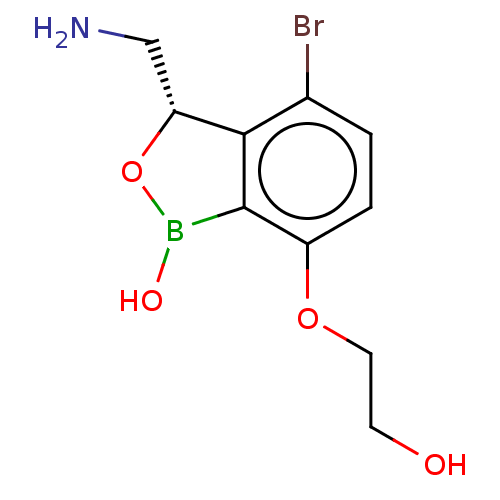 (CHEMBL4081207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241781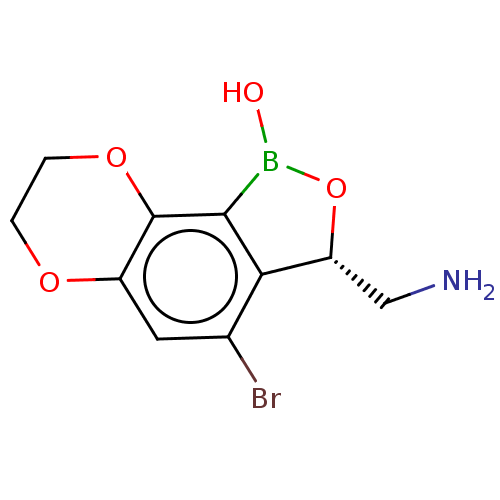 (CHEMBL4066833) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50018265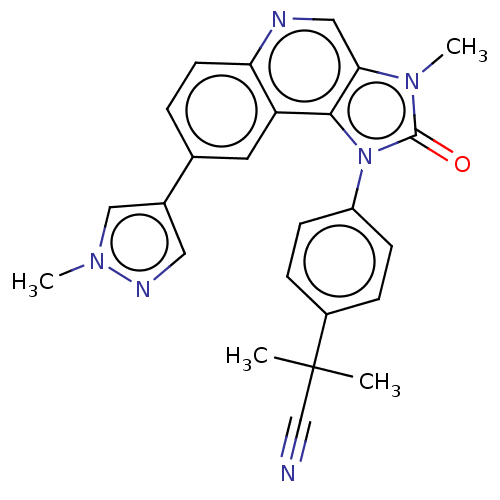 (CHEMBL3290293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human PI3K-gamma assessed as inhibition of Ptdlns(3,4,5)P3 phosphorylation after 1 hr by TR-FRET analysis | J Med Chem 57: 4834-48 (2014) Article DOI: 10.1021/jm500361r BindingDB Entry DOI: 10.7270/Q2CJ8G1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241789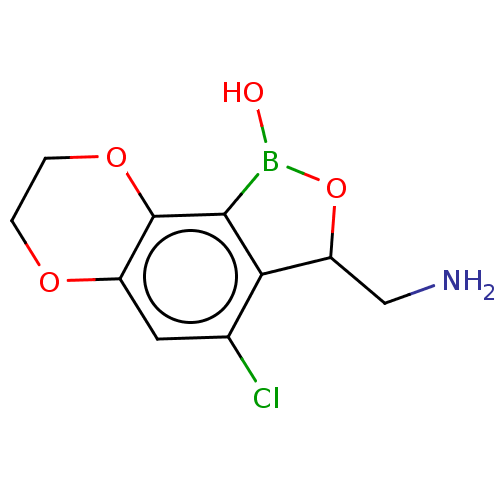 (CHEMBL4084478) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241755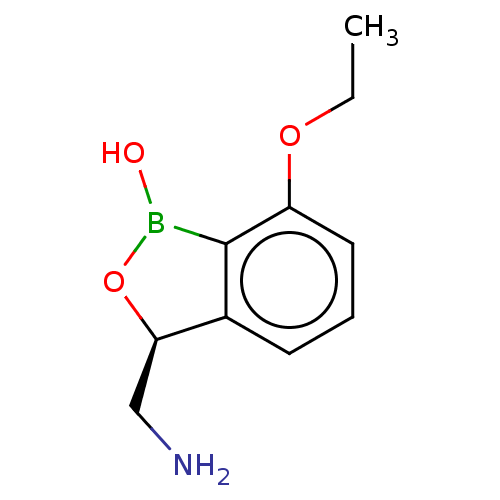 (CHEMBL4081755) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against sigma receptor from guinea pig brain, using [3H](+)-pentazocine as radioligand. | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241772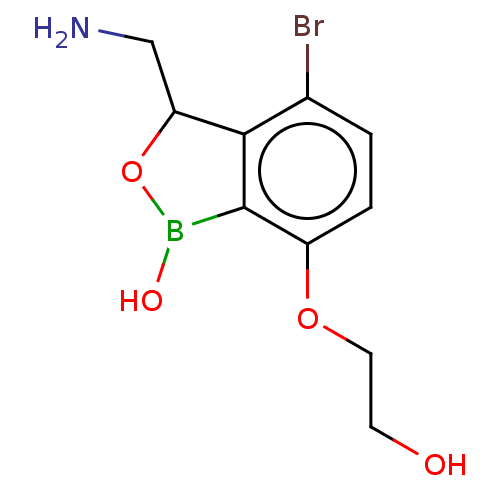 (CHEMBL4105458) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241757 (CHEMBL4066786) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM8126 (N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK4 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM8133 (N-(4-tert-butylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of human CDK2 using biotinylated-aminohexyl-Ala-Ala-Ala-Lys-Arg-Arg-Glu-Ile-Leu-Ser-Arg-Arg-Pro-Ser(PO3)-Tyr-Arg-amide as substrate after ... | J Med Chem 63: 756-783 (2020) Article DOI: 10.1021/acs.jmedchem.9b01741 BindingDB Entry DOI: 10.7270/Q2PR809G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50241769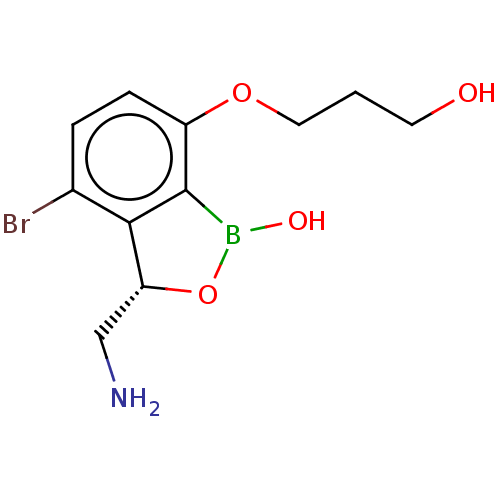 (CHEMBL4090012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv N-terminal 6His-tagged LeuRS expressed in Escherichia coli BL21(DE3) assessed as L-[14C]leucine incorp... | J Med Chem 60: 8011-8026 (2017) Article DOI: 10.1021/acs.jmedchem.7b00631 BindingDB Entry DOI: 10.7270/Q2XD13TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 217 total ) | Next | Last >> |