 Found 115 hits with Last Name = 'bergum' and Initial = 'pw'
Found 115 hits with Last Name = 'bergum' and Initial = 'pw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087645
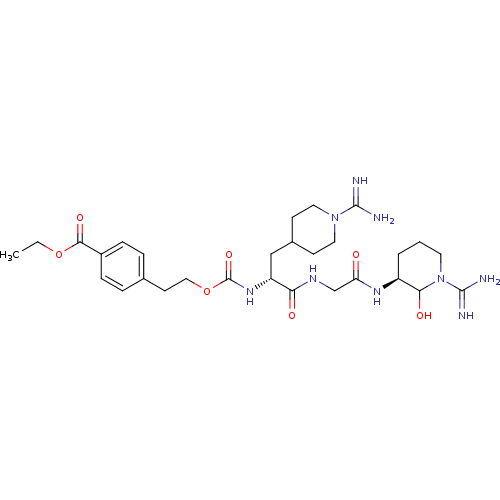
(4-{2-[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piper...)Show SMILES CCOC(=O)c1ccc(CCOC(=O)N[C@H](CC2CCN(CC2)C(N)=N)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)cc1 Show InChI InChI=1S/C29H45N9O7/c1-2-44-26(42)20-7-5-18(6-8-20)11-15-45-29(43)36-22(16-19-9-13-37(14-10-19)27(30)31)24(40)34-17-23(39)35-21-4-3-12-38(25(21)41)28(32)33/h5-8,19,21-22,25,41H,2-4,9-17H2,1H3,(H3,30,31)(H3,32,33)(H,34,40)(H,35,39)(H,36,43)/t21-,22+,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor X |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087641
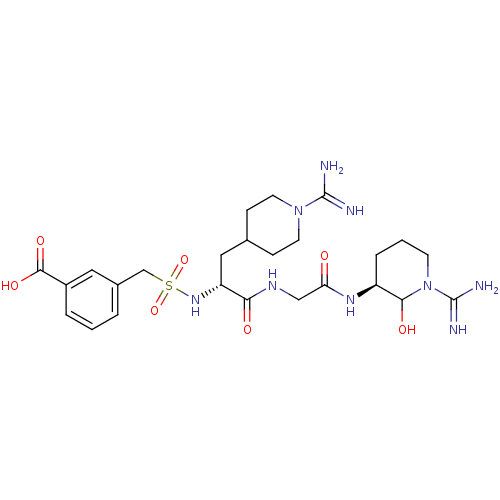
(3-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C25H39N9O7S/c26-24(27)33-9-6-15(7-10-33)12-19(32-42(40,41)14-16-3-1-4-17(11-16)23(38)39)21(36)30-13-20(35)31-18-5-2-8-34(22(18)37)25(28)29/h1,3-4,11,15,18-19,22,32,37H,2,5-10,12-14H2,(H3,26,27)(H3,28,29)(H,30,36)(H,31,35)(H,38,39)/t18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087639
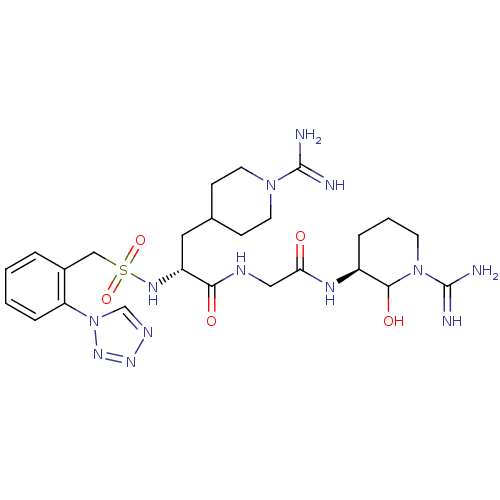
((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2ccccc2-n2cnnn2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C25H39N13O5S/c26-24(27)36-10-7-16(8-11-36)12-19(22(40)30-13-21(39)32-18-5-3-9-37(23(18)41)25(28)29)33-44(42,43)14-17-4-1-2-6-20(17)38-15-31-34-35-38/h1-2,4,6,15-16,18-19,23,33,41H,3,5,7-14H2,(H3,26,27)(H3,28,29)(H,30,40)(H,32,39)/t18-,19+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Concentration of the compound required for classical fast inhibition of cleavage of the chromogenic substrate by human enzyme Coagulation factor X in... |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087647
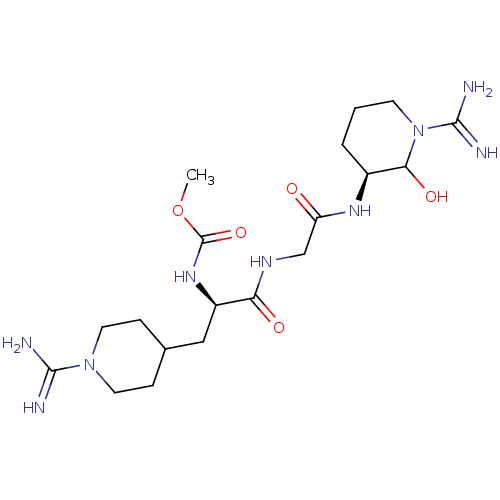
(CHEMBL162277 | [(R)-1-{[((S)-1-Carbamimidoyl-2-hyd...)Show SMILES COC(=O)N[C@H](CC1CCN(CC1)C(N)=N)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C19H35N9O5/c1-33-19(32)26-13(9-11-4-7-27(8-5-11)17(20)21)15(30)24-10-14(29)25-12-3-2-6-28(16(12)31)18(22)23/h11-13,16,31H,2-10H2,1H3,(H3,20,21)(H3,22,23)(H,24,30)(H,25,29)(H,26,32)/t12-,13+,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor X |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50073429
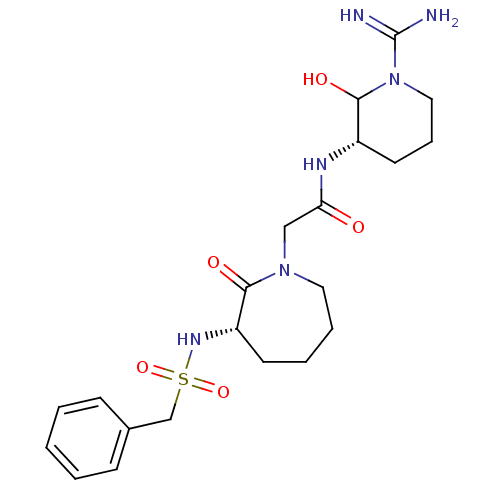
(CHEMBL82032 | CHEMBL84575 | N-((S)-1-Carbamimidoyl...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2CCCC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C21H32N6O5S/c22-21(23)27-12-6-10-16(20(27)30)24-18(28)13-26-11-5-4-9-17(19(26)29)25-33(31,32)14-15-7-2-1-3-8-15/h1-3,7-8,16-17,20,25,30H,4-6,9-14H2,(H3,22,23)(H,24,28)/t16-,17-,20?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme thrombin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087644

((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2ccccc2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C24H39N9O5S/c25-23(26)32-11-8-16(9-12-32)13-19(31-39(37,38)15-17-5-2-1-3-6-17)21(35)29-14-20(34)30-18-7-4-10-33(22(18)36)24(27)28/h1-3,5-6,16,18-19,22,31,36H,4,7-15H2,(H3,25,26)(H3,27,28)(H,29,35)(H,30,34)/t18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087635
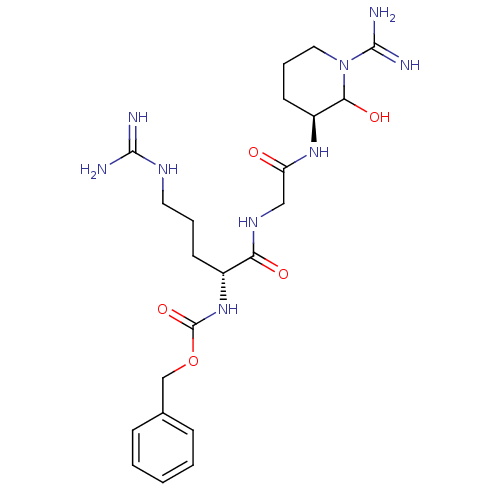
(((R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C22H35N9O5/c23-20(24)27-10-4-8-15(30-22(35)36-13-14-6-2-1-3-7-14)18(33)28-12-17(32)29-16-9-5-11-31(19(16)34)21(25)26/h1-3,6-7,15-16,19,34H,4-5,8-13H2,(H3,25,26)(H,28,33)(H,29,32)(H,30,35)(H4,23,24,27)/t15-,16+,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087635

(((R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C22H35N9O5/c23-20(24)27-10-4-8-15(30-22(35)36-13-14-6-2-1-3-7-14)18(33)28-12-17(32)29-16-9-5-11-31(19(16)34)21(25)26/h1-3,6-7,15-16,19,34H,4-5,8-13H2,(H3,25,26)(H,28,33)(H,29,32)(H,30,35)(H4,23,24,27)/t15-,16+,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM and Ramos cell VLA-4 interaction |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087635

(((R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C22H35N9O5/c23-20(24)27-10-4-8-15(30-22(35)36-13-14-6-2-1-3-7-14)18(33)28-12-17(32)29-16-9-5-11-31(19(16)34)21(25)26/h1-3,6-7,15-16,19,34H,4-5,8-13H2,(H3,25,26)(H,28,33)(H,29,32)(H,30,35)(H4,23,24,27)/t15-,16+,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM and Ramos cell VLA-4 interaction |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087638

((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C21H35N9O5S/c22-20(23)26-10-4-8-15(29-36(34,35)13-14-6-2-1-3-7-14)18(32)27-12-17(31)28-16-9-5-11-30(19(16)33)21(24)25/h1-3,6-7,15-16,19,29,33H,4-5,8-13H2,(H3,24,25)(H,27,32)(H,28,31)(H4,22,23,26)/t15-,16+,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087636
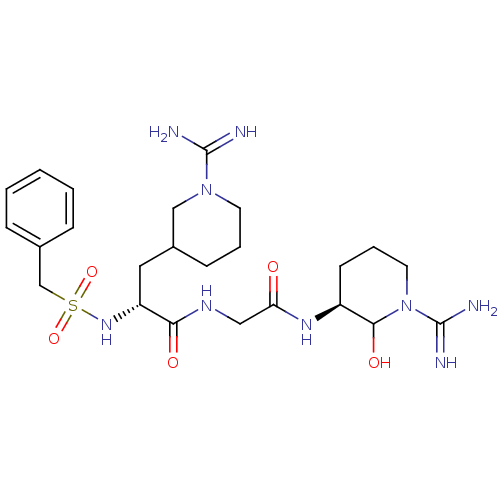
((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCCC(C[C@@H](NS(=O)(=O)Cc2ccccc2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)C1 Show InChI InChI=1S/C24H39N9O5S/c25-23(26)32-10-4-8-17(14-32)12-19(31-39(37,38)15-16-6-2-1-3-7-16)21(35)29-13-20(34)30-18-9-5-11-33(22(18)36)24(27)28/h1-3,6-7,17-19,22,31,36H,4-5,8-15H2,(H3,25,26)(H3,27,28)(H,29,35)(H,30,34)/t17?,18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087636

((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCCC(C[C@@H](NS(=O)(=O)Cc2ccccc2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)C1 Show InChI InChI=1S/C24H39N9O5S/c25-23(26)32-10-4-8-17(14-32)12-19(31-39(37,38)15-16-6-2-1-3-7-16)21(35)29-13-20(34)30-18-9-5-11-33(22(18)36)24(27)28/h1-3,6-7,17-19,22,31,36H,4-5,8-15H2,(H3,25,26)(H3,27,28)(H,29,35)(H,30,34)/t17?,18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM and Ramos cell VLA-4 interaction |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087643
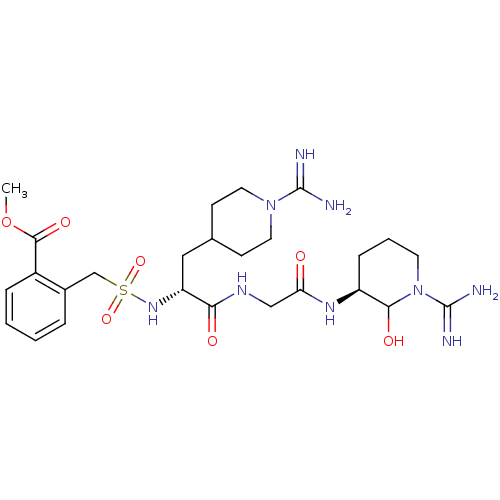
(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)N[C@H](CC1CCN(CC1)C(N)=N)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C26H41N9O7S/c1-42-24(39)18-6-3-2-5-17(18)15-43(40,41)33-20(13-16-8-11-34(12-9-16)25(27)28)22(37)31-14-21(36)32-19-7-4-10-35(23(19)38)26(29)30/h2-3,5-6,16,19-20,23,33,38H,4,7-15H2,1H3,(H3,27,28)(H3,29,30)(H,31,37)(H,32,36)/t19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087642
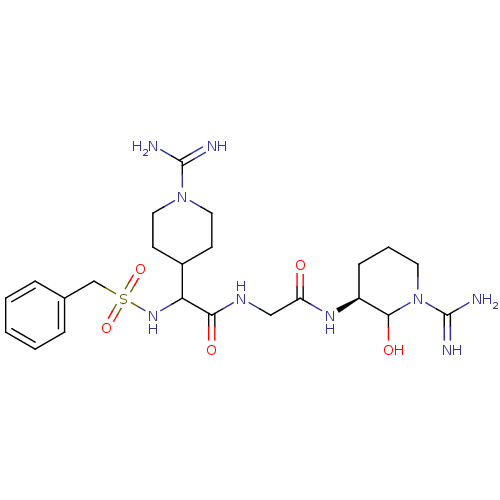
(CHEMBL163251 | N-[((S)-1-Carbamimidoyl-2-hydroxy-p...)Show SMILES NC(=N)N1CCC(CC1)C(NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C23H37N9O5S/c24-22(25)31-11-8-16(9-12-31)19(30-38(36,37)14-15-5-2-1-3-6-15)20(34)28-13-18(33)29-17-7-4-10-32(21(17)35)23(26)27/h1-3,5-6,16-17,19,21,30,35H,4,7-14H2,(H3,24,25)(H3,26,27)(H,28,34)(H,29,33)/t17-,19?,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087640

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)N[C@H](CC1CCCN(C1)C(N)=N)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C26H41N9O7S/c1-42-24(39)18-8-3-2-7-17(18)15-43(40,41)33-20(12-16-6-4-10-34(14-16)25(27)28)22(37)31-13-21(36)32-19-9-5-11-35(23(19)38)26(29)30/h2-3,7-8,16,19-20,23,33,38H,4-6,9-15H2,1H3,(H3,27,28)(H3,29,30)(H,31,37)(H,32,36)/t16?,19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISA |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087640

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)N[C@H](CC1CCCN(C1)C(N)=N)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C26H41N9O7S/c1-42-24(39)18-8-3-2-7-17(18)15-43(40,41)33-20(12-16-6-4-10-34(14-16)25(27)28)22(37)31-13-21(36)32-19-9-5-11-35(23(19)38)26(29)30/h2-3,7-8,16,19-20,23,33,38H,4-6,9-15H2,1H3,(H3,27,28)(H3,29,30)(H,31,37)(H,32,36)/t16?,19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081092
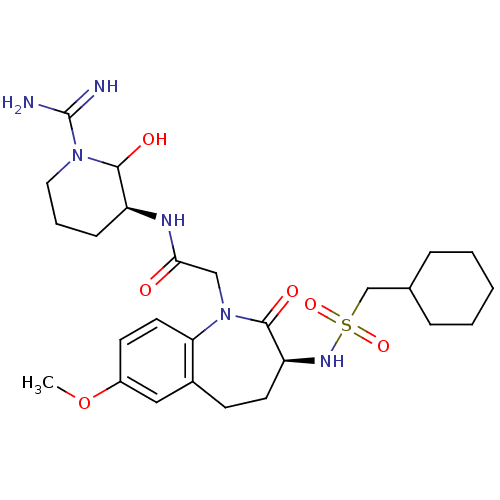
(CHEMBL85238 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)CC1CCCCC1 Show InChI InChI=1S/C26H40N6O6S/c1-38-19-10-12-22-18(14-19)9-11-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h10,12,14,17,20-21,24,30,34H,2-9,11,13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme trypsin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081092

(CHEMBL85238 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)CC1CCCCC1 Show InChI InChI=1S/C26H40N6O6S/c1-38-19-10-12-22-18(14-19)9-11-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h10,12,14,17,20-21,24,30,34H,2-9,11,13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087637
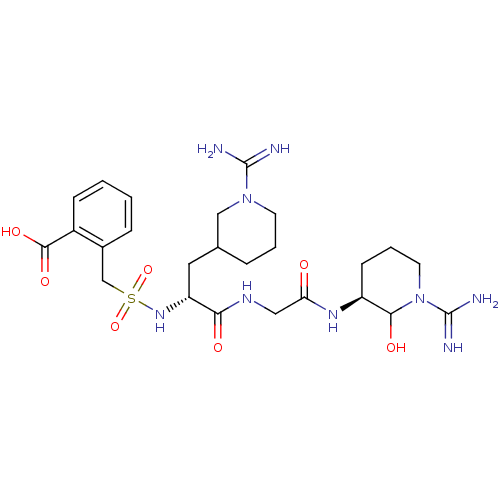
(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES NC(=N)N1CCCC(C[C@@H](NS(=O)(=O)Cc2ccccc2C(O)=O)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)C1 Show InChI InChI=1S/C25H39N9O7S/c26-24(27)33-9-3-5-15(13-33)11-19(32-42(40,41)14-16-6-1-2-7-17(16)23(38)39)21(36)30-12-20(35)31-18-8-4-10-34(22(18)37)25(28)29/h1-2,6-7,15,18-19,22,32,37H,3-5,8-14H2,(H3,26,27)(H3,28,29)(H,30,36)(H,31,35)(H,38,39)/t15?,18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISA |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087637

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES NC(=N)N1CCCC(C[C@@H](NS(=O)(=O)Cc2ccccc2C(O)=O)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)C1 Show InChI InChI=1S/C25H39N9O7S/c26-24(27)33-9-3-5-15(13-33)11-19(32-42(40,41)14-16-6-1-2-7-17(16)23(38)39)21(36)30-12-20(35)31-18-8-4-10-34(22(18)37)25(28)29/h1-2,6-7,15,18-19,22,32,37H,3-5,8-14H2,(H3,26,27)(H3,28,29)(H,30,36)(H,31,35)(H,38,39)/t15?,18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087634
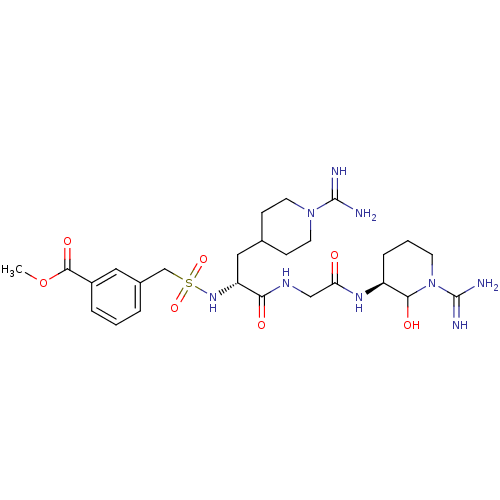
(3-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES COC(=O)c1cccc(CS(=O)(=O)N[C@H](CC2CCN(CC2)C(N)=N)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)c1 Show InChI InChI=1S/C26H41N9O7S/c1-42-24(39)18-5-2-4-17(12-18)15-43(40,41)33-20(13-16-7-10-34(11-8-16)25(27)28)22(37)31-14-21(36)32-19-6-3-9-35(23(19)38)26(29)30/h2,4-5,12,16,19-20,23,33,38H,3,6-11,13-15H2,1H3,(H3,27,28)(H3,29,30)(H,31,37)(H,32,36)/t19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087639

((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2ccccc2-n2cnnn2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C25H39N13O5S/c26-24(27)36-10-7-16(8-11-36)12-19(22(40)30-13-21(39)32-18-5-3-9-37(23(18)41)25(28)29)33-44(42,43)14-17-4-1-2-6-20(17)38-15-31-34-35-38/h1-2,4,6,15-16,18-19,23,33,41H,3,5,7-14H2,(H3,26,27)(H3,28,29)(H,30,40)(H,32,39)/t18-,19+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISA |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081088
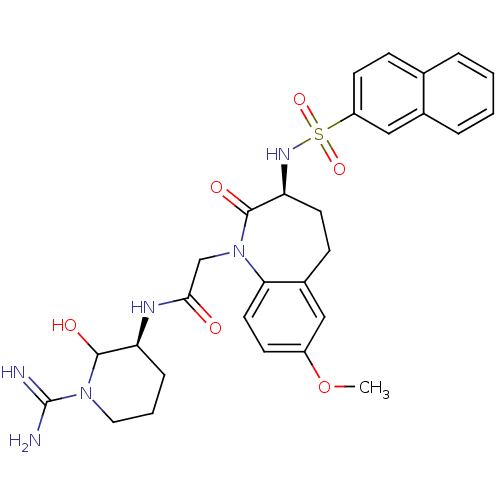
(CHEMBL316213 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C29H34N6O6S/c1-41-21-10-13-25-20(15-21)9-12-24(33-42(39,40)22-11-8-18-5-2-3-6-19(18)16-22)28(38)35(25)17-26(36)32-23-7-4-14-34(27(23)37)29(30)31/h2-3,5-6,8,10-11,13,15-16,23-24,27,33,37H,4,7,9,12,14,17H2,1H3,(H3,30,31)(H,32,36)/t23-,24-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human prothrombinase |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087641

(3-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C25H39N9O7S/c26-24(27)33-9-6-15(7-10-33)12-19(32-42(40,41)14-16-3-1-4-17(11-16)23(38)39)21(36)30-13-20(35)31-18-5-2-8-34(22(18)37)25(28)29/h1,3-4,11,15,18-19,22,32,37H,2,5-10,12-14H2,(H3,26,27)(H3,28,29)(H,30,36)(H,31,35)(H,38,39)/t18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087646

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2ccccc2C(O)=O)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C25H39N9O7S/c26-24(27)33-10-7-15(8-11-33)12-19(32-42(40,41)14-16-4-1-2-5-17(16)23(38)39)21(36)30-13-20(35)31-18-6-3-9-34(22(18)37)25(28)29/h1-2,4-5,15,18-19,22,32,37H,3,6-14H2,(H3,26,27)(H3,28,29)(H,30,36)(H,31,35)(H,38,39)/t18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit the cleavage of the chromogenic substrate by human enzyme Coagulation factor X in vitro |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087646

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES NC(=N)N1CCC(C[C@@H](NS(=O)(=O)Cc2ccccc2C(O)=O)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)CC1 Show InChI InChI=1S/C25H39N9O7S/c26-24(27)33-10-7-15(8-11-33)12-19(32-42(40,41)14-16-4-1-2-5-17(16)23(38)39)21(36)30-13-20(35)31-18-6-3-9-34(22(18)37)25(28)29/h1-2,4-5,15,18-19,22,32,37H,3,6-14H2,(H3,26,27)(H3,28,29)(H,30,36)(H,31,35)(H,38,39)/t18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human plasmin. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081087
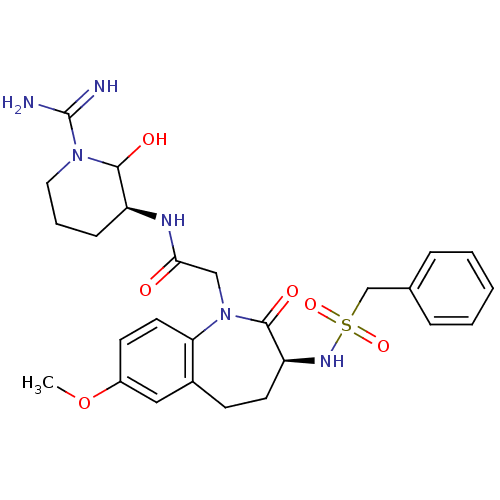
(CHEMBL87947 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H34N6O6S/c1-38-19-10-12-22-18(14-19)9-11-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h2-4,6-7,10,12,14,20-21,24,30,34H,5,8-9,11,13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081086
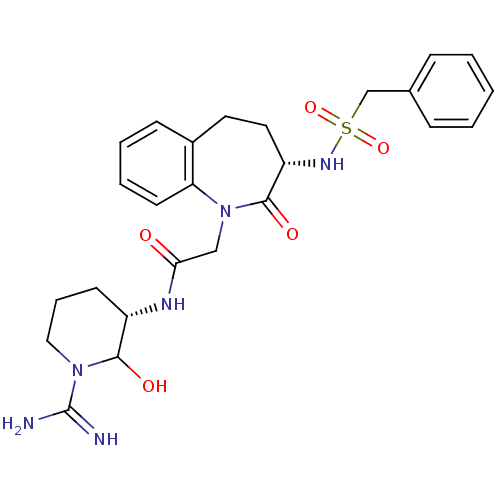
(CHEMBL314576 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2c3ccccc3CC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C25H32N6O5S/c26-25(27)30-14-6-10-19(23(30)33)28-22(32)15-31-21-11-5-4-9-18(21)12-13-20(24(31)34)29-37(35,36)16-17-7-2-1-3-8-17/h1-5,7-9,11,19-20,23,29,33H,6,10,12-16H2,(H3,26,27)(H,28,32)/t19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against prothrombinase |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081090
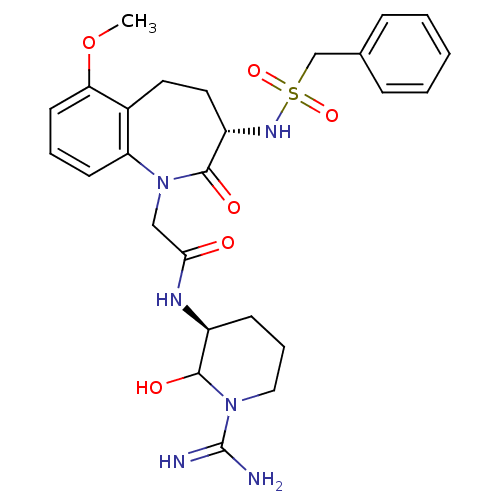
(CHEMBL87502 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1cccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc12)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H34N6O6S/c1-38-22-11-5-10-21-18(22)12-13-20(30-39(36,37)16-17-7-3-2-4-8-17)25(35)32(21)15-23(33)29-19-9-6-14-31(24(19)34)26(27)28/h2-5,7-8,10-11,19-20,24,30,34H,6,9,12-16H2,1H3,(H3,27,28)(H,29,33)/t19-,20-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081092

(CHEMBL85238 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)CC1CCCCC1 Show InChI InChI=1S/C26H40N6O6S/c1-38-19-10-12-22-18(14-19)9-11-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h10,12,14,17,20-21,24,30,34H,2-9,11,13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human prothrombinase |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50087640

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)N[C@H](CC1CCCN(C1)C(N)=N)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C26H41N9O7S/c1-42-24(39)18-8-3-2-7-17(18)15-43(40,41)33-20(12-16-6-4-10-34(14-16)25(27)28)22(37)31-13-21(36)32-19-9-5-11-35(23(19)38)26(29)30/h2-3,7-8,16,19-20,23,33,38H,4-6,9-15H2,1H3,(H3,27,28)(H3,29,30)(H,31,37)(H,32,36)/t16?,19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50087640

(2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)N[C@H](CC1CCCN(C1)C(N)=N)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C26H41N9O7S/c1-42-24(39)18-8-3-2-7-17(18)15-43(40,41)33-20(12-16-6-4-10-34(14-16)25(27)28)22(37)31-13-21(36)32-19-9-5-11-35(23(19)38)26(29)30/h2-3,7-8,16,19-20,23,33,38H,4-6,9-15H2,1H3,(H3,27,28)(H3,29,30)(H,31,37)(H,32,36)/t16?,19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human VCAM and Ramos cell VLA-4 interaction |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081086

(CHEMBL314576 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2c3ccccc3CC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C25H32N6O5S/c26-25(27)30-14-6-10-19(23(30)33)28-22(32)15-31-21-11-5-4-9-18(21)12-13-20(24(31)34)29-37(35,36)16-17-7-2-1-3-8-17/h1-5,7-9,11,19-20,23,29,33H,6,10,12-16H2,(H3,26,27)(H,28,32)/t19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human prothrombinase |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50081093
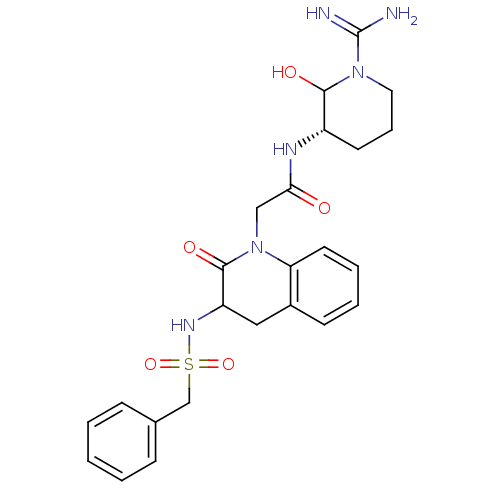
(CHEMBL88079 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2C(=O)C(Cc3ccccc23)NS(=O)(=O)Cc2ccccc2)C1O Show InChI InChI=1S/C24H30N6O5S/c25-24(26)29-12-6-10-18(22(29)32)27-21(31)14-30-20-11-5-4-9-17(20)13-19(23(30)33)28-36(34,35)15-16-7-2-1-3-8-16/h1-5,7-9,11,18-19,22,28,32H,6,10,12-15H2,(H3,25,26)(H,27,31)/t18-,19?,22?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme thrombin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081088

(CHEMBL316213 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C29H34N6O6S/c1-41-21-10-13-25-20(15-21)9-12-24(33-42(39,40)22-11-8-18-5-2-3-6-19(18)16-22)28(38)35(25)17-26(36)32-23-7-4-14-34(27(23)37)29(30)31/h2-3,5-6,8,10-11,13,15-16,23-24,27,33,37H,4,7,9,12,14,17H2,1H3,(H3,30,31)(H,32,36)/t23-,24-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human prothrombinase |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50073429

(CHEMBL82032 | CHEMBL84575 | N-((S)-1-Carbamimidoyl...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2CCCC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C21H32N6O5S/c22-21(23)27-12-6-10-16(20(27)30)24-18(28)13-26-11-5-4-9-17(19(26)29)25-33(31,32)14-15-7-2-1-3-8-15/h1-3,7-8,16-17,20,25,30H,4-6,9-14H2,(H3,22,23)(H,24,28)/t16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50087642

(CHEMBL163251 | N-[((S)-1-Carbamimidoyl-2-hydroxy-p...)Show SMILES NC(=N)N1CCC(CC1)C(NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C23H37N9O5S/c24-22(25)31-11-8-16(9-12-31)19(30-38(36,37)14-15-5-2-1-3-6-15)20(34)28-13-18(33)29-17-7-4-10-32(21(17)35)23(26)27/h1-3,5-6,16-17,19,21,30,35H,4,7-14H2,(H3,24,25)(H3,26,27)(H,28,34)(H,29,33)/t17-,19?,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081086

(CHEMBL314576 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2c3ccccc3CC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C25H32N6O5S/c26-25(27)30-14-6-10-19(23(30)33)28-22(32)15-31-21-11-5-4-9-18(21)12-13-20(24(31)34)29-37(35,36)16-17-7-2-1-3-8-17/h1-5,7-9,11,19-20,23,29,33H,6,10,12-16H2,(H3,26,27)(H,28,32)/t19-,20-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme trypsin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081088

(CHEMBL316213 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C29H34N6O6S/c1-41-21-10-13-25-20(15-21)9-12-24(33-42(39,40)22-11-8-18-5-2-3-6-19(18)16-22)28(38)35(25)17-26(36)32-23-7-4-14-34(27(23)37)29(30)31/h2-3,5-6,8,10-11,13,15-16,23-24,27,33,37H,4,7,9,12,14,17H2,1H3,(H3,30,31)(H,32,36)/t23-,24-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro evaluation of inhibition of cleavage of the chromogenic substrate by human enzyme trypsin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081090

(CHEMBL87502 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1cccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc12)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H34N6O6S/c1-38-22-11-5-10-21-18(22)12-13-20(30-39(36,37)16-17-7-3-2-4-8-17)25(35)32(21)15-23(33)29-19-9-6-14-31(24(19)34)26(27)28/h2-5,7-8,10-11,19-20,24,30,34H,6,9,12-16H2,1H3,(H3,27,28)(H,29,33)/t19-,20-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human prothrombinase |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081089
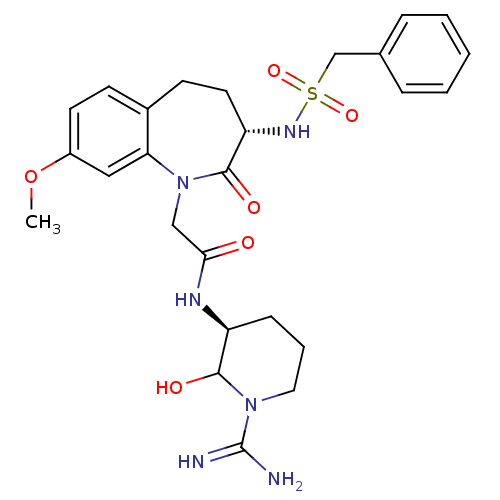
(CHEMBL315454 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES COc1ccc2CC[C@H](NS(=O)(=O)Cc3ccccc3)C(=O)N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)c2c1 Show InChI InChI=1S/C26H34N6O6S/c1-38-19-11-9-18-10-12-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22(18)14-19)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h2-4,6-7,9,11,14,20-21,24,30,34H,5,8,10,12-13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50087636

((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCCC(C[C@@H](NS(=O)(=O)Cc2ccccc2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)C1 Show InChI InChI=1S/C24H39N9O5S/c25-23(26)32-10-4-8-17(14-32)12-19(31-39(37,38)15-16-6-2-1-3-7-16)21(35)29-13-20(34)30-18-9-5-11-33(22(18)36)24(27)28/h1-3,6-7,17-19,22,31,36H,4-5,8-15H2,(H3,25,26)(H3,27,28)(H,29,35)(H,30,34)/t17?,18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50087636

((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...)Show SMILES NC(=N)N1CCCC(C[C@@H](NS(=O)(=O)Cc2ccccc2)C(=O)NCC(=O)N[C@H]2CCCN(C2O)C(N)=N)C1 Show InChI InChI=1S/C24H39N9O5S/c25-23(26)32-10-4-8-17(14-32)12-19(31-39(37,38)15-16-6-2-1-3-7-16)21(35)29-13-20(34)30-18-9-5-11-33(22(18)36)24(27)28/h1-3,6-7,17-19,22,31,36H,4-5,8-15H2,(H3,25,26)(H3,27,28)(H,29,35)(H,30,34)/t17?,18-,19+,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Coagulation factor X. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081087

(CHEMBL87947 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1ccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc2c1)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H34N6O6S/c1-38-19-10-12-22-18(14-19)9-11-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h2-4,6-7,10,12,14,20-21,24,30,34H,5,8-9,11,13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081089

(CHEMBL315454 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES COc1ccc2CC[C@H](NS(=O)(=O)Cc3ccccc3)C(=O)N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)c2c1 Show InChI InChI=1S/C26H34N6O6S/c1-38-19-11-9-18-10-12-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22(18)14-19)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h2-4,6-7,9,11,14,20-21,24,30,34H,5,8,10,12-13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro evaluation of inhibition of cleavage of the chromogenic substrate by human enzyme trypsin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081091
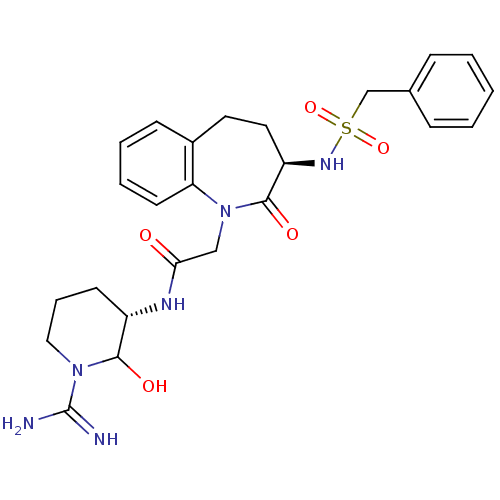
(CHEMBL316376 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2c3ccccc3CC[C@@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C25H32N6O5S/c26-25(27)30-14-6-10-19(23(30)33)28-22(32)15-31-21-11-5-4-9-18(21)12-13-20(24(31)34)29-37(35,36)16-17-7-2-1-3-8-17/h1-5,7-9,11,19-20,23,29,33H,6,10,12-16H2,(H3,26,27)(H,28,32)/t19-,20+,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081089

(CHEMBL315454 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...)Show SMILES COc1ccc2CC[C@H](NS(=O)(=O)Cc3ccccc3)C(=O)N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)c2c1 Show InChI InChI=1S/C26H34N6O6S/c1-38-19-11-9-18-10-12-21(30-39(36,37)16-17-6-3-2-4-7-17)25(35)32(22(18)14-19)15-23(33)29-20-8-5-13-31(24(20)34)26(27)28/h2-4,6-7,9,11,14,20-21,24,30,34H,5,8,10,12-13,15-16H2,1H3,(H3,27,28)(H,29,33)/t20-,21-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme Coagulation factor X |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50073429

(CHEMBL82032 | CHEMBL84575 | N-((S)-1-Carbamimidoyl...)Show SMILES NC(=N)N1CCC[C@H](NC(=O)CN2CCCC[C@H](NS(=O)(=O)Cc3ccccc3)C2=O)C1O Show InChI InChI=1S/C21H32N6O5S/c22-21(23)27-12-6-10-16(20(27)30)24-18(28)13-26-11-5-4-9-17(19(26)29)25-33(31,32)14-15-7-2-1-3-8-15/h1-3,7-8,16-17,20,25,30H,4-6,9-14H2,(H3,22,23)(H,24,28)/t16-,17-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against human enzyme trypsin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50087638

((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...)Show SMILES NC(=N)NCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)N[C@H]1CCCN(C1O)C(N)=N Show InChI InChI=1S/C21H35N9O5S/c22-20(23)26-10-4-8-15(29-36(34,35)13-14-6-2-1-3-7-14)18(32)27-12-17(31)28-16-9-5-11-30(19(16)33)21(24)25/h1-3,6-7,15-16,19,29,33H,4-5,8-13H2,(H3,24,25)(H,27,32)(H,28,31)(H4,22,23,26)/t15-,16+,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 10: 745-9 (2000)
BindingDB Entry DOI: 10.7270/Q2D50M5R |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50081090

(CHEMBL87502 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...)Show SMILES COc1cccc2N(CC(=O)N[C@H]3CCCN(C3O)C(N)=N)C(=O)[C@H](CCc12)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C26H34N6O6S/c1-38-22-11-5-10-21-18(22)12-13-20(30-39(36,37)16-17-7-3-2-4-8-17)25(35)32(21)15-23(33)29-19-9-6-14-31(24(19)34)26(27)28/h2-5,7-8,10-11,19-20,24,30,34H,6,9,12-16H2,1H3,(H3,27,28)(H,29,33)/t19-,20-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc.
Curated by ChEMBL
| Assay Description
In vitro evaluation of inhibition of cleavage of the chromogenic substrate by human enzyme trypsin |
Bioorg Med Chem Lett 9: 2573-8 (1999)
BindingDB Entry DOI: 10.7270/Q26M361R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data