 Found 496 hits with Last Name = 'berry' and Initial = 'p'
Found 496 hits with Last Name = 'berry' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50044424
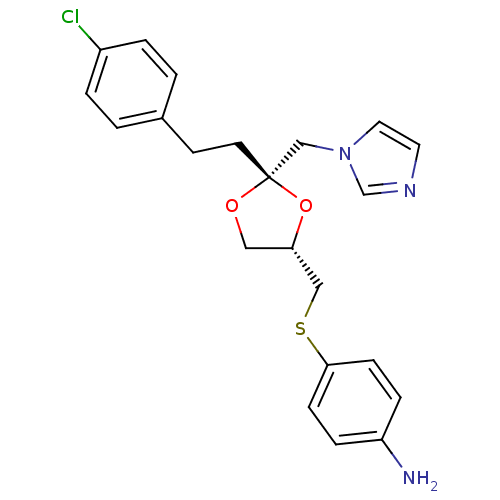
(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 19A1 |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM50161586

(4-{(2S,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Apparent Ki for rat Lanosterol 14-alpha demethylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 19A1 |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for corticoid 11-beta-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 6-beta-hydroxylase of hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B1, mitochondrial
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for corticoid 11-beta-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase of rat hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 17alpha,20-lyase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase in hamster hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Inhibition of lanosterol 14-alpha-demethylase of rat hepatic microsomes |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044425

(4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazo...)Show SMILES Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for cholesterol 17-alpha-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Rattus norvegicus) | BDBM31653

(CHEMBL421109 | imidazole-dioxolane, 5)Show SMILES Nc1ccc(SC[C@@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 |r| Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Apparent Ki for rat Lanosterol 14-alpha demethylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for progesterone 17-alpha,20-lyase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50044424

(4-(((2S,4S)-2-((1H-imidazol-1-yl)methyl)-2-(4-chlo...)Show SMILES Nc1ccc(SC[C@@H]2CO[C@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1 Show InChI InChI=1S/C22H24ClN3O2S/c23-18-3-1-17(2-4-18)9-10-22(15-26-12-11-25-16-26)27-13-20(28-22)14-29-21-7-5-19(24)6-8-21/h1-8,11-12,16,20H,9-10,13-15,24H2/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by ChEMBL
| Assay Description
Binding affinity for cholesterol 17-alpha-hydroxylase |
J Med Chem 36: 2235-7 (1993)
BindingDB Entry DOI: 10.7270/Q2ZW1K0P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426601
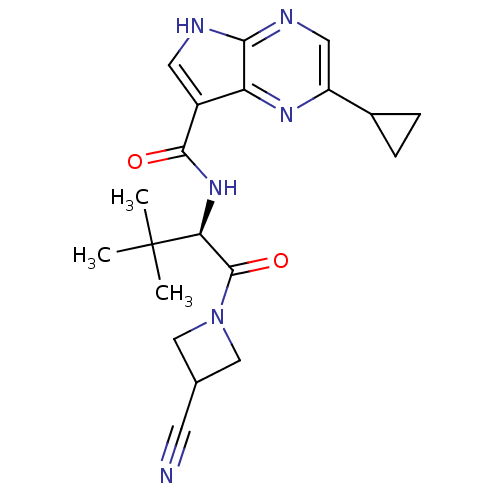
(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426599
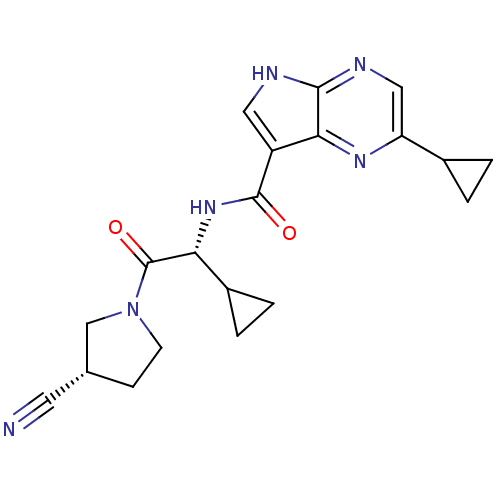
(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50426607
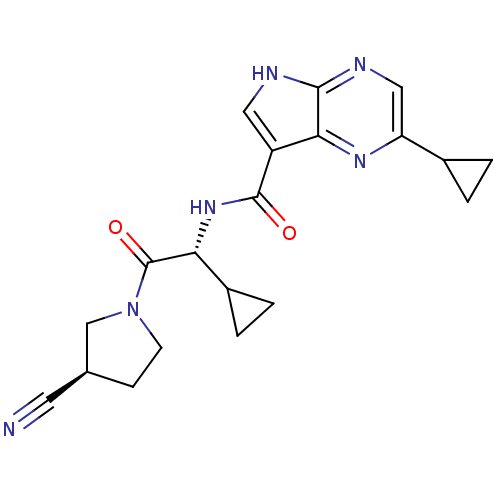
(CHEMBL2325897)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50426599

(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50426599

(CHEMBL2325898)Show SMILES O=C(N[C@H](C1CC1)C(=O)N1CC[C@@H](C1)C#N)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C20H22N6O2/c21-7-11-5-6-26(10-11)20(28)16(13-3-4-13)25-19(27)14-8-22-18-17(14)24-15(9-23-18)12-1-2-12/h8-9,11-13,16H,1-6,10H2,(H,22,23)(H,25,27)/t11-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50426601

(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112575
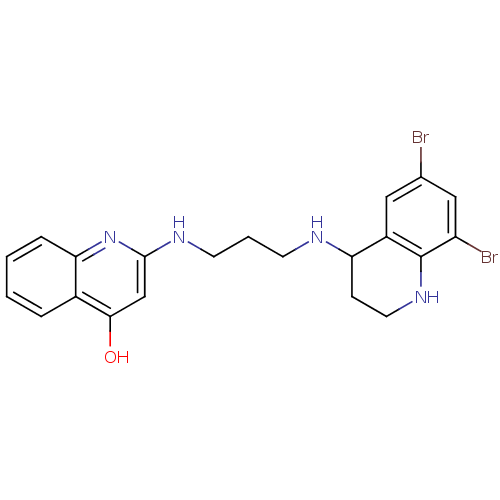
(2-[3-(6,8-Dibromo-1,2,3,4-tetrahydro-quinolin-4-yl...)Show SMILES Oc1cc(NCCCNC2CCNc3c(Br)cc(Br)cc23)nc2ccccc12 Show InChI InChI=1S/C21H22Br2N4O/c22-13-10-15-17(6-9-26-21(15)16(23)11-13)24-7-3-8-25-20-12-19(28)14-4-1-2-5-18(14)27-20/h1-2,4-5,10-12,17,24,26H,3,6-9H2,(H2,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305799

(5-((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbo...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C31H35F2N5O2/c1-20-10-26(13-34)35-21(2)27(20)29(40)37-16-23-14-36(15-24(23)17-37)9-8-30(25-6-4-3-5-7-25)18-38(19-30)28(39)22-11-31(32,33)12-22/h3-7,10,22-24H,8-9,11-12,14-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50329256
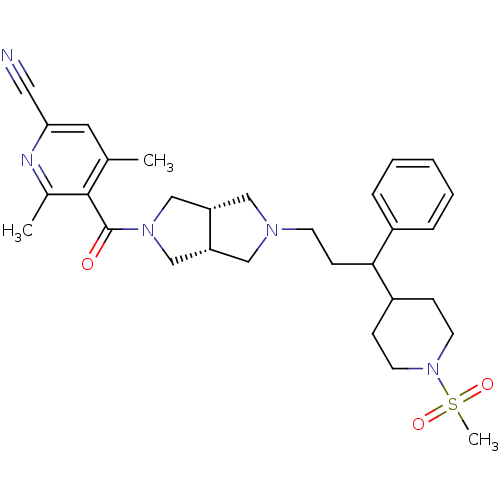
(4,6-dimethyl-5-((3aR,6aS)-5-(3-(1-(methylsulfonyl)...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC(C3CCN(CC3)S(C)(=O)=O)c3ccccc3)C[C@@H]2C1)C#N |r| Show InChI InChI=1S/C30H39N5O3S/c1-21-15-27(16-31)32-22(2)29(21)30(36)34-19-25-17-33(18-26(25)20-34)12-11-28(23-7-5-4-6-8-23)24-9-13-35(14-10-24)39(3,37)38/h4-8,15,24-26,28H,9-14,17-20H2,1-3H3/t25-,26+,28? | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CCR5 by cell-cell fusion inhibition assay |
Bioorg Med Chem Lett 20: 6802-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.118
BindingDB Entry DOI: 10.7270/Q29G5N1F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426606
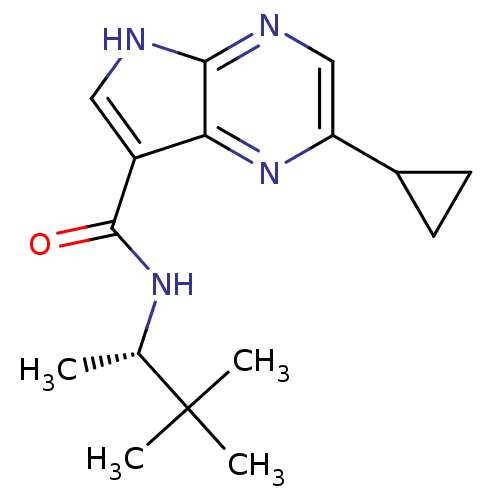
(CHEMBL2325903)Show SMILES C[C@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(C)(C)C |r| Show InChI InChI=1S/C16H22N4O/c1-9(16(2,3)4)19-15(21)11-7-17-14-13(11)20-12(8-18-14)10-5-6-10/h7-10H,5-6H2,1-4H3,(H,17,18)(H,19,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426604

(CHEMBL2325906)Show SMILES C[C@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(C)(C)C#N |r| Show InChI InChI=1S/C16H19N5O/c1-9(16(2,3)8-17)20-15(22)11-6-18-14-13(11)21-12(7-19-14)10-4-5-10/h6-7,9-10H,4-5H2,1-3H3,(H,18,19)(H,20,22)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305800
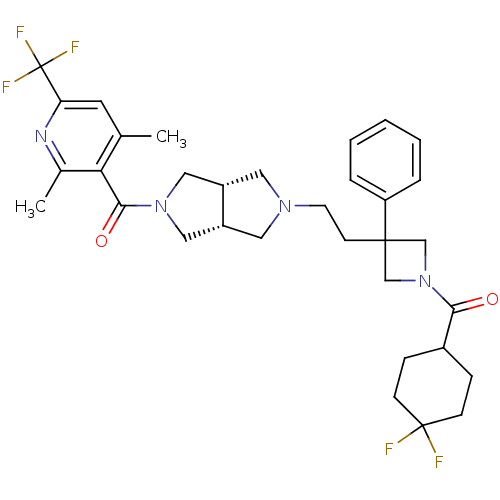
(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C33H39F5N4O2/c1-21-14-27(33(36,37)38)39-22(2)28(21)30(44)41-17-24-15-40(16-25(24)18-41)13-12-31(26-6-4-3-5-7-26)19-42(20-31)29(43)23-8-10-32(34,35)11-9-23/h3-7,14,23-25H,8-13,15-20H2,1-2H3/t24-,25+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318447
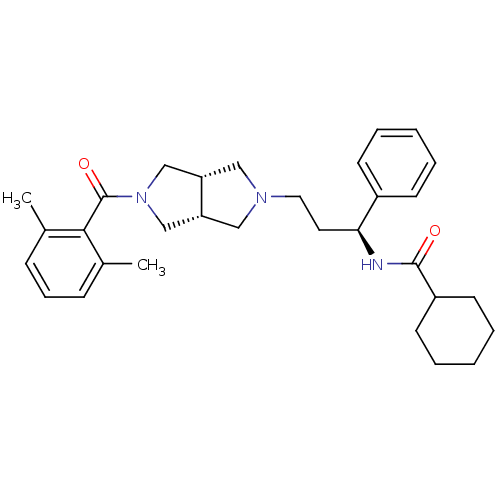
(CHEMBL1096764 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H41N3O2/c1-22-10-9-11-23(2)29(22)31(36)34-20-26-18-33(19-27(26)21-34)17-16-28(24-12-5-3-6-13-24)32-30(35)25-14-7-4-8-15-25/h3,5-6,9-13,25-28H,4,7-8,14-21H2,1-2H3,(H,32,35)/t26-,27+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305801
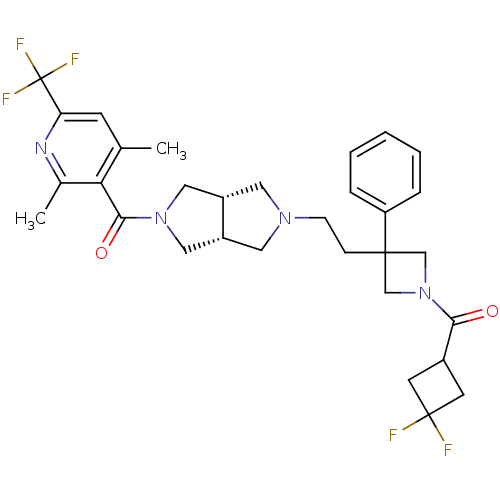
(((3aR,6aS)-5-(2-(1-(3,3-difluorocyclobutanecarbony...)Show SMILES Cc1cc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CC(F)(F)C3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C31H35F5N4O2/c1-19-10-25(31(34,35)36)37-20(2)26(19)28(42)39-15-22-13-38(14-23(22)16-39)9-8-29(24-6-4-3-5-7-24)17-40(18-29)27(41)21-11-30(32,33)12-21/h3-7,10,21-23H,8-9,11-18H2,1-2H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50426601

(CHEMBL2325895)Show SMILES CC(C)(C)[C@@H](NC(=O)c1c[nH]c2ncc(nc12)C1CC1)C(=O)N1CC(C1)C#N |r| Show InChI InChI=1S/C20H24N6O2/c1-20(2,3)16(19(28)26-9-11(6-21)10-26)25-18(27)13-7-22-17-15(13)24-14(8-23-17)12-4-5-12/h7-8,11-12,16H,4-5,9-10H2,1-3H3,(H,22,23)(H,25,27)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha/beta subunit
(Staphylococcus aureus) | BDBM18116
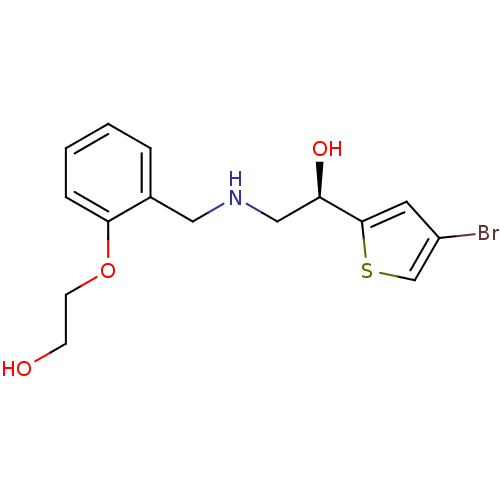
(2-[2-({[(2R)-2-(4-bromothiophen-2-yl)-2-hydroxyeth...)Show InChI InChI=1S/C15H18BrNO3S/c16-12-7-15(21-10-12)13(19)9-17-8-11-3-1-2-4-14(11)20-6-5-18/h1-4,7,10,13,17-19H,5-6,8-9H2/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 2.0 | n/a |
GSK
| Assay Description
The reactions were performed in 96-well Packard 384-well Packard Optiplates. After 10 min of incubation, the reaction was stopped by addition of a co... |
Bioorg Med Chem Lett 15: 2305-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.003
BindingDB Entry DOI: 10.7270/Q25D8Q3C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318446
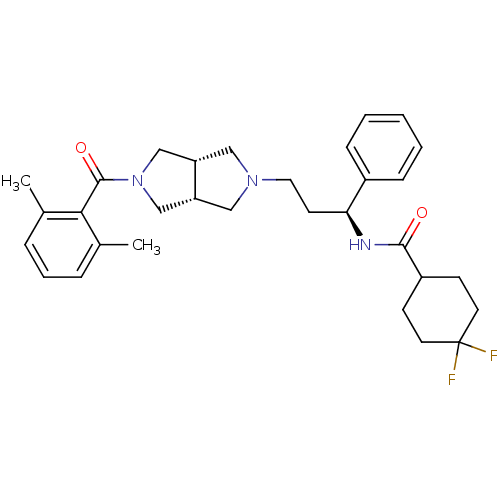
(CHEMBL1096765 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H39F2N3O2/c1-21-7-6-8-22(2)28(21)30(38)36-19-25-17-35(18-26(25)20-36)16-13-27(23-9-4-3-5-10-23)34-29(37)24-11-14-31(32,33)15-12-24/h3-10,24-27H,11-20H2,1-2H3,(H,34,37)/t25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50305794

(((3aR,6aS)-5-(2-(1-(4,4-difluorocyclohexanecarbony...)Show SMILES Cc1nc(nc(C)c1C(=O)N1C[C@@H]2CN(CCC3(CN(C3)C(=O)C3CCC(F)(F)CC3)c3ccccc3)C[C@@H]2C1)C(F)(F)F |r| Show InChI InChI=1S/C32H38F5N5O2/c1-20-26(21(2)39-29(38-20)32(35,36)37)28(44)41-16-23-14-40(15-24(23)17-41)13-12-30(25-6-4-3-5-7-25)18-42(19-30)27(43)22-8-10-31(33,34)11-9-22/h3-7,22-24H,8-19H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CCR5 by cell-cell fusion assay |
Bioorg Med Chem Lett 20: 704-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.072
BindingDB Entry DOI: 10.7270/Q22R3RSC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50319434
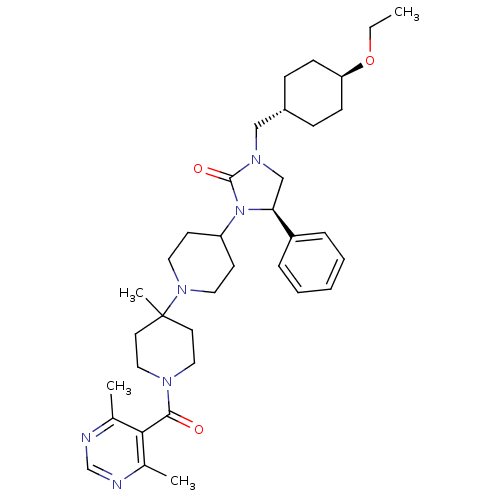
((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...)Show SMILES CCO[C@H]1CC[C@H](CN2C[C@H](N(C3CCN(CC3)C3(C)CCN(CC3)C(=O)c3c(C)ncnc3C)C2=O)c2ccccc2)CC1 |r,wU:10.40,6.6,wD:3.2,(13.9,-8.06,;13.92,-6.52,;15.26,-5.76,;15.27,-4.22,;16.61,-3.46,;16.63,-1.92,;15.29,-1.14,;15.3,.4,;16.64,1.16,;16.57,2.7,;18.01,3.24,;18.97,2.04,;20.51,2.11,;21.23,3.48,;22.77,3.56,;23.6,2.25,;22.89,.87,;21.34,.81,;25.13,2.32,;24.35,3.65,;25.96,1.01,;27.49,1.07,;28.21,2.44,;27.38,3.74,;25.84,3.68,;29.75,2.5,;30.46,3.86,;30.57,1.19,;32.1,1.26,;32.81,2.63,;32.92,-.04,;32.21,-1.41,;30.67,-1.47,;29.85,-.17,;28.31,-.22,;18.12,.75,;18.67,-.69,;18.79,4.57,;20.33,4.56,;21.11,5.89,;20.34,7.22,;18.8,7.23,;18.03,5.9,;13.96,-1.9,;13.95,-3.44,)| Show InChI InChI=1S/C36H52N6O3/c1-5-45-31-13-11-28(12-14-31)23-40-24-32(29-9-7-6-8-10-29)42(35(40)44)30-15-19-41(20-16-30)36(4)17-21-39(22-18-36)34(43)33-26(2)37-25-38-27(33)3/h6-10,25,28,30-32H,5,11-24H2,1-4H3/t28-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay |
Bioorg Med Chem Lett 20: 3219-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.077
BindingDB Entry DOI: 10.7270/Q2FT8M63 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318451

(CHEMBL1096445 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H39N3O2/c1-21-9-8-10-22(2)28(21)30(35)33-19-25-17-32(18-26(25)20-33)16-15-27(23-11-4-3-5-12-23)31-29(34)24-13-6-7-14-24/h3-5,8-12,24-27H,6-7,13-20H2,1-2H3,(H,31,34)/t25-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318439

(CHEMBL1097815 | N-((S)-3-((3aR,6aS)-5-(4-fluoro-2,...)Show SMILES Cc1cc(F)cc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C30H38FN3O2/c1-20-14-26(31)15-21(2)28(20)30(36)34-18-24-16-33(17-25(24)19-34)13-12-27(22-8-4-3-5-9-22)32-29(35)23-10-6-7-11-23/h3-5,8-9,14-15,23-25,27H,6-7,10-13,16-19H2,1-2H3,(H,32,35)/t24-,25+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50115528
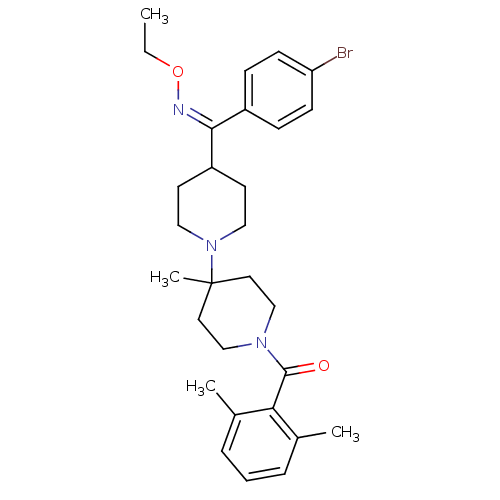
((Z)-(4-((4-bromophenyl)(ethoxyimino)methyl)-4'-met...)Show SMILES CCO\N=C(\C1CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cccc1C)c1ccc(Br)cc1 Show InChI InChI=1S/C29H38BrN3O2/c1-5-35-31-27(23-9-11-25(30)12-10-23)24-13-17-33(18-14-24)29(4)15-19-32(20-16-29)28(34)26-21(2)7-6-8-22(26)3/h6-12,24H,5,13-20H2,1-4H3/b31-27+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50310731
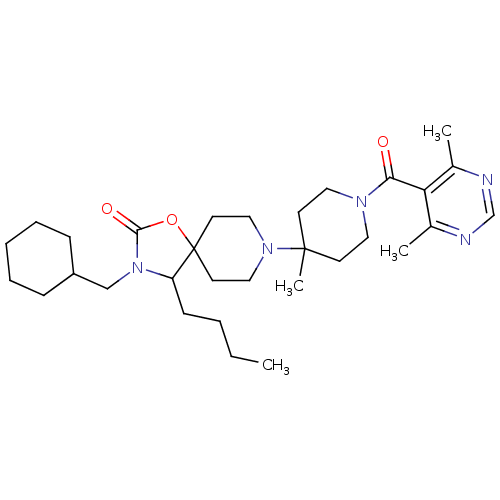
(4-butyl-3-(cyclohexylmethyl)-8-(1-(4,6-dimethylpyr...)Show SMILES CCCCC1N(CC2CCCCC2)C(=O)OC11CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C Show InChI InChI=1S/C31H49N5O3/c1-5-6-12-26-31(39-29(38)36(26)21-25-10-8-7-9-11-25)15-19-35(20-16-31)30(4)13-17-34(18-14-30)28(37)27-23(2)32-22-33-24(27)3/h22,25-26H,5-21H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells |
Bioorg Med Chem Lett 19: 5401-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.122
BindingDB Entry DOI: 10.7270/Q25H7GD5 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318448

(CHEMBL1096768 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H37N3O2/c1-20-8-6-9-21(2)27(20)29(34)32-18-24-16-31(17-25(24)19-32)15-14-26(22-10-4-3-5-11-22)30-28(33)23-12-7-13-23/h3-6,8-11,23-26H,7,12-19H2,1-2H3,(H,30,33)/t24-,25+,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318436

(CHEMBL1097169 | N-((S)-3-((3aR,6aS)-5-(2,4-dimethy...)Show SMILES Cc1ccnc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)C3CCCC3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C29H38N4O2/c1-20-12-14-30-21(2)27(20)29(35)33-18-24-16-32(17-25(24)19-33)15-13-26(22-8-4-3-5-9-22)31-28(34)23-10-6-7-11-23/h3-5,8-9,12,14,23-26H,6-7,10-11,13,15-19H2,1-2H3,(H,31,34)/t24-,25+,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50426616

(CHEMBL2325899)Show SMILES CC(C)[C@H](C)NC(=O)c1c[nH]c2ncc(nc12)C1CC1 |r| Show InChI InChI=1S/C15H20N4O/c1-8(2)9(3)18-15(20)11-6-16-14-13(11)19-12(7-17-14)10-4-5-10/h6-10H,4-5H2,1-3H3,(H,16,17)(H,18,20)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... |
J Med Chem 56: 345-56 (2013)
Article DOI: 10.1021/jm301646k
BindingDB Entry DOI: 10.7270/Q2Q241JX |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50319451
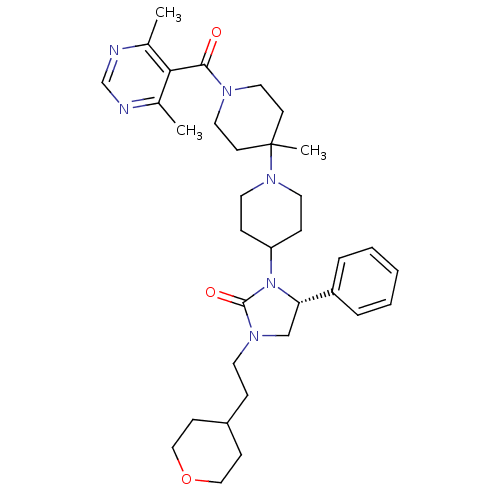
((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...)Show SMILES Cc1ncnc(C)c1C(=O)N1CCC(C)(CC1)N1CCC(CC1)N1[C@@H](CN(CCC2CCOCC2)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C34H48N6O3/c1-25-31(26(2)36-24-35-25)32(41)37-19-14-34(3,15-20-37)39-17-10-29(11-18-39)40-30(28-7-5-4-6-8-28)23-38(33(40)42)16-9-27-12-21-43-22-13-27/h4-8,24,27,29-30H,9-23H2,1-3H3/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay |
Bioorg Med Chem Lett 20: 3219-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.077
BindingDB Entry DOI: 10.7270/Q2FT8M63 |
More data for this
Ligand-Target Pair | |
Phenylalanine--tRNA ligase alpha/beta subunit
(Staphylococcus aureus) | BDBM18093

(2-[2-({[2-(4-bromothiophen-2-yl)-2-hydroxyethyl]am...)Show InChI InChI=1S/C15H18BrNO3S/c16-12-7-15(21-10-12)13(19)9-17-8-11-3-1-2-4-14(11)20-6-5-18/h1-4,7,10,13,17-19H,5-6,8-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.9 | 37 |
GSK
| Assay Description
The reactions were performed in 96-well Packard 384-well Packard Optiplates. After 10 min of incubation, the reaction was stopped by addition of a co... |
Bioorg Med Chem Lett 15: 2305-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.003
BindingDB Entry DOI: 10.7270/Q25D8Q3C |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50318445

(CHEMBL1096766 | N-((S)-3-((3aR,6aS)-5-(2,6-dimethy...)Show SMILES Cc1cccc(C)c1C(=O)N1C[C@@H]2CN(CC[C@H](NC(=O)c3ccccc3)c3ccccc3)C[C@@H]2C1 |r| Show InChI InChI=1S/C31H35N3O2/c1-22-10-9-11-23(2)29(22)31(36)34-20-26-18-33(19-27(26)21-34)17-16-28(24-12-5-3-6-13-24)32-30(35)25-14-7-4-8-15-25/h3-15,26-28H,16-21H2,1-2H3,(H,32,35)/t26-,27+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Binding affinity at CCR5 receptor by radiolabeled RANTES binding assay |
Bioorg Med Chem Lett 20: 3116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.095
BindingDB Entry DOI: 10.7270/Q27S7PQ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data