

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212110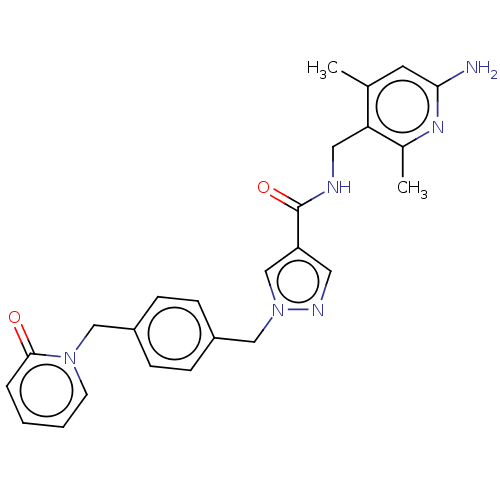 (US9290485, 142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0224 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212116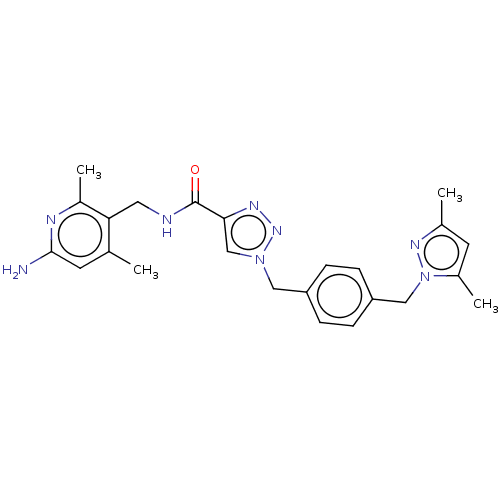 (US9290485, 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212114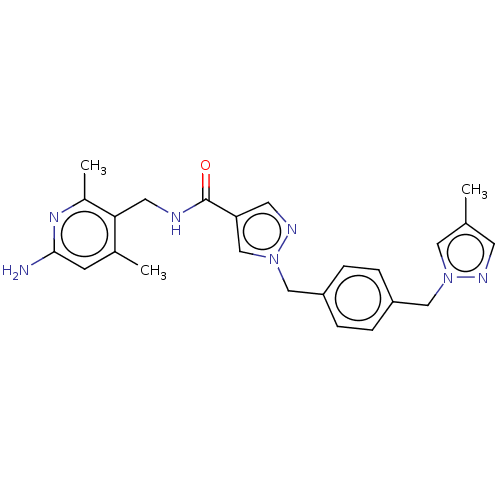 (US9290485, 146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM211977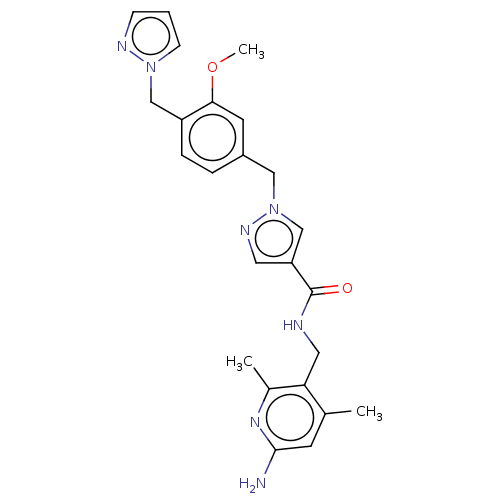 (US9290485, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0912 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212115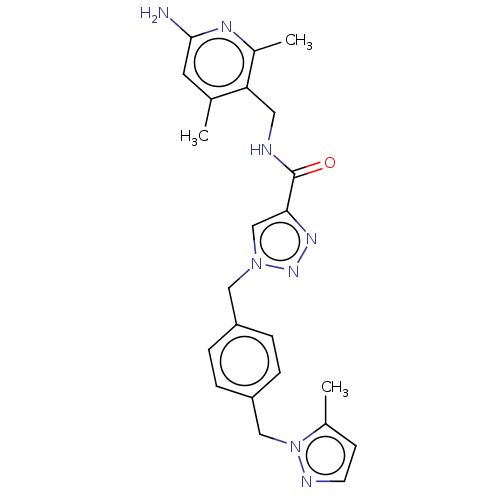 (US9290485, 147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212136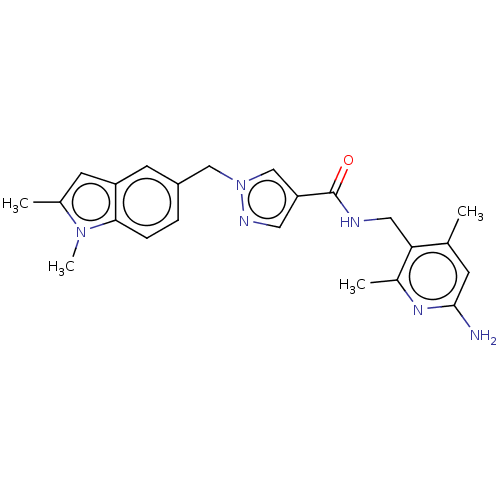 (US9290485, 168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212004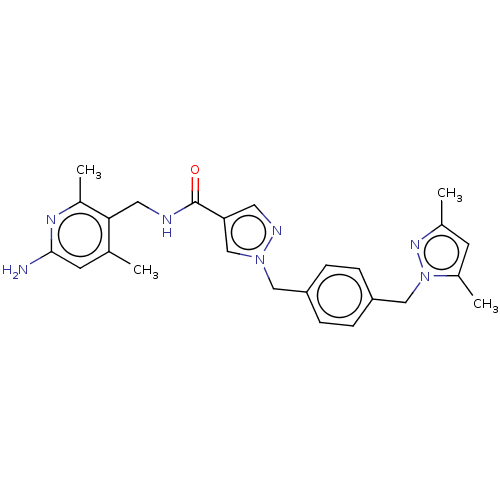 (US9290485, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212135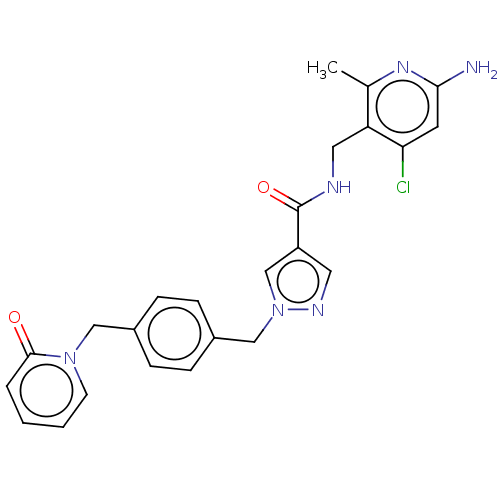 (US9290485, 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212134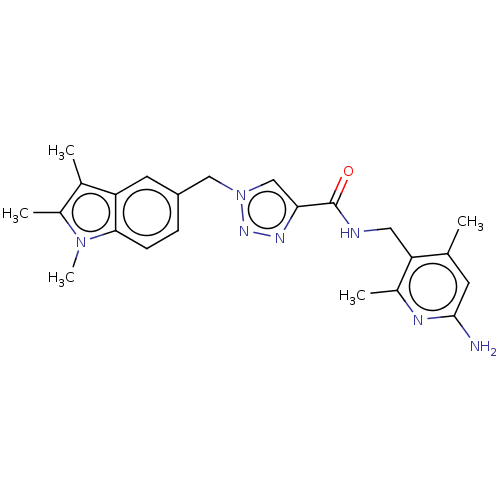 (US9290485, 166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM211974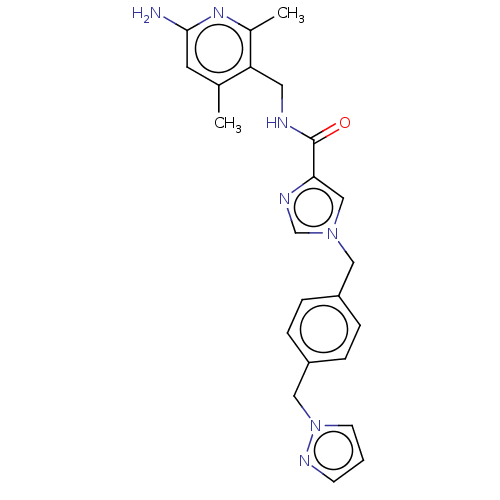 (US9290485, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212113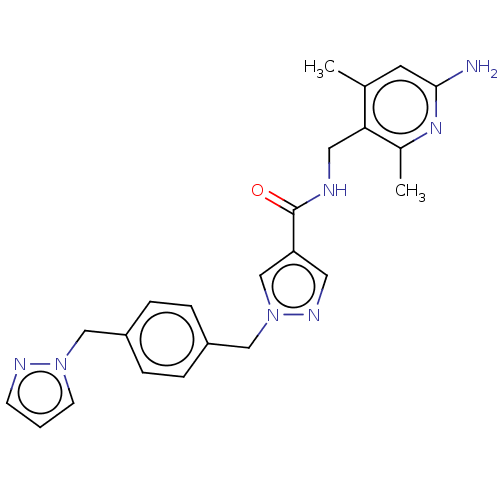 (US9290485, 145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM196124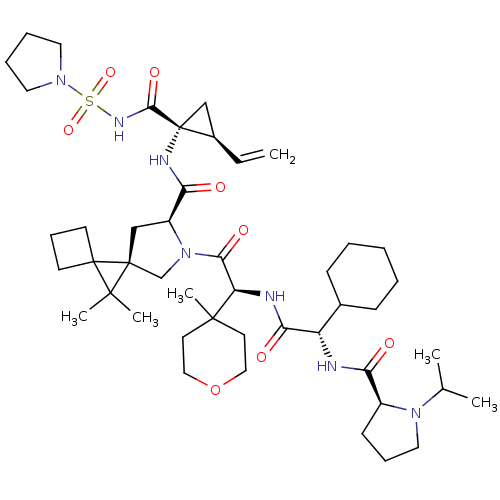 (US9206232, 125) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110357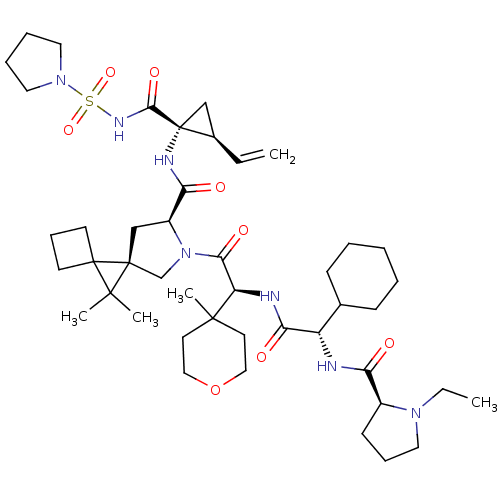 (US8613914, 125 | US9206232, 129) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212141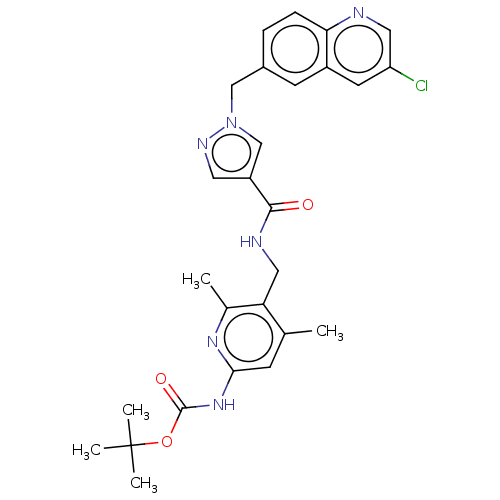 (US9290485, 173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110396 (US8613914, 170 | US9206232, 170) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110396 (US8613914, 170 | US9206232, 170) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110400 (US8613914, 174 | US9206232, 174) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110400 (US8613914, 174 | US9206232, 174) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110393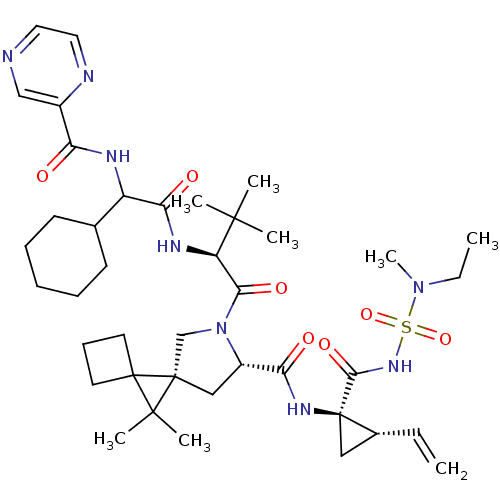 (US8613914, 167 | US9206232, 167) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110393 (US8613914, 167 | US9206232, 167) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110388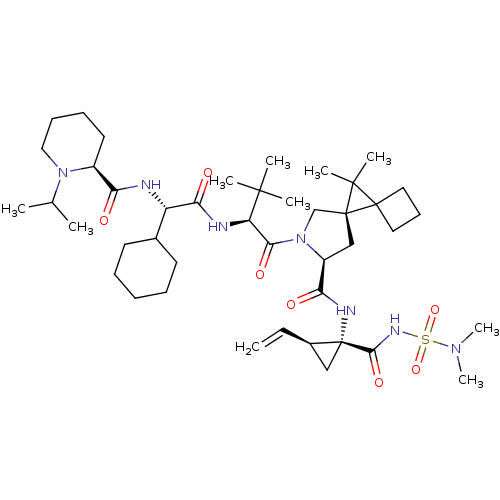 (US8613914, 162 | US9206232, 162) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110280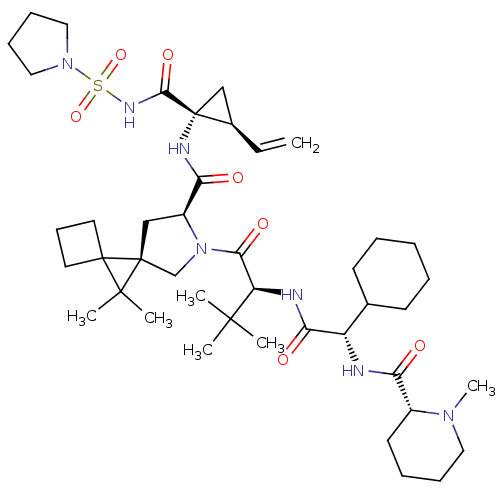 (US8613914, 48 | US9206232, 48) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110273 (US8613914, 41 | US9206232, 41) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110388 (US8613914, 162 | US9206232, 162) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM196120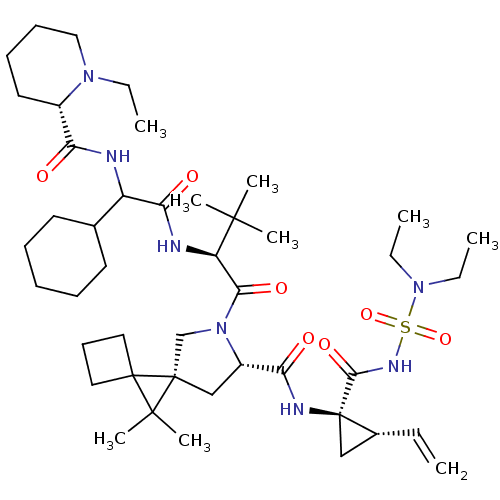 (US9206232, 77) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110388 (US8613914, 162 | US9206232, 162) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110309 (US8613914, 77) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110280 (US8613914, 48 | US9206232, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110273 (US8613914, 41 | US9206232, 41) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212124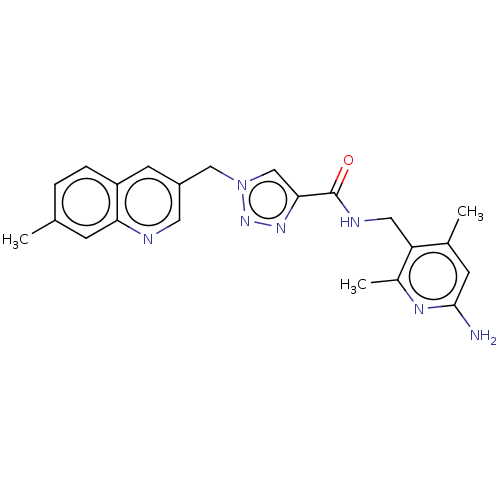 (US9290485, 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50329791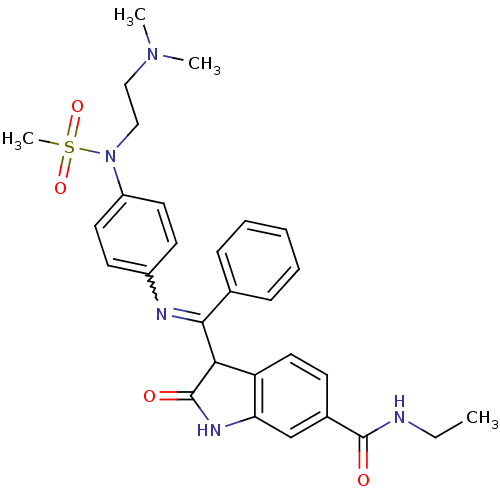 ((Z)-3-((4-(N-(2-(dimethylamino)ethyl)methylsulfona...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG Curated by ChEMBL | Assay Description Inhibition of TGFbeta receptor | J Med Chem 53: 7287-95 (2010) Article DOI: 10.1021/jm100812a BindingDB Entry DOI: 10.7270/Q2F47PCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110294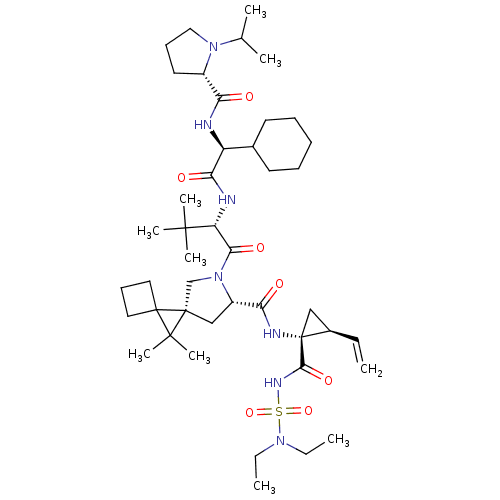 (US8613914, 62 | US9206232, 62) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110277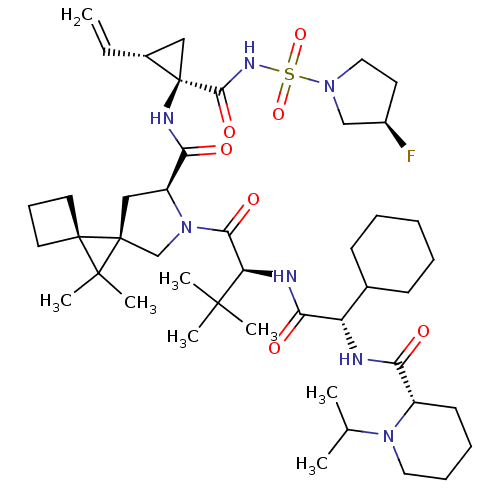 (US8613914, 45 | US9206232, 45) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110264 (US8613914, 32 | US9206232, 32) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110397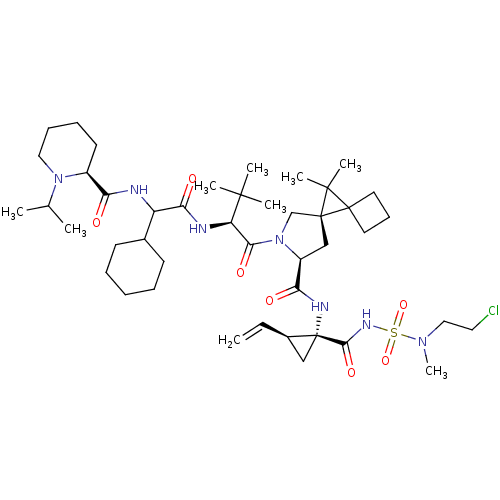 (US8613914, 171 | US9206232, 171) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110383 (US8613914, 157 | US9206232, 157) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110294 (US8613914, 62 | US9206232, 62) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110277 (US8613914, 45 | US9206232, 45) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110264 (US8613914, 32 | US9206232, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110383 (US8613914, 157 | US9206232, 157) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110397 (US8613914, 171 | US9206232, 171) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110378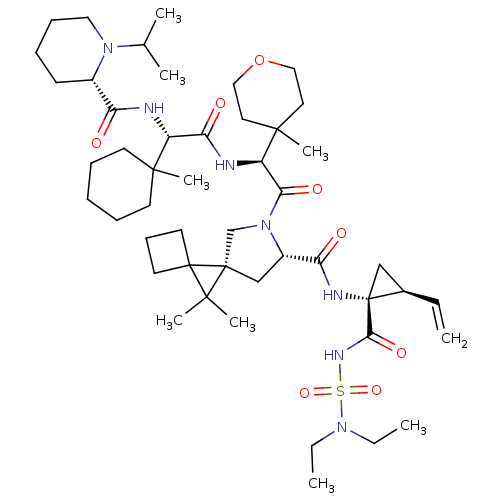 (US8613914, 152 | US9206232, 152) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110378 (US8613914, 152 | US9206232, 152) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110295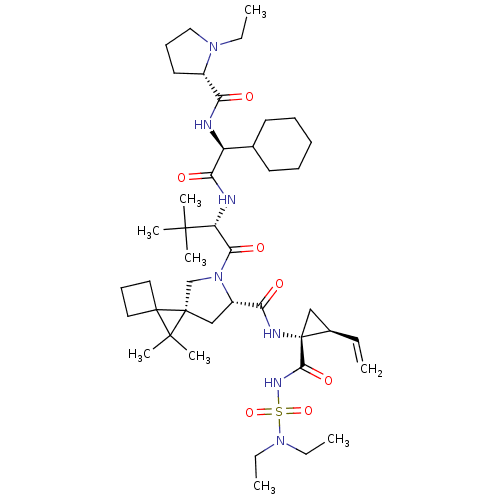 (US8613914, 63 | US9206232, 63) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212109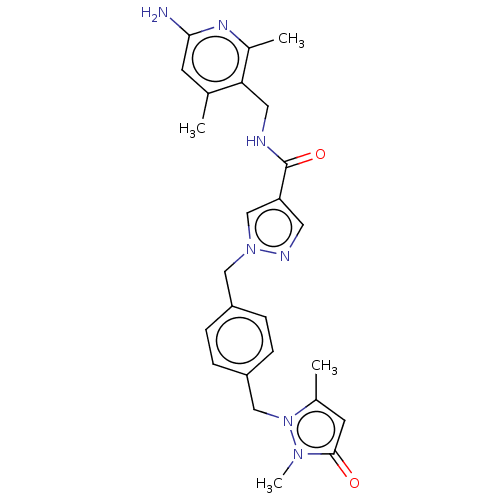 (US9290485, 141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110295 (US8613914, 63 | US9206232, 63) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110382 (US8613914, 156 | US9206232, 156) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110382 (US8613914, 156 | US9206232, 156) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110360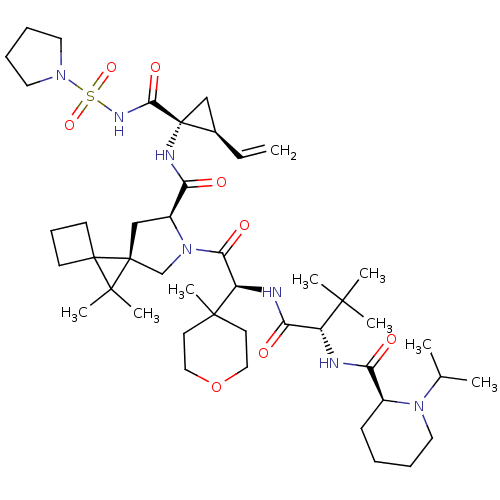 (US8613914, 128 | US9206232, 128) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM110287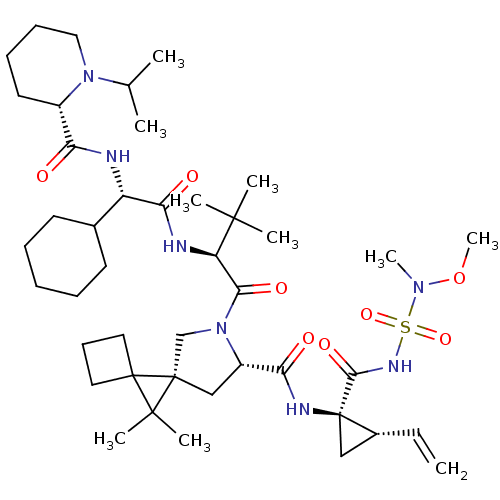 (US8613914, 55 | US9206232, 55) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 847 total ) | Next | Last >> |