

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075807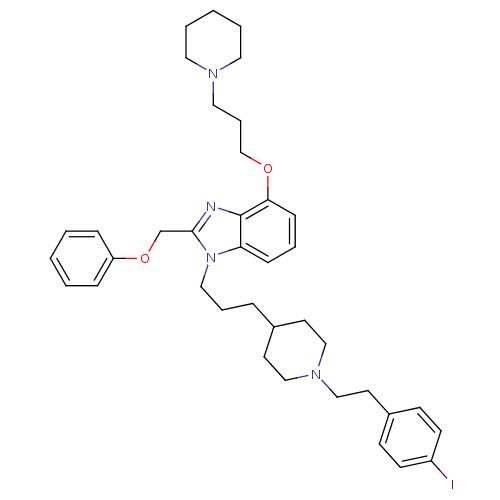 (1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075796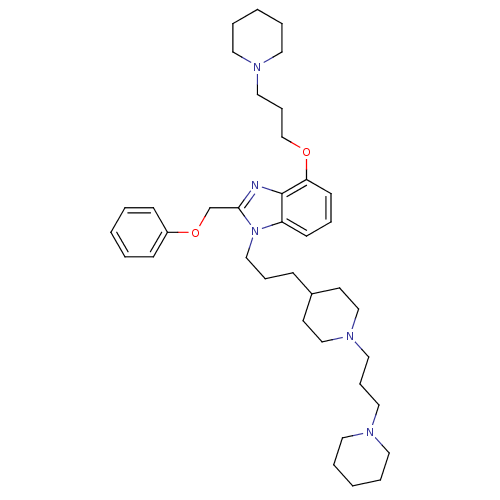 (2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50077798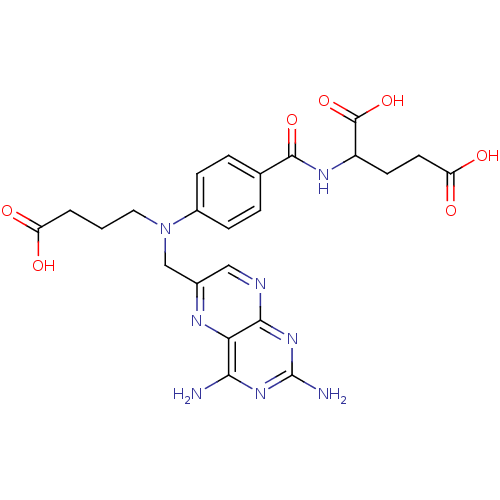 (2-{4-[(3-Carboxy-propyl)-(2,4-diamino-pteridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075811 (3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50077796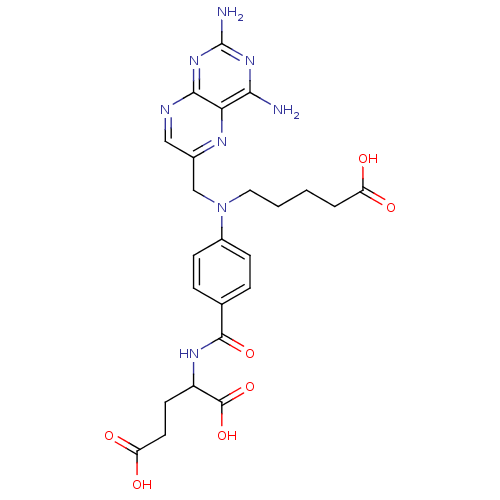 (2-{4-[(4-Carboxy-butyl)-(2,4-diamino-pteridin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075803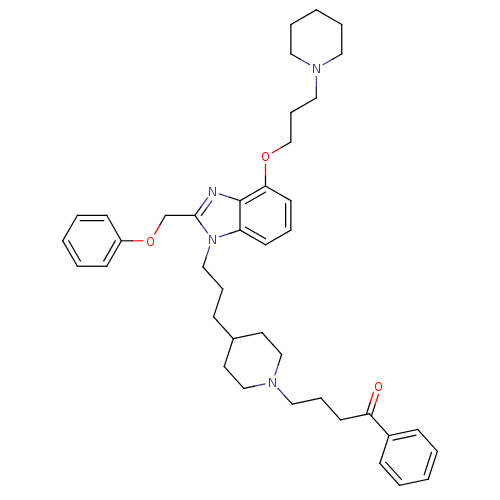 (4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 0.179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase in humans | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50077799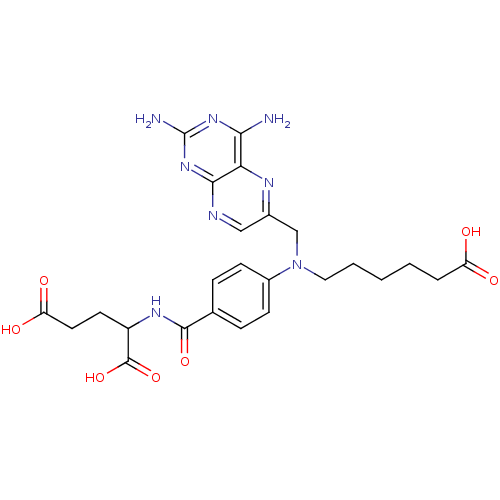 (2-{4-[(5-Carboxy-pentyl)-(2,4-diamino-pteridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi dihydrofolate reductase | Bioorg Med Chem Lett 9: 1463-8 (1999) BindingDB Entry DOI: 10.7270/Q2BZ657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075812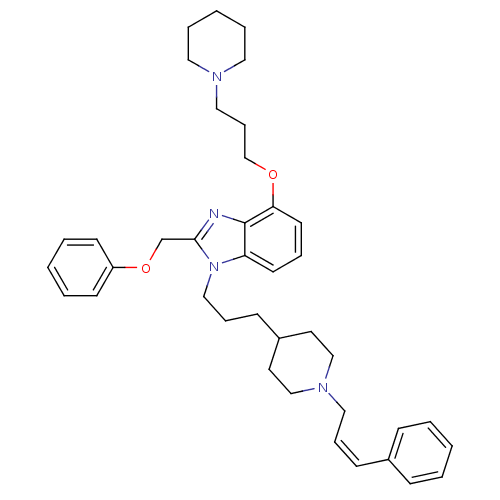 (2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075809 (1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202918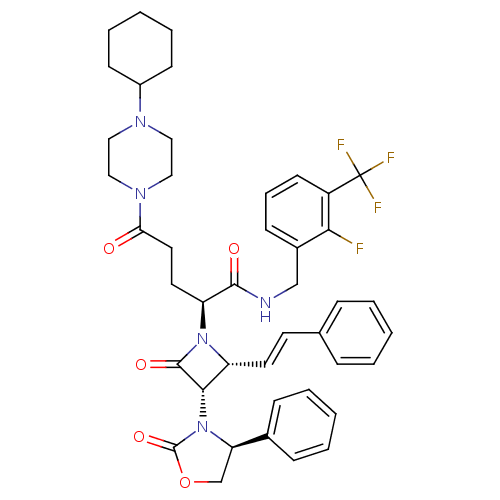 ((S)-N-(2-fluoro-3-(trifluoromethyl)benzyl)-5-(4-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50452573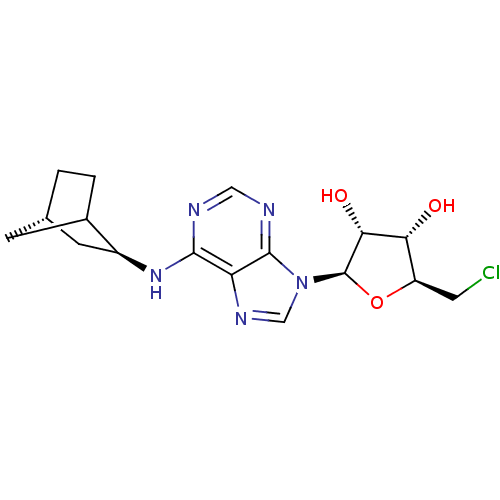 (CHEMBL3038260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202900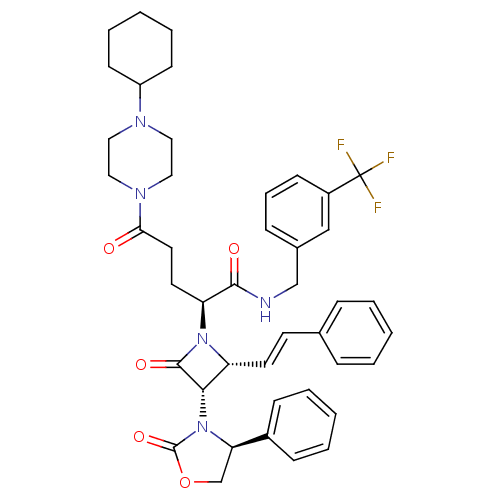 ((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-cyclohexylp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075797 (1-{3-[1-(2-Cyclohexyl-ethyl)-piperidin-4-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075806 (1-{3-[1-(3-Methyl-butyl)-piperidin-4-yl]-propyl}-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50452575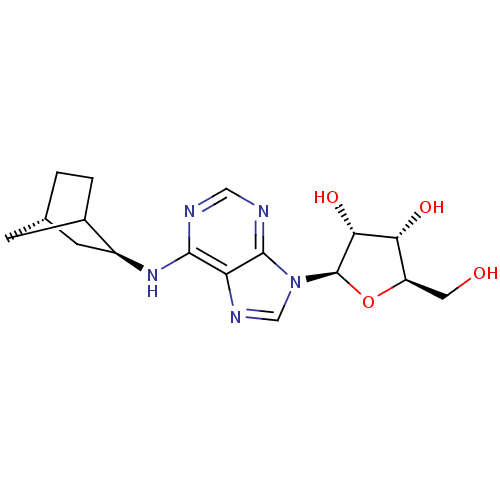 (CHEMBL2112182) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202920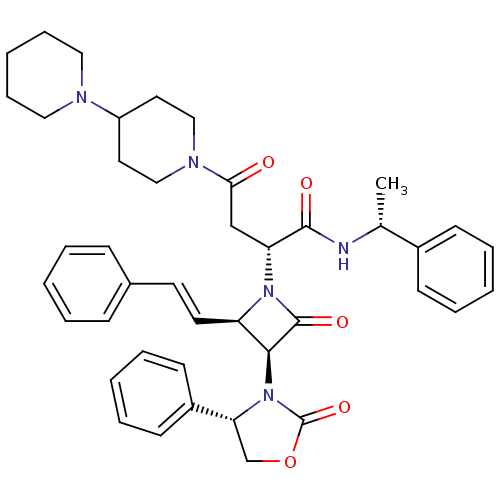 (2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075802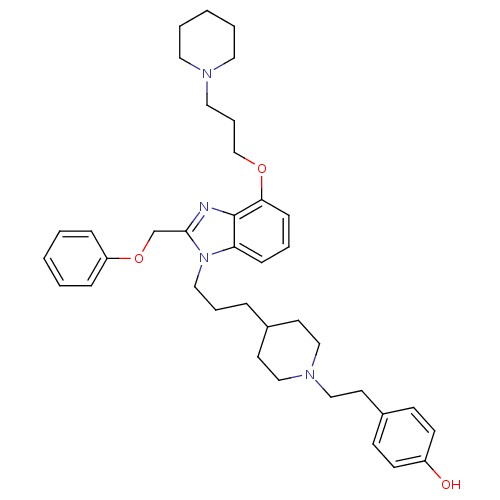 (4-[2-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075799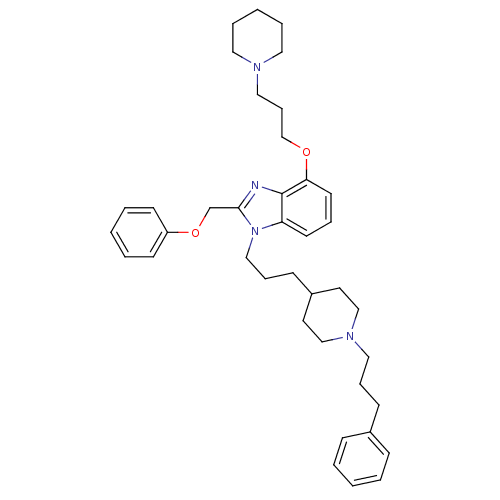 (2-Phenoxymethyl-1-{3-[1-(3-phenyl-propyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202882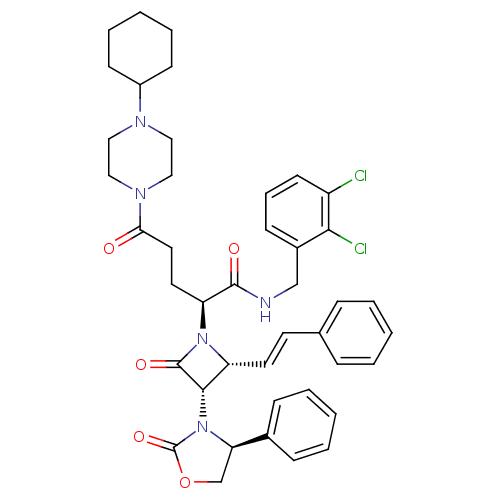 ((S)-N-(2,3-dichlorobenzyl)-5-(4-cyclohexylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075798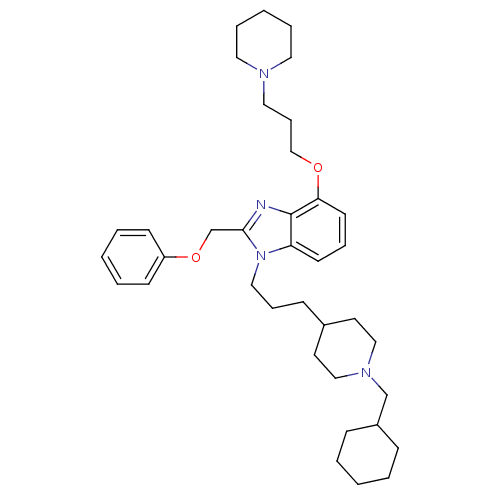 (1-[3-(1-Cyclohexylmethyl-piperidin-4-yl)-propyl]-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202902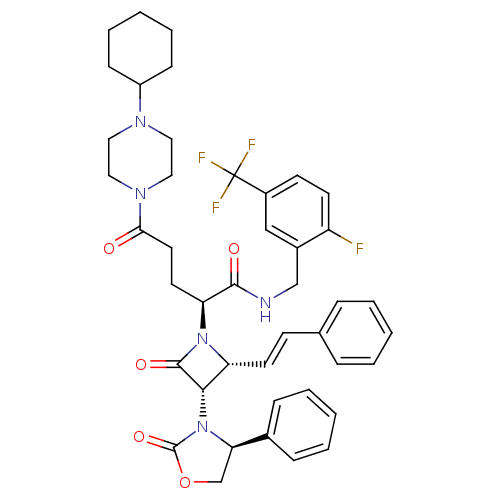 ((S)-N-(2-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50367846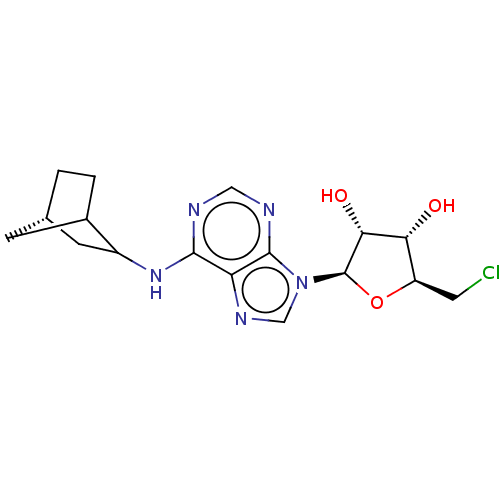 (CHEMBL3038256) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50267577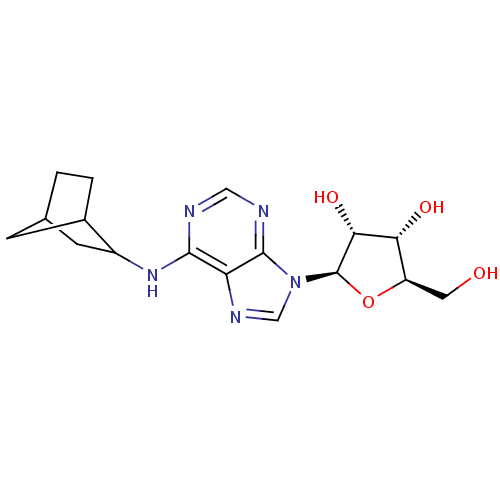 (CHEMBL489640 | N6-((+/-)-endo-norborn-2-yl)adenosi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202873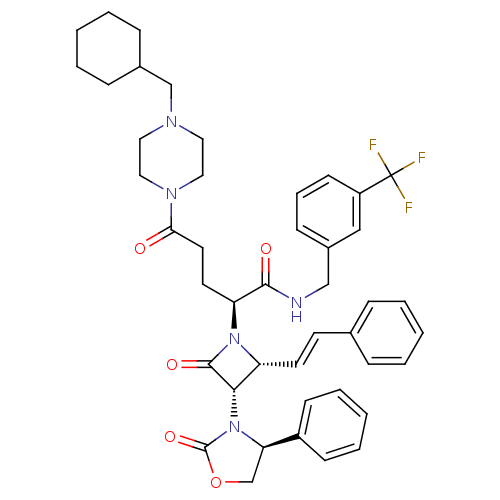 ((S)-N-(3-(trifluoromethyl)benzyl)-5-(4-(cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202916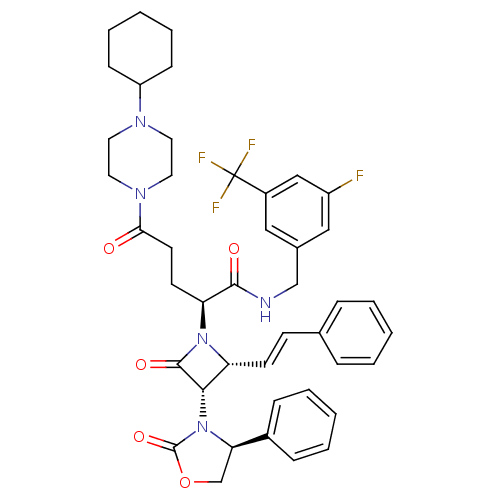 ((S)-N-(3-fluoro-5-(trifluoromethyl)benzyl)-5-(4-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50085672 ((2R,3R,4S,5R)-2-(6-Amino-2-cyclopentylamino-purin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | Mol Pharmacol 29: 331-46 (1986) BindingDB Entry DOI: 10.7270/Q2MK6BDV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CHA from adenosine A1 receptor of rat whole brain | J Med Chem 28: 1383-4 (1985) BindingDB Entry DOI: 10.7270/Q2GH9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity at adenosine A1 receptor from rat brain membranes by [3H]N6-cyclohexyladenosine displacement. | J Med Chem 31: 1282-5 (1988) BindingDB Entry DOI: 10.7270/Q2KK9CCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of binding of [3H]N6-cyclohexyladenosine to adenosine A1 receptor of rat whole brain membranes. | J Med Chem 32: 1667-73 (1989) Checked by Author BindingDB Entry DOI: 10.7270/Q2BR8SS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor on rat whole brain membrane using [3H]N6-cyclohexyladenosine | J Med Chem 31: 271-3 (1988) BindingDB Entry DOI: 10.7270/Q23R0TFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202860 (2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202871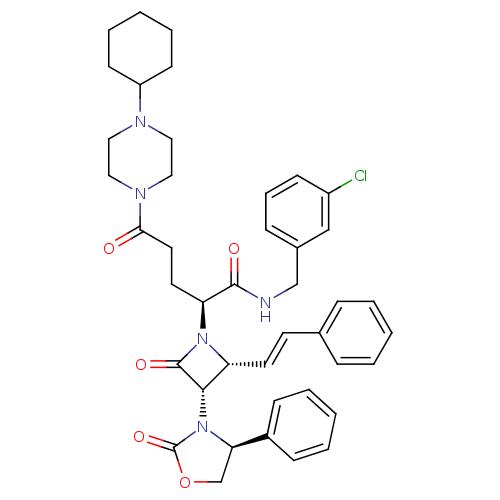 ((S)-N-(3-chlorobenzyl)-5-(4-cyclohexylpiperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075795 (1-[3-(1-But-3-enyl-piperidin-4-yl)-propyl]-2-pheno...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.707 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50267393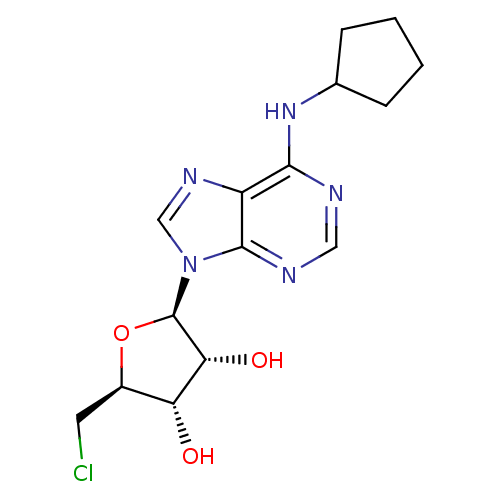 (CHEMBL477444 | N6-Cyclopentyl-9H-(5-chloro-5-deoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060725 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060725 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells | J Med Chem 40: 3712-4 (1997) Article DOI: 10.1021/jm970512x BindingDB Entry DOI: 10.7270/Q2HQ3Z1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075808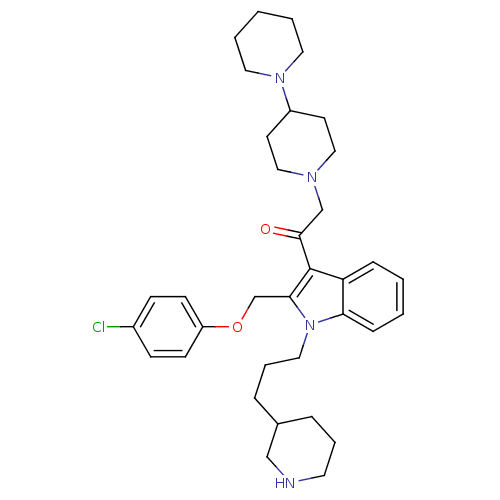 (2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50452201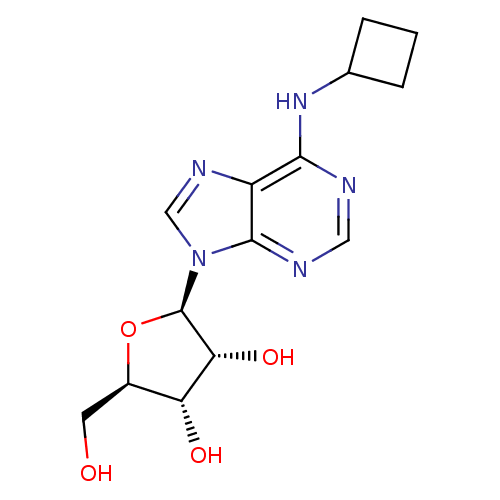 (CHEMBL569275) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.777 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]CHA from adenosine A1 receptor of rat whole brain | J Med Chem 28: 1383-4 (1985) BindingDB Entry DOI: 10.7270/Q2GH9JHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202877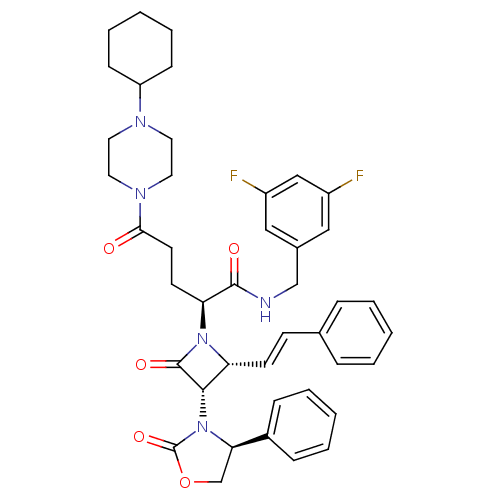 ((S)-N-(3,5-difluorobenzyl)-5-(4-cyclohexylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50075804 (1-[3-(1-Isobutyl-piperidin-4-yl)-propyl]-2-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50452574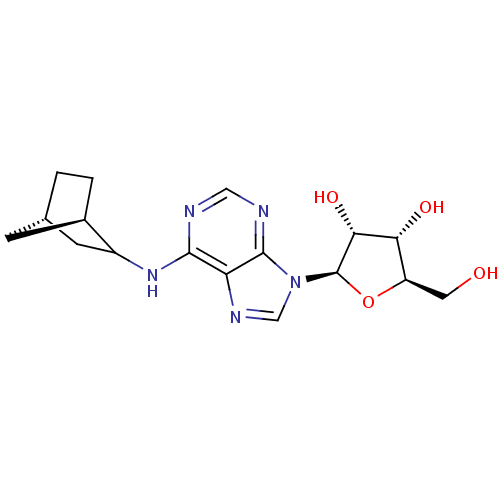 (CHEMBL2112183) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor using [3H]CHA in rat brain membranes | J Med Chem 32: 8-11 (1989) BindingDB Entry DOI: 10.7270/Q2TX3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202869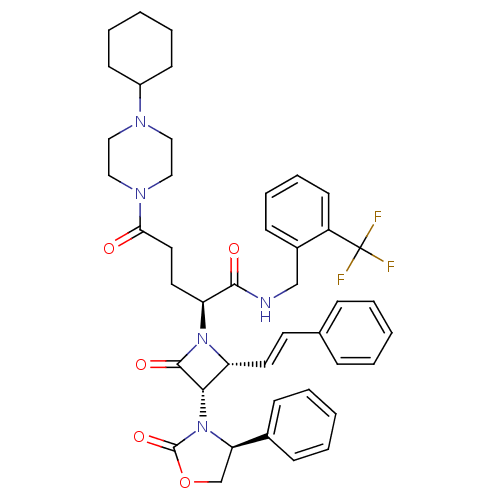 ((S)-N-(2-(trifluoromethyl)benzyl)-5-(4-cyclohexylp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263570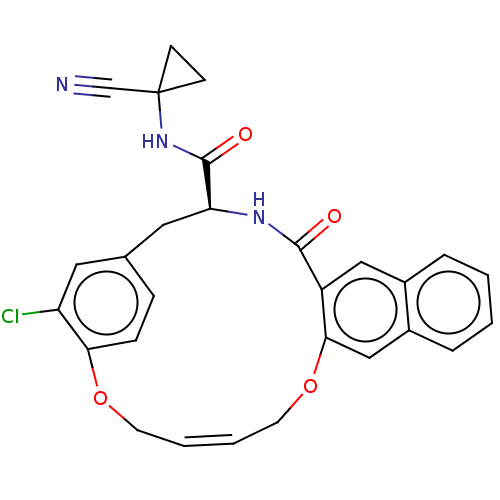 (CHEMBL4066422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50118810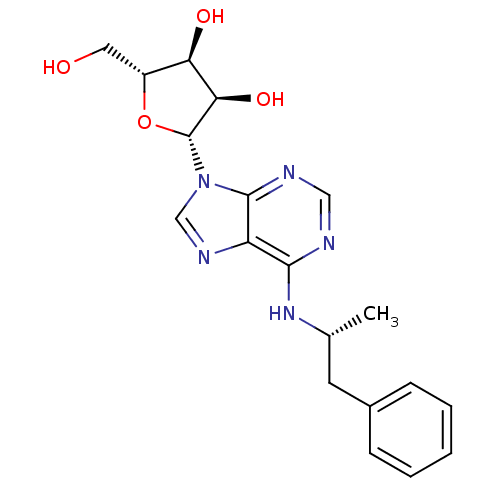 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor in rat brain membranes using [3H]CHA as radioligand | J Med Chem 29: 346-53 (1986) BindingDB Entry DOI: 10.7270/Q28P612K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202864 (4-amino-1-benzyl-1-[(3S)-3-[(3S,4R)-2-oxo-3-[(4S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202858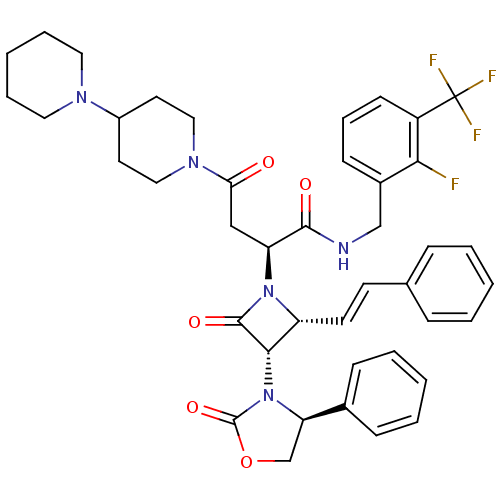 (2(R)-[[4-(piperidin-1-yl)piperidin-1-yl]carbonylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50202899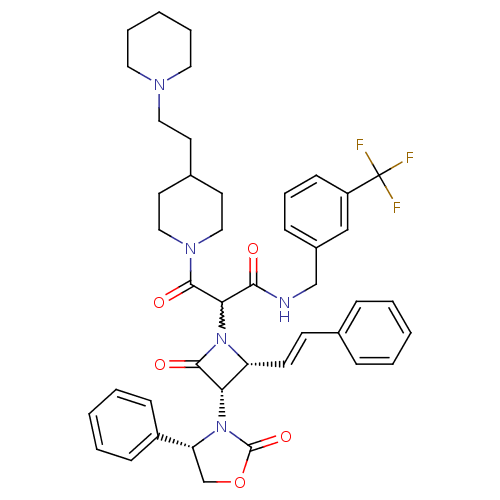 (CHEMBL241826 | N-(3-(trifluoromethyl)benzyl)-3-oxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lehigh University Curated by ChEMBL | Assay Description Binding affinity for human cloned vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem 15: 2054-80 (2007) Article DOI: 10.1016/j.bmc.2006.12.031 BindingDB Entry DOI: 10.7270/Q2GX4B6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2723 total ) | Next | Last >> |