 Found 128 hits with Last Name = 'bundesmann' and Initial = 'mw'
Found 128 hits with Last Name = 'bundesmann' and Initial = 'mw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398789
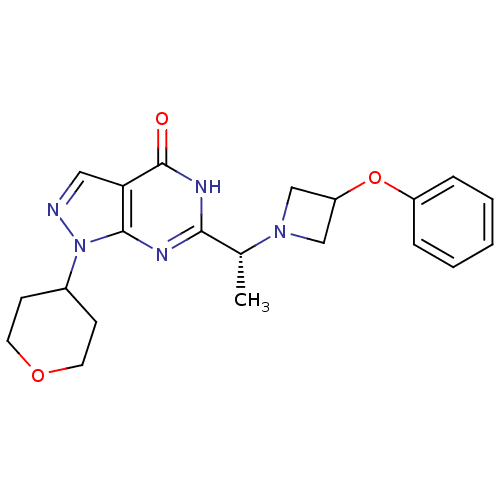
(CHEMBL2180073)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H25N5O3/c1-14(25-12-17(13-25)29-16-5-3-2-4-6-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-9-28-10-8-15/h2-6,11,14-15,17H,7-10,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398807
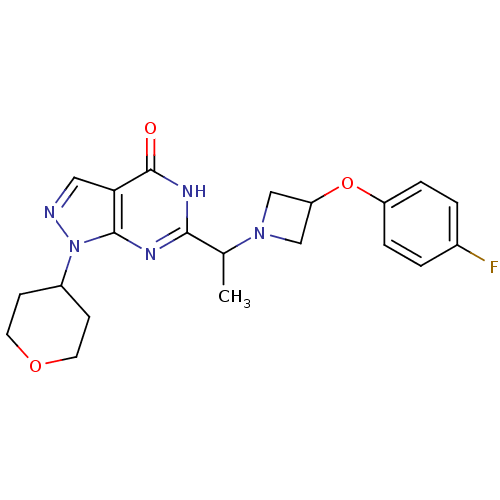
(CHEMBL2180074)Show SMILES CC(N1CC(C1)Oc1ccc(F)cc1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C21H24FN5O3/c1-13(26-11-17(12-26)30-16-4-2-14(22)3-5-16)19-24-20-18(21(28)25-19)10-23-27(20)15-6-8-29-9-7-15/h2-5,10,13,15,17H,6-9,11-12H2,1H3,(H,24,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398804
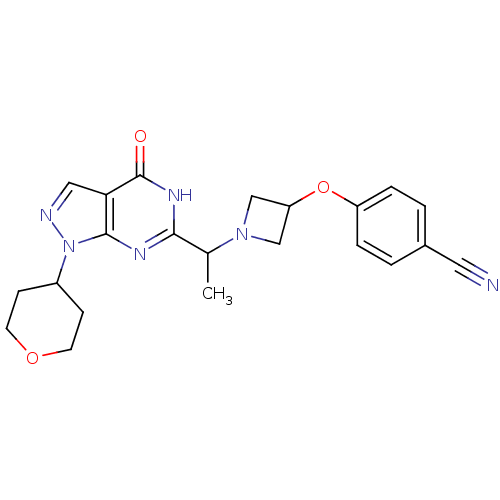
(CHEMBL2177497)Show SMILES CC(N1CC(C1)Oc1ccc(cc1)C#N)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C22H24N6O3/c1-14(27-12-18(13-27)31-17-4-2-15(10-23)3-5-17)20-25-21-19(22(29)26-20)11-24-28(21)16-6-8-30-9-7-16/h2-5,11,14,16,18H,6-9,12-13H2,1H3,(H,25,26,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50398800
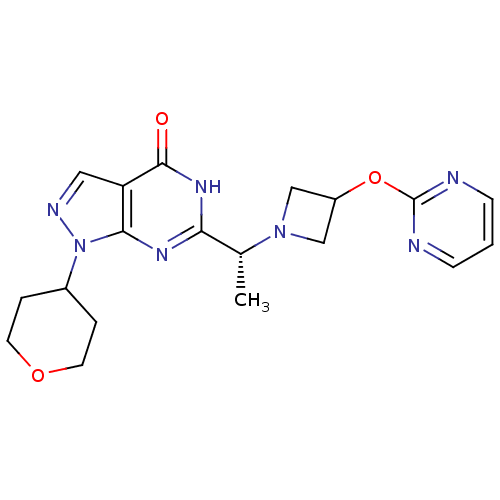
(CHEMBL2180069)Show SMILES C[C@@H](N1CC(C1)Oc1ncccn1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C19H23N7O3/c1-12(25-10-14(11-25)29-19-20-5-2-6-21-19)16-23-17-15(18(27)24-16)9-22-26(17)13-3-7-28-8-4-13/h2,5-6,9,12-14H,3-4,7-8,10-11H2,1H3,(H,23,24,27)/t12-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human DAT |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136576
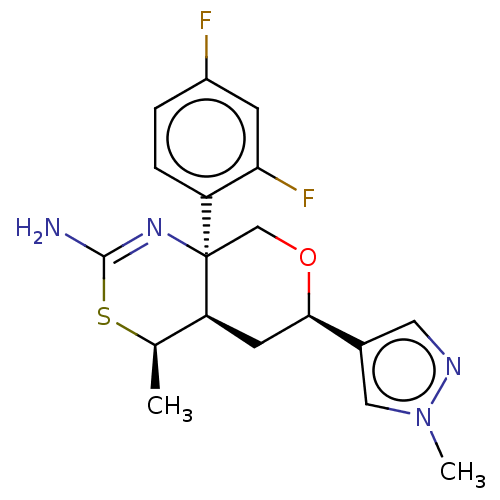
(US8865706, 16)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cnn(C)c1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H20F2N4OS/c1-10-14-6-16(11-7-22-24(2)8-11)25-9-18(14,23-17(21)26-10)13-4-3-12(19)5-15(13)20/h3-5,7-8,10,14,16H,6,9H2,1-2H3,(H2,21,23)/t10-,14+,16-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081645

(CHEMBL3422237)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cc(C)no1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-4-15(24-22-9)14-5-10-7-25-16(20)21-17(10,8-23-14)12-3-2-11(18)6-13(12)19/h2-4,6,10,14H,5,7-8H2,1H3,(H2,20,21)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081646
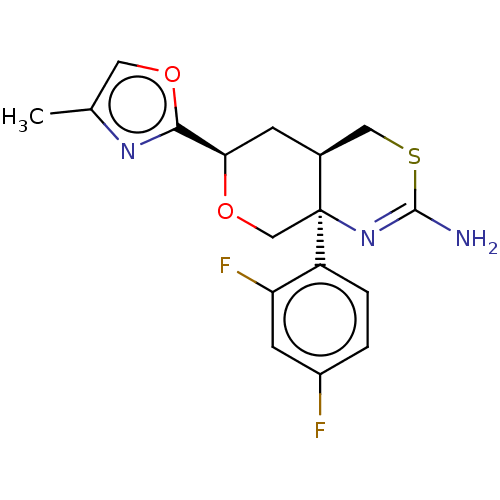
(CHEMBL3422236)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1nc(C)co1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-6-23-15(21-9)14-4-10-7-25-16(20)22-17(10,8-24-14)12-3-2-11(18)5-13(12)19/h2-3,5-6,10,14H,4,7-8H2,1H3,(H2,20,22)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM41536
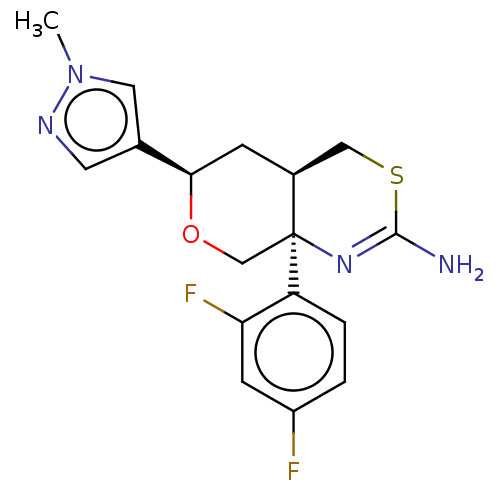
(US8865706, 15)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2CSC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:13| Show InChI InChI=1S/C19H15F6N5O/c20-12-3-13(21)18(23)17(22)11(12)7-29-6-10(5-26-29)27-16(31)8-30-15(19(24)25)4-14(28-30)9-1-2-9/h3-6,9,19H,1-2,7-8H2,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM148176

(US8962616, 22 | US8962616, 4)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1nc(C)co1)c1ccc(F)cc1F |r,c:4| Show InChI InChI=1S/C18H19F2N3O2S/c1-9-7-24-16(22-9)15-6-13-10(2)26-17(21)23-18(13,8-25-15)12-4-3-11(19)5-14(12)20/h3-5,7,10,13,15H,6,8H2,1-2H3,(H2,21,23)/t10-,13+,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136570
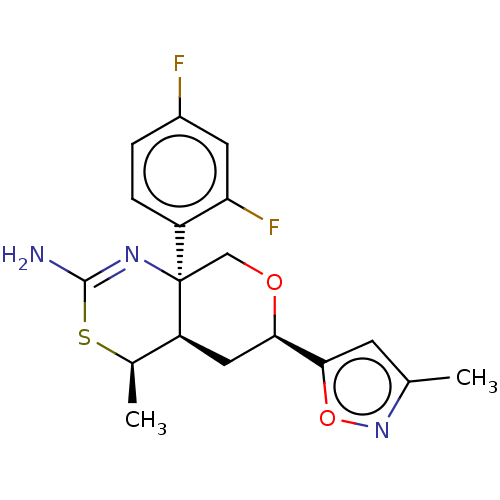
(US8865706, 9)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cc(C)no1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H19F2N3O2S/c1-9-5-16(25-23-9)15-7-13-10(2)26-17(21)22-18(13,8-24-15)12-4-3-11(19)6-14(12)20/h3-6,10,13,15H,7-8H2,1-2H3,(H2,21,22)/t10-,13+,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136583

(US8865706, 22)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2[C@@H](CF)SC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:15| Show InChI InChI=1S/C18H19F3N4OS/c1-25-8-10(7-23-25)15-5-13-16(6-19)27-17(22)24-18(13,9-26-15)12-3-2-11(20)4-14(12)21/h2-4,7-8,13,15-16H,5-6,9H2,1H3,(H2,22,24)/t13-,15+,16+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398800

(CHEMBL2180069)Show SMILES C[C@@H](N1CC(C1)Oc1ncccn1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C19H23N7O3/c1-12(25-10-14(11-25)29-19-20-5-2-6-21-19)16-23-17-15(18(27)24-16)9-22-26(17)13-3-7-28-8-4-13/h2,5-6,9,12-14H,3-4,7-8,10-11H2,1H3,(H,23,24,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081642
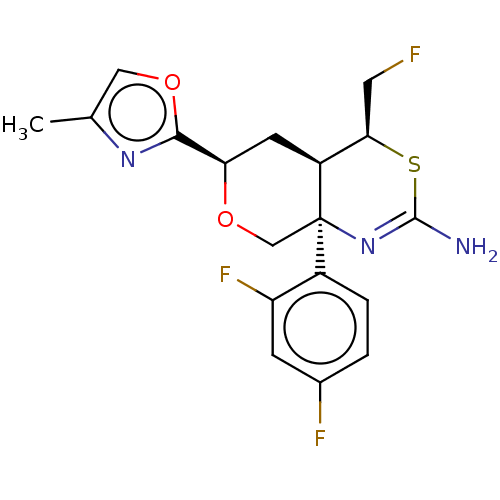
(CHEMBL3422244)Show SMILES [H][C@@]12C[C@@H](OC[C@@]1(N=C(N)S[C@@H]2CF)c1ccc(F)cc1F)c1nc(C)co1 |r,t:8| Show InChI InChI=1S/C18H18F3N3O2S/c1-9-7-25-16(23-9)14-5-12-15(6-19)27-17(22)24-18(12,8-26-14)11-3-2-10(20)4-13(11)21/h2-4,7,12,14-15H,5-6,8H2,1H3,(H2,22,24)/t12-,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398790

(CHEMBL2180072)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H25N5O2/c1-14(25-12-17(13-25)28-16-9-3-2-4-10-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-5-6-8-15/h2-4,9-11,14-15,17H,5-8,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136574
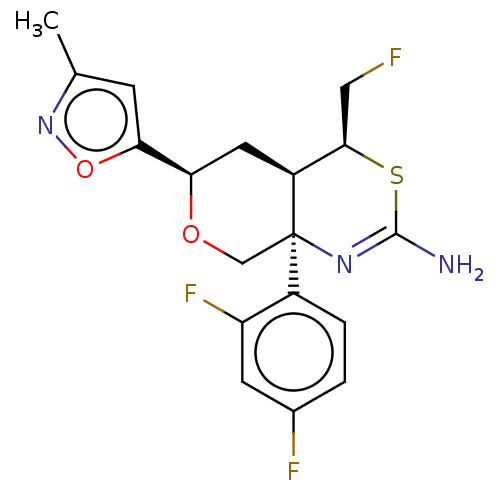
(US8865706, 13)Show SMILES Cc1cc(on1)[C@H]1C[C@H]2[C@@H](CF)SC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:15| Show InChI InChI=1S/C18H18F3N3O2S/c1-9-4-15(26-24-9)14-6-12-16(7-19)27-17(22)23-18(12,8-25-14)11-3-2-10(20)5-13(11)21/h2-5,12,14,16H,6-8H2,1H3,(H2,22,23)/t12-,14+,16+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398802
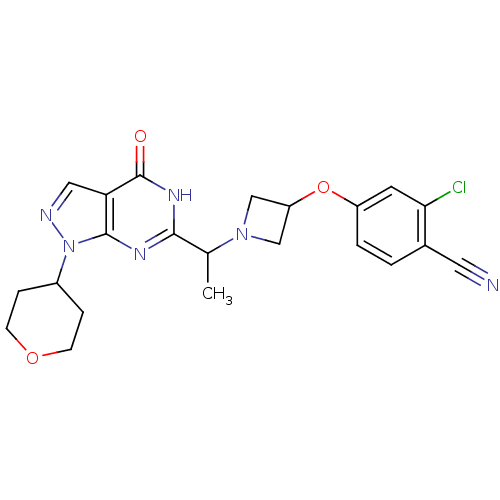
(CHEMBL2180066)Show SMILES CC(N1CC(C1)Oc1ccc(C#N)c(Cl)c1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C22H23ClN6O3/c1-13(28-11-17(12-28)32-16-3-2-14(9-24)19(23)8-16)20-26-21-18(22(30)27-20)10-25-29(21)15-4-6-31-7-5-15/h2-3,8,10,13,15,17H,4-7,11-12H2,1H3,(H,26,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398789

(CHEMBL2180073)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C21H25N5O3/c1-14(25-12-17(13-25)29-16-5-3-2-4-6-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-9-28-10-8-15/h2-6,11,14-15,17H,7-10,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM148173
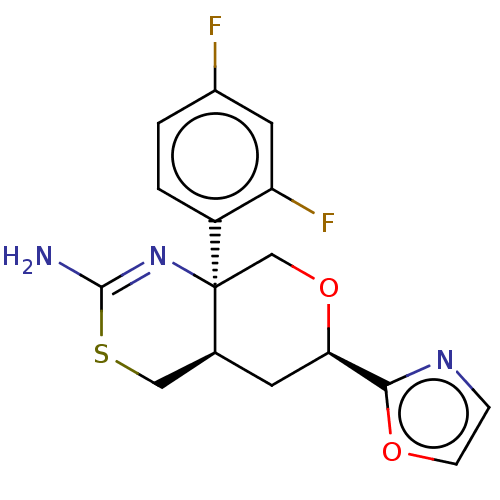
(US8962616, 1)Show SMILES NC1=N[C@]2(CO[C@H](C[C@H]2CS1)c1ncco1)c1ccc(F)cc1F |r,t:1| Show InChI InChI=1S/C16H15F2N3O2S/c17-10-1-2-11(12(18)6-10)16-8-23-13(14-20-3-4-22-14)5-9(16)7-24-15(19)21-16/h1-4,6,9,13H,5,7-8H2,(H2,19,21)/t9-,13+,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398799
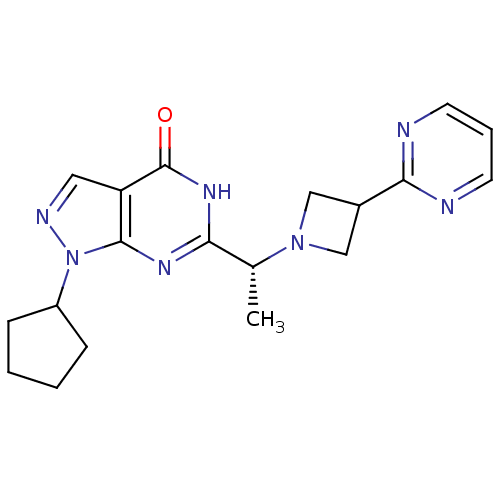
(CHEMBL2180070)Show SMILES C[C@@H](N1CC(C1)c1ncccn1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C19H23N7O/c1-12(25-10-13(11-25)17-20-7-4-8-21-17)16-23-18-15(19(27)24-16)9-22-26(18)14-5-2-3-6-14/h4,7-9,12-14H,2-3,5-6,10-11H2,1H3,(H,23,24,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136576

(US8865706, 16)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cnn(C)c1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H20F2N4OS/c1-10-14-6-16(11-7-22-24(2)8-11)25-9-18(14,23-17(21)26-10)13-4-3-12(19)5-15(13)20/h3-5,7-8,10,14,16H,6,9H2,1-2H3,(H2,21,23)/t10-,14+,16-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398803
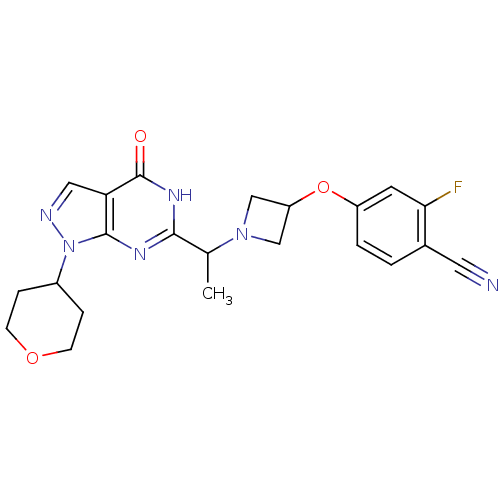
(CHEMBL2180065)Show SMILES CC(N1CC(C1)Oc1ccc(C#N)c(F)c1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C22H23FN6O3/c1-13(28-11-17(12-28)32-16-3-2-14(9-24)19(23)8-16)20-26-21-18(22(30)27-20)10-25-29(21)15-4-6-31-7-5-15/h2-3,8,10,13,15,17H,4-7,11-12H2,1H3,(H,26,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398790

(CHEMBL2180072)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H25N5O2/c1-14(25-12-17(13-25)28-16-9-3-2-4-10-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-5-6-8-15/h2-4,9-11,14-15,17H,5-8,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398801

(CHEMBL2180067)Show SMILES CC(N1CC(C1)Oc1ccc(F)c(c1)C#N)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C22H23FN6O3/c1-13(28-11-17(12-28)32-16-2-3-19(23)14(8-16)9-24)20-26-21-18(22(30)27-20)10-25-29(21)15-4-6-31-7-5-15/h2-3,8,10,13,15,17H,4-7,11-12H2,1H3,(H,26,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398807

(CHEMBL2180074)Show SMILES CC(N1CC(C1)Oc1ccc(F)cc1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C21H24FN5O3/c1-13(26-11-17(12-26)30-16-4-2-14(22)3-5-16)19-24-20-18(21(28)25-19)10-23-27(20)15-6-8-29-9-7-15/h2-5,10,13,15,17H,6-9,11-12H2,1H3,(H,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM41536

(US8865706, 15)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2CSC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:13| Show InChI InChI=1S/C19H15F6N5O/c20-12-3-13(21)18(23)17(22)11(12)7-29-6-10(5-26-29)27-16(31)8-30-15(19(24)25)4-14(28-30)9-1-2-9/h3-6,9,19H,1-2,7-8H2,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136574

(US8865706, 13)Show SMILES Cc1cc(on1)[C@H]1C[C@H]2[C@@H](CF)SC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:15| Show InChI InChI=1S/C18H18F3N3O2S/c1-9-4-15(26-24-9)14-6-12-16(7-19)27-17(22)23-18(12,8-25-14)11-3-2-10(20)5-13(11)21/h2-5,12,14,16H,6-8H2,1H3,(H2,22,23)/t12-,14+,16+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398806
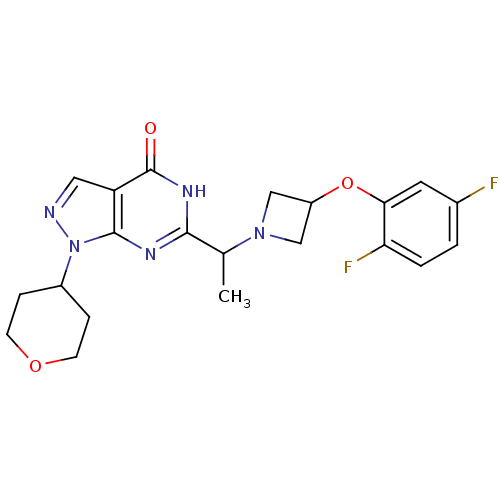
(CHEMBL2180075)Show SMILES CC(N1CC(C1)Oc1cc(F)ccc1F)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C21H23F2N5O3/c1-12(27-10-15(11-27)31-18-8-13(22)2-3-17(18)23)19-25-20-16(21(29)26-19)9-24-28(20)14-4-6-30-7-5-14/h2-3,8-9,12,14-15H,4-7,10-11H2,1H3,(H,25,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM142394

(US8933221, 4)Show SMILES NC1=N[C@]2(CO[C@@H](CF)C[C@H]2CS1)c1ccc(F)cc1F |t:1| Show InChI InChI=1S/C14H15F3N2OS/c15-5-10-3-8-6-21-13(18)19-14(8,7-20-10)11-2-1-9(16)4-12(11)17/h1-2,4,8,10H,3,5-7H2,(H2,18,19)/t8-,10+,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136583

(US8865706, 22)Show SMILES Cn1cc(cn1)[C@H]1C[C@H]2[C@@H](CF)SC(N)=N[C@]2(CO1)c1ccc(F)cc1F |c:15| Show InChI InChI=1S/C18H19F3N4OS/c1-25-8-10(7-23-25)15-5-13-16(6-19)27-17(22)24-18(13,9-26-15)12-3-2-11(20)4-14(12)21/h2-4,7-8,13,15-16H,5-6,9H2,1H3,(H2,22,24)/t13-,15+,16+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398800

(CHEMBL2180069)Show SMILES C[C@@H](N1CC(C1)Oc1ncccn1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C19H23N7O3/c1-12(25-10-14(11-25)29-19-20-5-2-6-21-19)16-23-17-15(18(27)24-16)9-22-26(17)13-3-7-28-8-4-13/h2,5-6,9,12-14H,3-4,7-8,10-11H2,1H3,(H,23,24,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398805
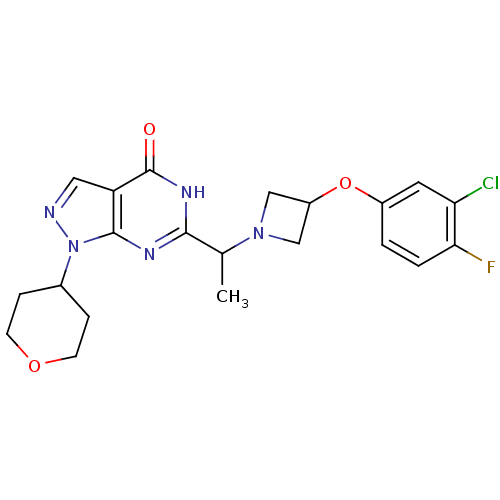
(CHEMBL2177496)Show SMILES CC(N1CC(C1)Oc1ccc(F)c(Cl)c1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C21H23ClFN5O3/c1-12(27-10-15(11-27)31-14-2-3-18(23)17(22)8-14)19-25-20-16(21(29)26-19)9-24-28(20)13-4-6-30-7-5-13/h2-3,8-9,12-13,15H,4-7,10-11H2,1H3,(H,25,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398804

(CHEMBL2177497)Show SMILES CC(N1CC(C1)Oc1ccc(cc1)C#N)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 Show InChI InChI=1S/C22H24N6O3/c1-14(27-12-18(13-27)31-17-4-2-15(10-23)3-5-17)20-25-21-19(22(29)26-20)11-24-28(21)16-6-8-30-9-7-16/h2-5,11,14,16,18H,6-9,12-13H2,1H3,(H,25,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM136570

(US8865706, 9)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1cc(C)no1)c1ccc(F)cc1F |c:4| Show InChI InChI=1S/C18H19F2N3O2S/c1-9-5-16(25-23-9)15-7-13-10(2)26-17(21)22-18(13,8-24-15)12-4-3-11(19)6-14(12)20/h3-6,10,13,15H,7-8H2,1-2H3,(H2,21,22)/t10-,13+,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081645

(CHEMBL3422237)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cc(C)no1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-4-15(24-22-9)14-5-10-7-25-16(20)21-17(10,8-23-14)12-3-2-11(18)6-13(12)19/h2-4,6,10,14H,5,7-8H2,1H3,(H2,20,21)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081646

(CHEMBL3422236)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1nc(C)co1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-6-23-15(21-9)14-4-10-7-25-16(20)22-17(10,8-24-14)12-3-2-11(18)5-13(12)19/h2-3,5-6,10,14H,4,7-8H2,1H3,(H2,20,22)/t10-,14+,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM148176

(US8962616, 22 | US8962616, 4)Show SMILES C[C@H]1SC(N)=N[C@]2(CO[C@H](C[C@@H]12)c1nc(C)co1)c1ccc(F)cc1F |r,c:4| Show InChI InChI=1S/C18H19F2N3O2S/c1-9-7-24-16(22-9)15-6-13-10(2)26-17(21)23-18(13,8-25-15)12-4-3-11(19)5-14(12)20/h3-5,7,10,13,15H,6,8H2,1-2H3,(H2,21,23)/t10-,13+,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50398790

(CHEMBL2180072)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H25N5O2/c1-14(25-12-17(13-25)28-16-9-3-2-4-10-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-5-6-8-15/h2-4,9-11,14-15,17H,5-8,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081642

(CHEMBL3422244)Show SMILES [H][C@@]12C[C@@H](OC[C@@]1(N=C(N)S[C@@H]2CF)c1ccc(F)cc1F)c1nc(C)co1 |r,t:8| Show InChI InChI=1S/C18H18F3N3O2S/c1-9-7-25-16(23-9)14-5-12-15(6-19)27-17(22)24-18(12,8-26-14)11-3-2-10(20)4-13(11)21/h2-4,7,12,14-15H,5-6,8H2,1H3,(H2,22,24)/t12-,14+,15+,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398791
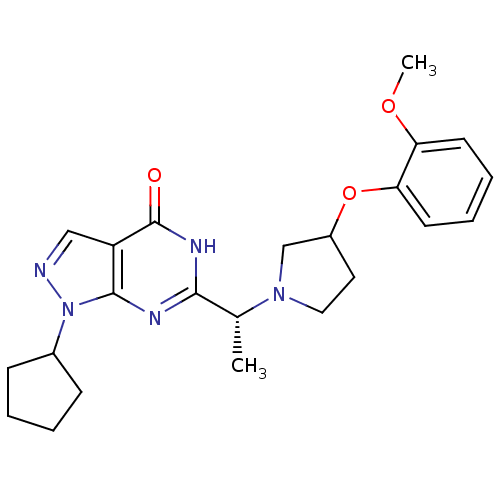
(CHEMBL2177504)Show SMILES COc1ccccc1OC1CCN(C1)[C@H](C)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C23H29N5O3/c1-15(27-12-11-17(14-27)31-20-10-6-5-9-19(20)30-2)21-25-22-18(23(29)26-21)13-24-28(22)16-7-3-4-8-16/h5-6,9-10,13,15-17H,3-4,7-8,11-12,14H2,1-2H3,(H,25,26,29)/t15-,17?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50081645

(CHEMBL3422237)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cc(C)no1)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C17H17F2N3O2S/c1-9-4-15(24-22-9)14-5-10-7-25-16(20)21-17(10,8-23-14)12-3-2-11(18)6-13(12)19/h2-4,6,10,14H,5,7-8H2,1H3,(H2,20,21)/t10-,14+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398792
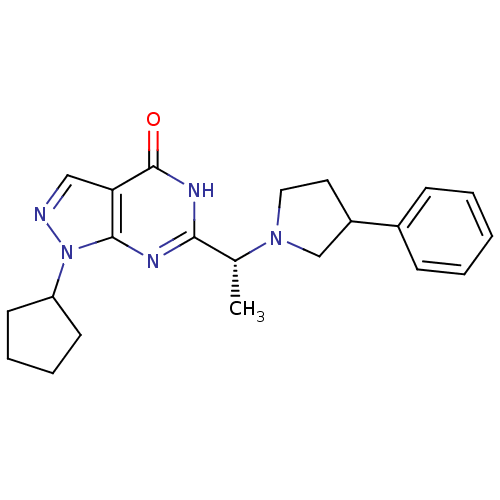
(CHEMBL2177503)Show SMILES C[C@@H](N1CCC(C1)c1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C22H27N5O/c1-15(26-12-11-17(14-26)16-7-3-2-4-8-16)20-24-21-19(22(28)25-20)13-23-27(21)18-9-5-6-10-18/h2-4,7-8,13,15,17-18H,5-6,9-12,14H2,1H3,(H,24,25,28)/t15-,17?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50398790

(CHEMBL2180072)Show SMILES C[C@@H](N1CC(C1)Oc1ccccc1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C21H25N5O2/c1-14(25-12-17(13-25)28-16-9-3-2-4-10-16)19-23-20-18(21(27)24-19)11-22-26(20)15-7-5-6-8-15/h2-4,9-11,14-15,17H,5-8,12-13H2,1H3,(H,23,24,27)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM148173

(US8962616, 1)Show SMILES NC1=N[C@]2(CO[C@H](C[C@H]2CS1)c1ncco1)c1ccc(F)cc1F |r,t:1| Show InChI InChI=1S/C16H15F2N3O2S/c17-10-1-2-11(12(18)6-10)16-8-23-13(14-20-3-4-22-14)5-9(16)7-24-15(19)21-16/h1-4,6,9,13H,5,7-8H2,(H2,19,21)/t9-,13+,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50081644
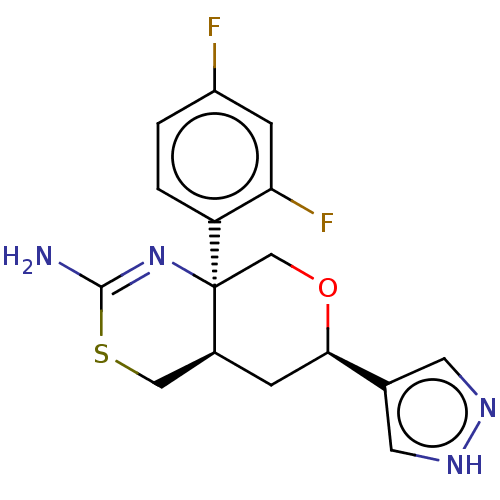
(CHEMBL3422239)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cn[nH]c1)c1ccc(F)cc1F |r,c:5| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM50398800

(CHEMBL2180069)Show SMILES C[C@@H](N1CC(C1)Oc1ncccn1)c1nc2n(ncc2c(=O)[nH]1)C1CCOCC1 |r| Show InChI InChI=1S/C19H23N7O3/c1-12(25-10-14(11-25)29-19-20-5-2-6-21-19)16-23-17-15(18(27)24-16)9-22-26(17)13-3-7-28-8-4-13/h2,5-6,9,12-14H,3-4,7-8,10-11H2,1H3,(H,23,24,27)/t12-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE1C |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A
(Homo sapiens (Human)) | BDBM50398795
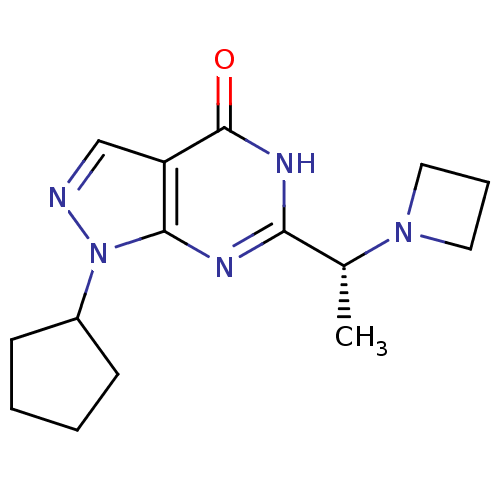
(CHEMBL2177500)Show SMILES C[C@@H](N1CCC1)c1nc2n(ncc2c(=O)[nH]1)C1CCCC1 |r| Show InChI InChI=1S/C15H21N5O/c1-10(19-7-4-8-19)13-17-14-12(15(21)18-13)9-16-20(14)11-5-2-3-6-11/h9-11H,2-8H2,1H3,(H,17,18,21)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 593 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE9A using [3H]cGMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 55: 9055-68 (2012)
Article DOI: 10.1021/jm3009635
BindingDB Entry DOI: 10.7270/Q2CR5VHK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081644

(CHEMBL3422239)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@H](C2)c1cn[nH]c1)c1ccc(F)cc1F |r,c:5| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 599 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM148173

(US8962616, 1)Show SMILES NC1=N[C@]2(CO[C@H](C[C@H]2CS1)c1ncco1)c1ccc(F)cc1F |r,t:1| Show InChI InChI=1S/C16H15F2N3O2S/c17-10-1-2-11(12(18)6-10)16-8-23-13(14-20-3-4-22-14)5-9(16)7-24-15(19)21-16/h1-4,6,9,13H,5,7-8H2,(H2,19,21)/t9-,13+,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 614 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50081643
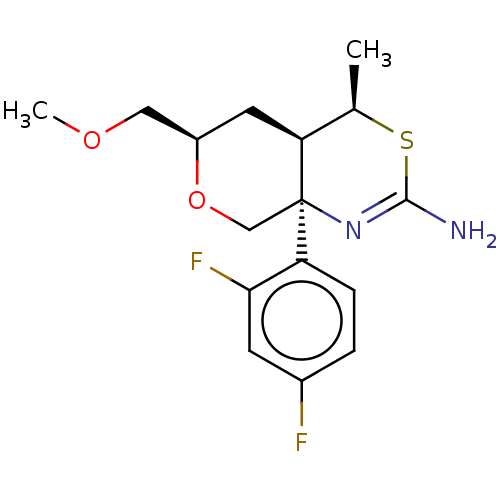
(CHEMBL3422240 | US9260455, 2)Show SMILES [H][C@@]12C[C@H](COC)OC[C@@]1(N=C(N)S[C@@H]2C)c1ccc(F)cc1F |r,t:11| Show InChI InChI=1S/C16H20F2N2O2S/c1-9-13-6-11(7-21-2)22-8-16(13,20-15(19)23-9)12-4-3-10(17)5-14(12)18/h3-5,9,11,13H,6-8H2,1-2H3,(H2,19,20)/t9-,11-,13+,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50078324
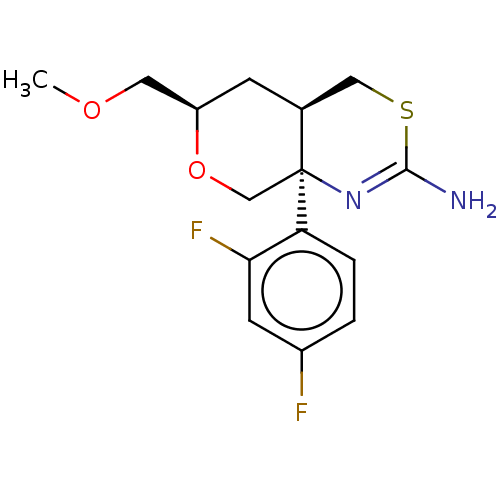
(CHEMBL3414708 | US9260455, 8)Show SMILES [H][C@]12CSC(N)=N[C@]1(CO[C@@H](COC)C2)c1ccc(F)cc1F |r,c:5| Show InChI InChI=1S/C15H18F2N2O2S/c1-20-6-11-4-9-7-22-14(18)19-15(9,8-21-11)12-3-2-10(16)5-13(12)17/h2-3,5,9,11H,4,6-8H2,1H3,(H2,18,19)/t9-,11+,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay |
J Med Chem 58: 3223-52 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00191
BindingDB Entry DOI: 10.7270/Q29W0H68 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data