 Found 983 hits with Last Name = 'camp' and Initial = 'h'
Found 983 hits with Last Name = 'camp' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291847

(7-[3-(Quinolin-2-ylmethoxy)-benzyloxy]-2-(1H-tetra...)Show SMILES O=C1CC(Oc2cc(OCc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C27H21N5O4/c33-24-14-26(27-29-31-32-30-27)36-25-13-21(10-11-22(24)25)34-15-17-4-3-6-20(12-17)35-16-19-9-8-18-5-1-2-7-23(18)28-19/h1-13,26H,14-16H2,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291855

(7-[3-(Quinolin-2-ylmethoxy)-phenoxymethyl]-2-(1H-t...)Show SMILES O=C1CC(Oc2cc(COc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C27H21N5O4/c33-24-14-26(27-29-31-32-30-27)36-25-12-17(8-11-22(24)25)15-34-20-5-3-6-21(13-20)35-16-19-10-9-18-4-1-2-7-23(18)28-19/h1-13,26H,14-16H2,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000485
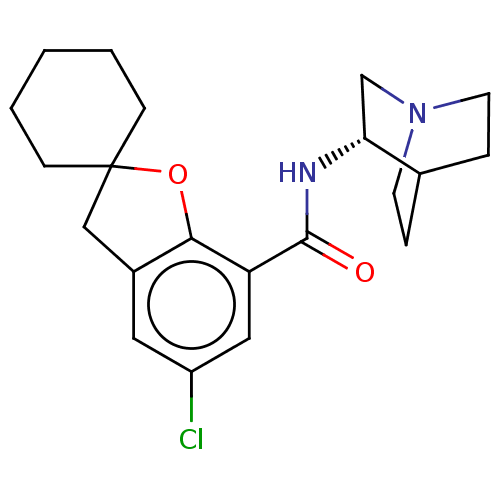
((S)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...)Show SMILES Clc1cc2CC3(CCCCC3)Oc2c(c1)C(=O)N[C@@H]1CN2CCC1CC2 |wU:18.20,(5.27,-4.79,;6.6,-5.56,;6.6,-7.11,;7.93,-7.88,;8.26,-9.38,;9.8,-9.54,;9.03,-10.87,;9.8,-12.21,;11.35,-12.21,;12.12,-10.87,;11.35,-9.52,;10.41,-8.14,;9.27,-7.11,;9.27,-5.55,;7.93,-4.79,;10.6,-4.77,;10.04,-3.34,;12.12,-5,;13.08,-3.79,;14.6,-4.1,;15.63,-2.95,;15.14,-1.5,;13.64,-1.18,;12.61,-2.34,;13.71,-3.05,;14.57,-2.32,)| Show InChI InChI=1S/C21H27ClN2O2/c22-16-10-15-12-21(6-2-1-3-7-21)26-19(15)17(11-16)20(25)23-18-13-24-8-4-14(18)5-9-24/h10-11,14,18H,1-9,12-13H2,(H,23,25)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50009075
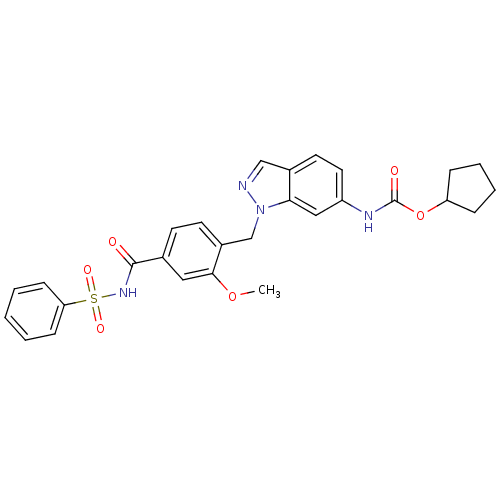
(CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H28N4O6S/c1-37-26-15-19(27(33)31-39(35,36)24-9-3-2-4-10-24)11-12-21(26)18-32-25-16-22(14-13-20(25)17-29-32)30-28(34)38-23-7-5-6-8-23/h2-4,9-17,23H,5-8,18H2,1H3,(H,30,34)(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against cysteinyl leukotriene D4 receptor from guinea pig lung membrane |
J Med Chem 33: 2828-41 (1990)
BindingDB Entry DOI: 10.7270/Q29C6WDM |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604954
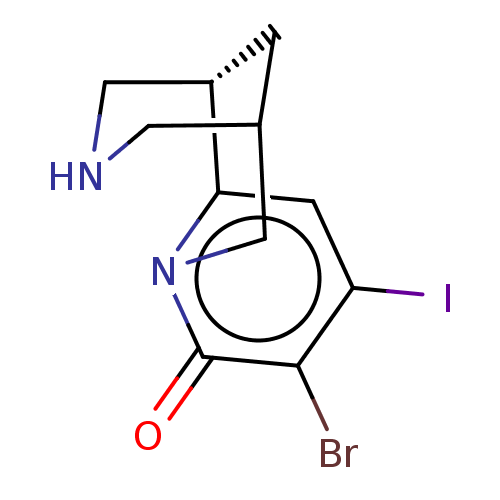
(US11667638, Example 160) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291848
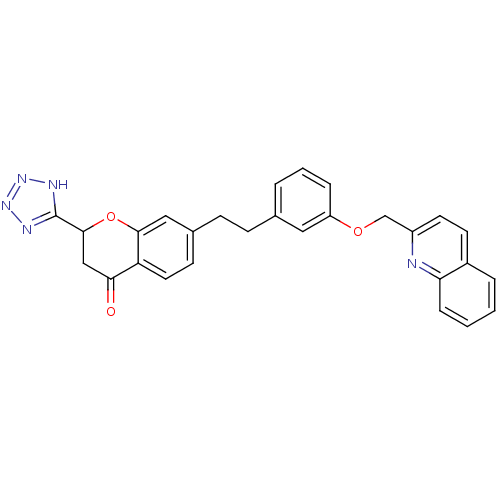
(7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-ethyl}-2-(1...)Show SMILES O=C1CC(Oc2cc(CCc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C28H23N5O3/c34-25-16-27(28-30-32-33-31-28)36-26-15-19(10-13-23(25)26)9-8-18-4-3-6-22(14-18)35-17-21-12-11-20-5-1-2-7-24(20)29-21/h1-7,10-15,27H,8-9,16-17H2,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009075

(CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H28N4O6S/c1-37-26-15-19(27(33)31-39(35,36)24-9-3-2-4-10-24)11-12-21(26)18-32-25-16-22(14-13-20(25)17-29-32)30-28(34)38-23-7-5-6-8-23/h2-4,9-17,23H,5-8,18H2,1H3,(H,30,34)(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604951

(US11667638, Example 127) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604955
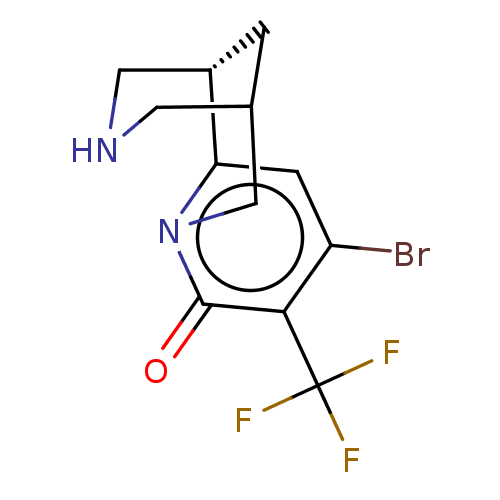
(US11667638, Example 162)Show SMILES FC(F)(F)c1c(Br)cc2[C@@H]3CNCC(C3)Cn2c1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000482
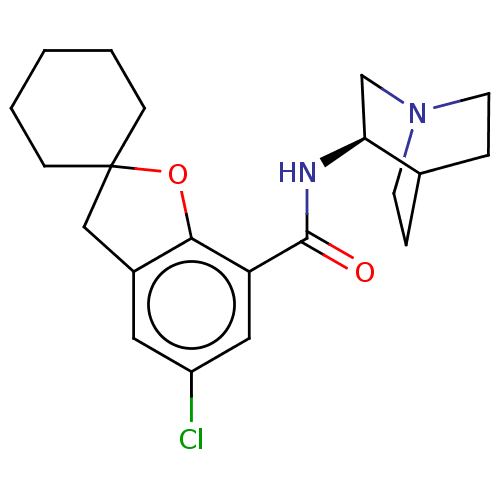
((R)-2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'...)Show SMILES Clc1cc2CC3(CCCCC3)Oc2c(c1)C(=O)N[C@H]1CN2CCC1CC2 |wD:18.20,(5.27,-4.79,;6.6,-5.56,;6.6,-7.11,;7.93,-7.88,;8.26,-9.38,;9.8,-9.54,;9.03,-10.87,;9.8,-12.21,;11.35,-12.21,;12.12,-10.87,;11.35,-9.52,;10.41,-8.14,;9.27,-7.11,;9.27,-5.55,;7.93,-4.79,;10.6,-4.77,;10.04,-3.34,;12.12,-5,;13.08,-3.79,;14.6,-4.1,;15.63,-2.95,;15.14,-1.5,;13.64,-1.18,;12.61,-2.34,;13.71,-3.05,;14.57,-2.32,)| Show InChI InChI=1S/C21H27ClN2O2/c22-16-10-15-12-21(6-2-1-3-7-21)26-19(15)17(11-16)20(25)23-18-13-24-8-4-14(18)5-9-24/h10-11,14,18H,1-9,12-13H2,(H,23,25)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291849
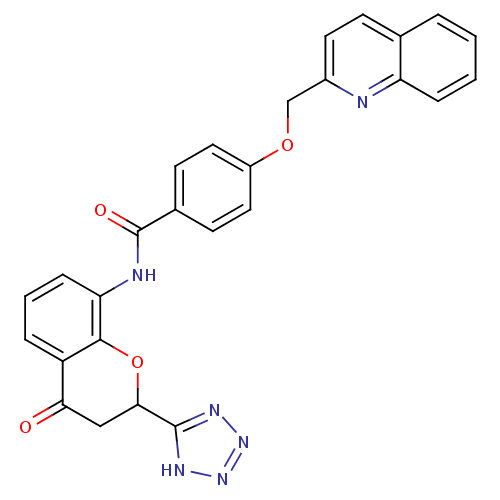
(CHEMBL47662 | N-[4-Oxo-2-(1H-tetrazol-5-yl)-chroma...)Show SMILES O=C(Nc1cccc2C(=O)CC(Oc12)c1nnn[nH]1)c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C27H20N6O4/c34-23-14-24(26-30-32-33-31-26)37-25-20(23)5-3-7-22(25)29-27(35)17-9-12-19(13-10-17)36-15-18-11-8-16-4-1-2-6-21(16)28-18/h1-13,24H,14-15H2,(H,29,35)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000480
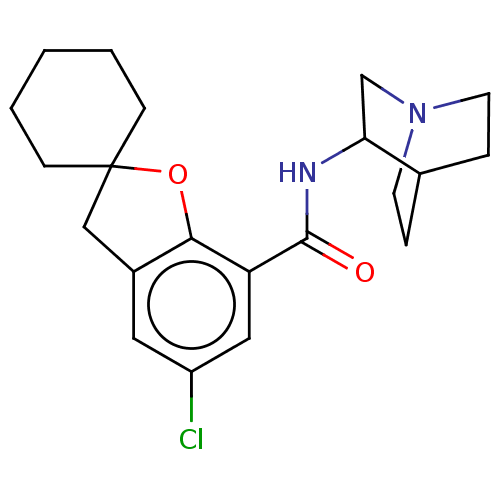
(2-(5-chlorospiro[2,3-dihydrobenzo[b]furan-2,1'-cyc...)Show SMILES Clc1cc2CC3(CCCCC3)Oc2c(c1)C(=O)NC1CN2CCC1CC2 |(5.27,-4.79,;6.6,-5.56,;6.6,-7.11,;7.93,-7.88,;8.26,-9.38,;9.8,-9.54,;9.03,-10.87,;9.8,-12.21,;11.35,-12.21,;12.12,-10.87,;11.35,-9.52,;10.41,-8.14,;9.27,-7.11,;9.27,-5.55,;7.93,-4.79,;10.6,-4.77,;10.04,-3.34,;12.12,-5,;13.08,-3.79,;14.6,-4.1,;15.63,-2.95,;15.14,-1.5,;13.64,-1.18,;12.61,-2.34,;13.71,-3.05,;14.57,-2.32,)| Show InChI InChI=1S/C21H27ClN2O2/c22-16-10-15-12-21(6-2-1-3-7-21)26-19(15)17(11-16)20(25)23-18-13-24-8-4-14(18)5-9-24/h10-11,14,18H,1-9,12-13H2,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000479
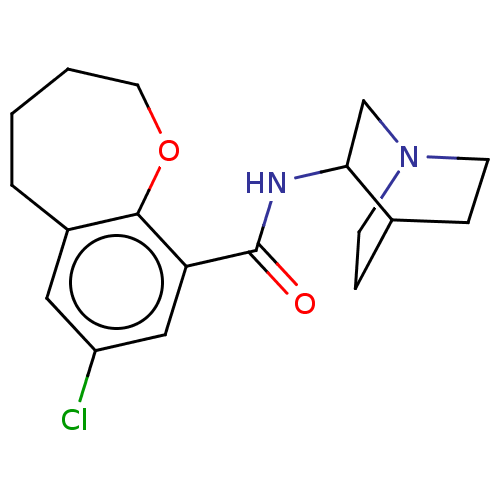
(7-Chloro-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carb...)Show SMILES Clc1cc2CCCCOc2c(c1)C(=O)NC1CN2CCC1CC2 |(3.81,-7.51,;5.15,-6.74,;6.48,-7.51,;7.82,-6.74,;9.03,-7.7,;10.52,-7.35,;11.18,-5.96,;10.52,-4.59,;9.03,-4.24,;7.82,-5.2,;6.48,-4.43,;5.15,-5.2,;6.48,-2.88,;5.15,-2.11,;7.82,-2.11,;7.82,-.57,;9.21,.13,;9.29,1.65,;8,2.52,;6.62,1.82,;6.53,.27,;7.84,.39,;8.07,1.5,)| Show InChI InChI=1S/C18H23ClN2O2/c19-14-9-13-3-1-2-8-23-17(13)15(10-14)18(22)20-16-11-21-6-4-12(16)5-7-21/h9-10,12,16H,1-8,11H2,(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291850
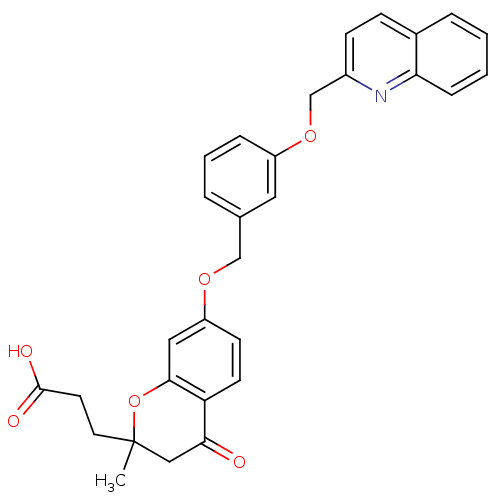
(3-{2-Methyl-4-oxo-7-[3-(quinolin-2-ylmethoxy)-benz...)Show SMILES CC1(CCC(O)=O)CC(=O)c2ccc(OCc3cccc(OCc4ccc5ccccc5n4)c3)cc2O1 Show InChI InChI=1S/C30H27NO6/c1-30(14-13-29(33)34)17-27(32)25-12-11-24(16-28(25)37-30)35-18-20-5-4-7-23(15-20)36-19-22-10-9-21-6-2-3-8-26(21)31-22/h2-12,15-16H,13-14,17-19H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604918
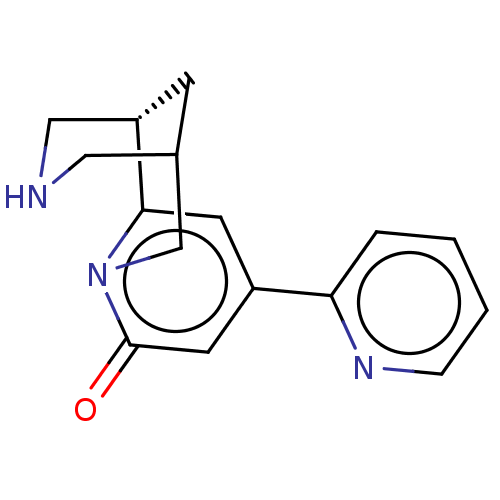
(US11667638, Example 101)Show SMILES O=c1cc(cc2[C@@H]3CNCC(C3)Cn12)-c1ccccn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM86311
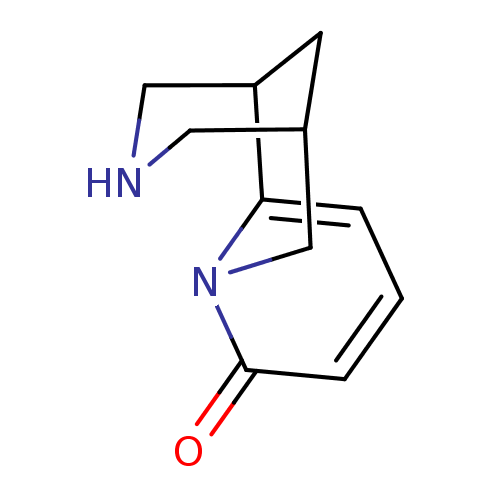
(CAS_485-35-8 | Cytisine | Cytisine-(-) | NSC_22407...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 1.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291854
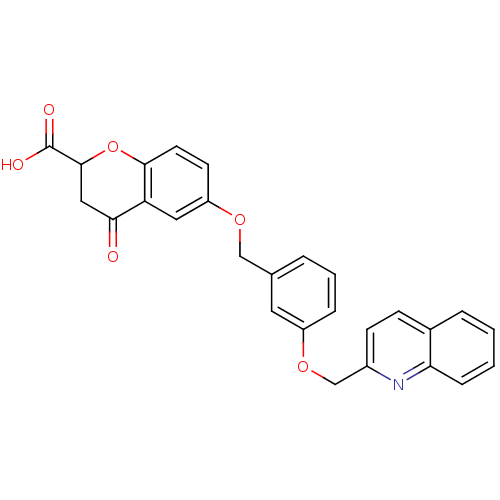
(4-Oxo-6-[3-(quinolin-2-ylmethoxy)-benzyloxy]-chrom...)Show SMILES OC(=O)C1CC(=O)c2cc(OCc3cccc(OCc4ccc5ccccc5n4)c3)ccc2O1 Show InChI InChI=1S/C27H21NO6/c29-24-14-26(27(30)31)34-25-11-10-21(13-22(24)25)32-15-17-4-3-6-20(12-17)33-16-19-9-8-18-5-1-2-7-23(18)28-19/h1-13,26H,14-16H2,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000483
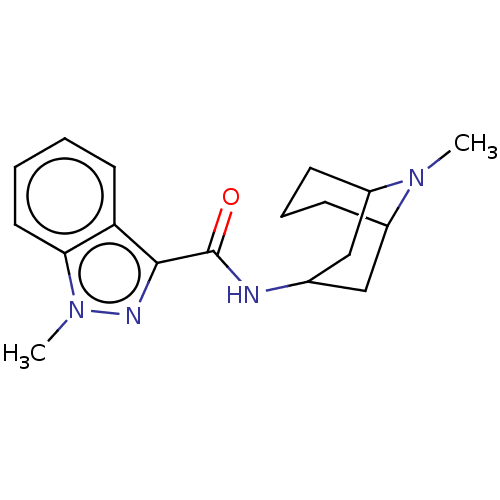
((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...)Show SMILES CN1C2CCCC1CC(C2)NC(=O)c1nn(C)c2ccccc12 |THB:10:8:1:3.5.4| Show InChI InChI=1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000495
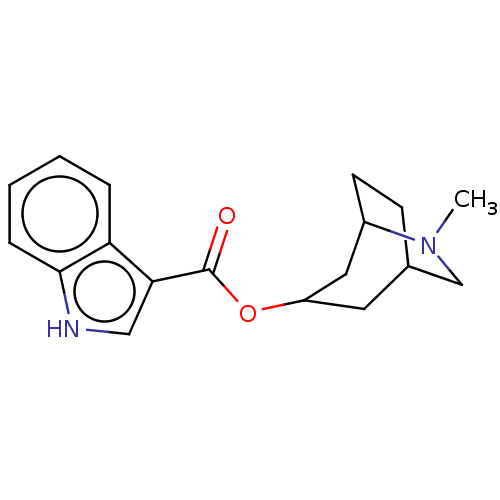
((ICS 205-930)1H-Indole-3-carboxylic acid 6-methyl-...)Show SMILES CN1CC2CCC1CC(C2)OC(=O)c1c[nH]c2ccccc12 |THB:0:1:8.7.9:5.4,10:8:1.2:5.4| Show InChI InChI=1S/C18H22N2O2/c1-20-11-12-6-7-13(20)9-14(8-12)22-18(21)16-10-19-17-5-3-2-4-15(16)17/h2-5,10,12-14,19H,6-9,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604901
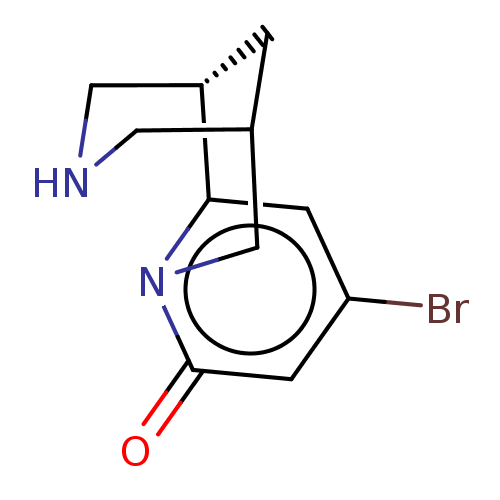
(US11667638, Example 62) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604937
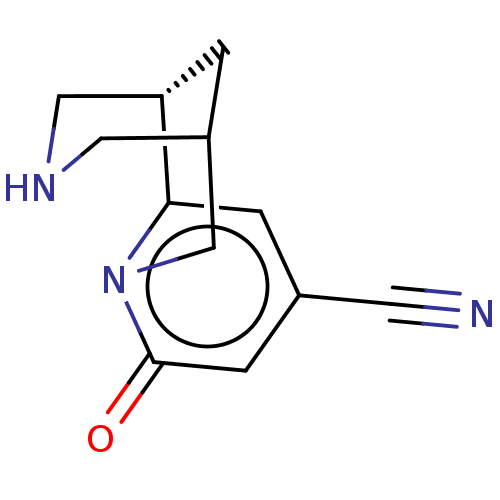
(US11667638, Example 140) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197404
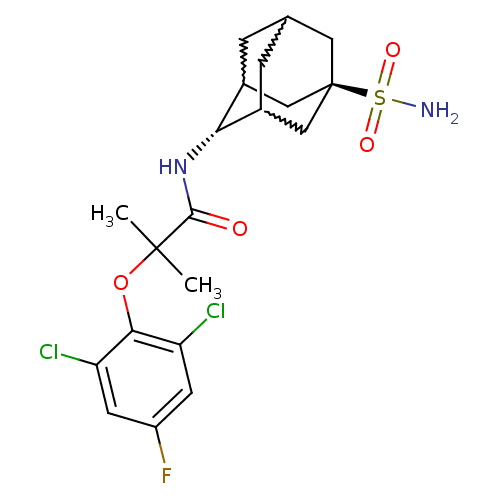
(2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...)Show SMILES CC(C)(Oc1c(Cl)cc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.19,17.28,21.21,wU:16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:17:24.19.20:22,16:21:24:25.18.17,TEB:18:19:22:25.17.16,20:21:25:24.19.18,(24.03,-36.5,;24.84,-37.8,;25.66,-39.11,;26.17,-37.02,;27.51,-37.78,;28.83,-37,;28.81,-35.46,;30.17,-37.75,;30.18,-39.3,;31.52,-40.06,;28.85,-40.08,;27.52,-39.32,;26.19,-40.1,;23.56,-38.65,;23.65,-40.19,;22.18,-37.97,;20.9,-38.82,;20.89,-40.35,;19.87,-41.62,;18.47,-41.06,;18.46,-39.47,;19.5,-38.24,;18.15,-38.72,;18.16,-40.2,;16.97,-41.48,;19.49,-40.69,;16.67,-39.79,;15.17,-39.39,;17.07,-38.31,;16.26,-41.28,)| Show InChI InChI=1S/C20H25Cl2FN2O4S/c1-19(2,29-17-14(21)5-13(23)6-15(17)22)18(26)25-16-11-3-10-4-12(16)9-20(7-10,8-11)30(24,27)28/h5-6,10-12,16H,3-4,7-9H2,1-2H3,(H,25,26)(H2,24,27,28)/t10?,11?,12?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197399
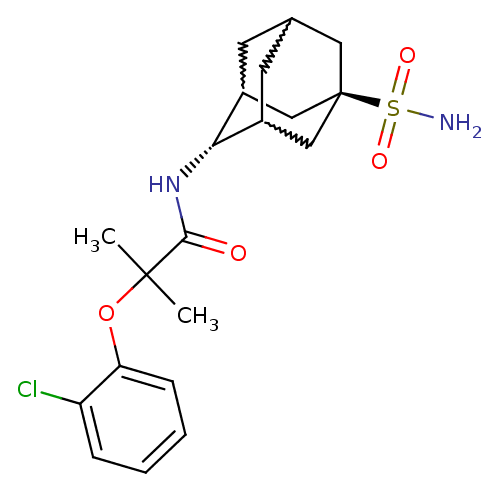
(2-(2-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(20.57,1.97,;21.38,.67,;22.2,-.64,;22.71,1.45,;24.05,.69,;24.05,-.85,;25.39,-1.61,;26.72,-.83,;26.71,.72,;25.37,1.47,;25.35,3.01,;20.1,-.18,;20.19,-1.72,;18.72,.5,;17.44,-.35,;17.42,-1.88,;16.41,-3.16,;15.01,-2.59,;15,-1,;16.04,.23,;14.69,-.25,;14.7,-1.73,;13.5,-3.01,;16.03,-2.22,;13.21,-1.32,;11.71,-.92,;13.61,.16,;12.8,-2.81,)| Show InChI InChI=1S/C20H27ClN2O4S/c1-19(2,27-16-6-4-3-5-15(16)21)18(24)23-17-13-7-12-8-14(17)11-20(9-12,10-13)28(22,25)26/h3-6,12-14,17H,7-11H2,1-2H3,(H,23,24)(H2,22,25,26)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50202094
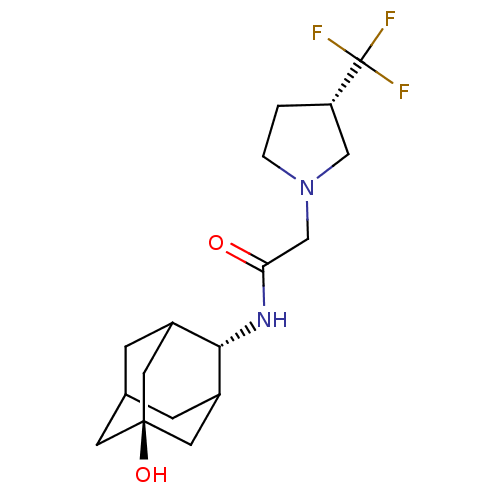
(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197418
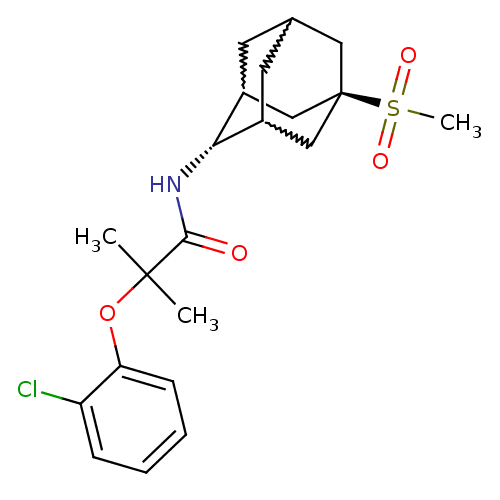
(2-(2-chloro-phenoxy)-N-(5-methanesulfonyl-adamanta...)Show SMILES CC(C)(Oc1ccccc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(C)(=O)=O |w:17.17,15.26,19.19,wU:14.14,wD:21.27,TLB:14:15:22:18.19.20,13:14:22.17.18:20,THB:16:15:22.17.18:20,14:19:22:23.16.15,TEB:16:17:20:23.15.14,18:19:23:22.17.16,(2.61,-15.76,;3.42,-17.07,;4.24,-18.37,;4.75,-16.28,;6.09,-17.05,;6.1,-18.59,;7.44,-19.35,;8.77,-18.57,;8.76,-17.02,;7.41,-16.26,;7.39,-14.72,;2.14,-17.92,;2.23,-19.46,;.76,-17.23,;-.53,-18.08,;-.54,-19.61,;-1.56,-20.89,;-2.97,-20.33,;-2.97,-18.74,;-1.93,-17.5,;-3.28,-17.98,;-3.27,-19.47,;-4.47,-20.75,;-1.94,-19.96,;-4.77,-19.06,;-6.26,-18.66,;-4.36,-17.57,;-5.17,-20.55,)| Show InChI InChI=1S/C21H28ClNO4S/c1-20(2,27-17-7-5-4-6-16(17)22)19(24)23-18-14-8-13-9-15(18)12-21(10-13,11-14)28(3,25)26/h4-7,13-15,18H,8-12H2,1-3H3,(H,23,24)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50013556
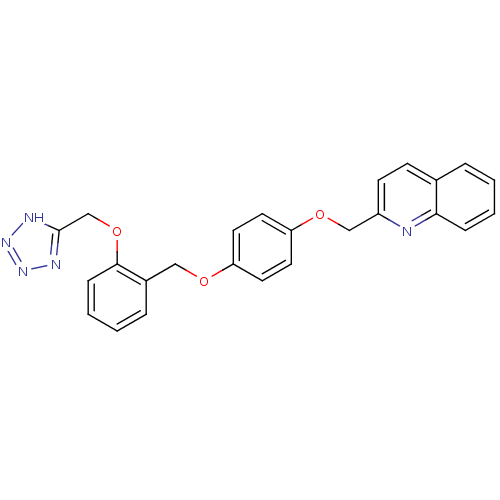
(2-{4-[2-(1H-Tetrazol-5-ylmethoxy)-benzyloxy]-pheno...)Show SMILES C(Oc1ccccc1COc1ccc(OCc2ccc3ccccc3n2)cc1)c1nnn[nH]1 Show InChI InChI=1S/C25H21N5O3/c1-3-7-23-18(5-1)9-10-20(26-23)16-32-22-13-11-21(12-14-22)31-15-19-6-2-4-8-24(19)33-17-25-27-29-30-28-25/h1-14H,15-17H2,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291856
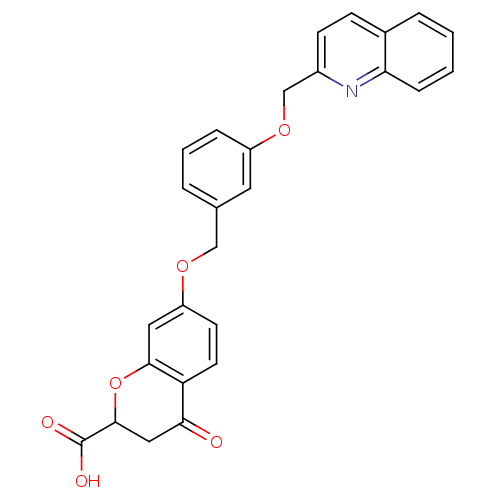
(4-Oxo-7-[3-(quinolin-2-ylmethoxy)-benzyloxy]-chrom...)Show SMILES OC(=O)C1CC(=O)c2ccc(OCc3cccc(OCc4ccc5ccccc5n4)c3)cc2O1 Show InChI InChI=1S/C27H21NO6/c29-24-14-26(27(30)31)34-25-13-21(10-11-22(24)25)32-15-17-4-3-6-20(12-17)33-16-19-9-8-18-5-1-2-7-23(18)28-19/h1-13,26H,14-16H2,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50000494

(7-Bromo-2,3,4,5-tetrahydro-benzo[b]oxepine-9-carbo...)Show SMILES Brc1cc2CCCCOc2c(c1)C(=O)NC1CN2CCC1CC2 |(3.81,-7.51,;5.15,-6.74,;6.48,-7.51,;7.82,-6.74,;9.03,-7.7,;10.52,-7.35,;11.18,-5.96,;10.52,-4.59,;9.03,-4.24,;7.82,-5.2,;6.48,-4.43,;5.15,-5.2,;6.48,-2.88,;5.15,-2.11,;7.82,-2.11,;7.82,-.57,;9.21,.13,;9.29,1.65,;8,2.52,;6.62,1.82,;6.53,.27,;7.84,.39,;8.07,1.5,)| Show InChI InChI=1S/C18H23BrN2O2/c19-14-9-13-3-1-2-8-23-17(13)15(10-14)18(22)20-16-11-21-6-4-12(16)5-7-21/h9-10,12,16H,1-8,11H2,(H,20,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Displacement of the 5-hydroxytryptamine 3 receptor ligand [3H]GR-65630 from rat brain cortical membranes. |
J Med Chem 35: 895-903 (1992)
BindingDB Entry DOI: 10.7270/Q2SJ1M77 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50013548
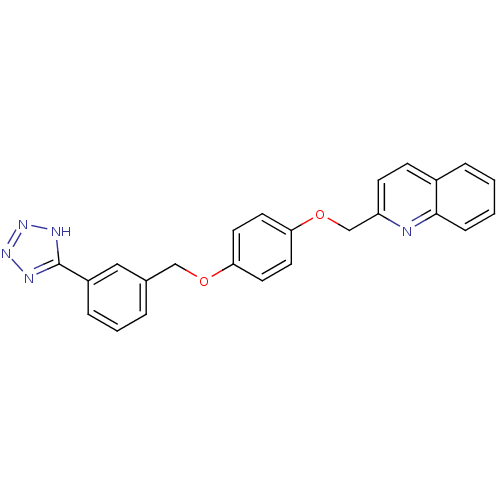
(2-{4-[3-(1H-Tetrazol-5-yl)-benzyloxy]-phenoxymethy...)Show SMILES C(Oc1ccc(OCc2ccc3ccccc3n2)cc1)c1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C24H19N5O2/c1-2-7-23-18(5-1)8-9-20(25-23)16-31-22-12-10-21(11-13-22)30-15-17-4-3-6-19(14-17)24-26-28-29-27-24/h1-14H,15-16H2,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50013559

(2-{3-[4-(1H-Tetrazol-5-ylmethoxy)-benzyloxy]-pheno...)Show SMILES C(Oc1ccc(COc2cccc(OCc3ccc4ccccc4n3)c2)cc1)c1nnn[nH]1 Show InChI InChI=1S/C25H21N5O3/c1-2-7-24-19(4-1)10-11-20(26-24)16-32-23-6-3-5-22(14-23)31-15-18-8-12-21(13-9-18)33-17-25-27-29-30-28-25/h1-14H,15-17H2,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604900
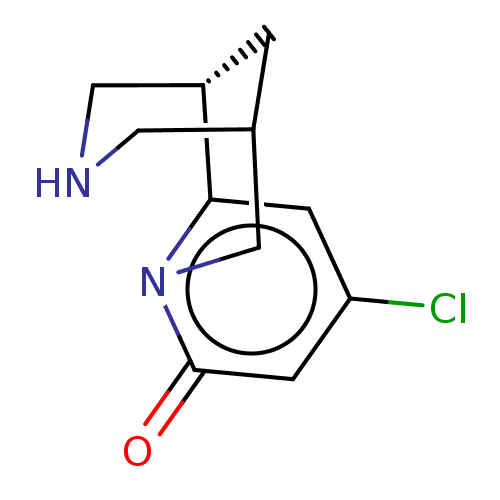
(US11667638, Example 60) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291858
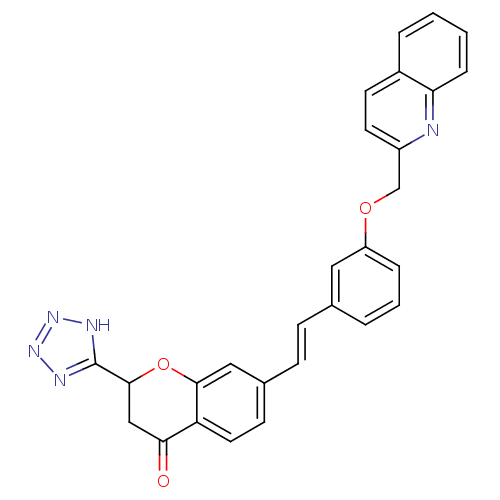
(7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-vinyl}-2-(1...)Show SMILES O=C1CC(Oc2cc(\C=C\c3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C28H21N5O3/c34-25-16-27(28-30-32-33-31-28)36-26-15-19(10-13-23(25)26)9-8-18-4-3-6-22(14-18)35-17-21-12-11-20-5-1-2-7-24(20)29-21/h1-15,27H,16-17H2,(H,30,31,32,33)/b9-8+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50013570
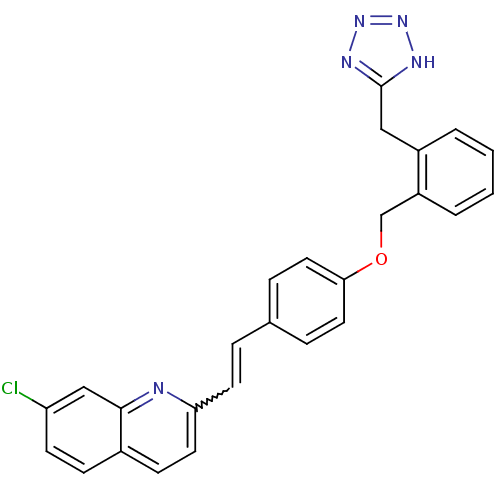
(7-Chloro-2-(2-{4-[2-(1H-tetrazol-5-ylmethyl)-benzy...)Show SMILES Clc1ccc2ccc(C=Cc3ccc(OCc4ccccc4Cc4nnn[nH]4)cc3)nc2c1 |w:8.7| Show InChI InChI=1S/C26H20ClN5O/c27-22-10-8-19-9-12-23(28-25(19)16-22)11-5-18-6-13-24(14-7-18)33-17-21-4-2-1-3-20(21)15-26-29-31-32-30-26/h1-14,16H,15,17H2,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50013562
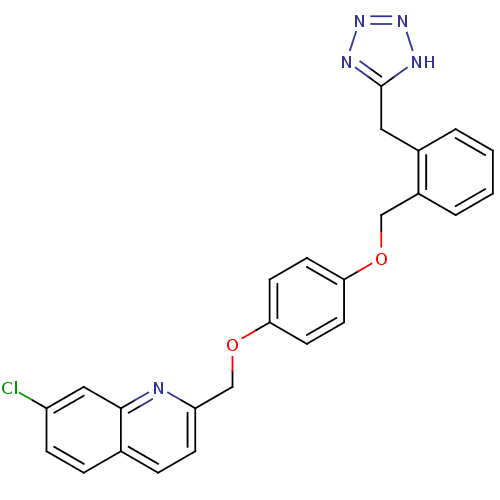
(7-Chloro-2-{4-[2-(1H-tetrazol-5-ylmethyl)-benzylox...)Show SMILES Clc1ccc2ccc(COc3ccc(OCc4ccccc4Cc4nnn[nH]4)cc3)nc2c1 Show InChI InChI=1S/C25H20ClN5O2/c26-20-7-5-17-6-8-21(27-24(17)14-20)16-33-23-11-9-22(10-12-23)32-15-19-4-2-1-3-18(19)13-25-28-30-31-29-25/h1-12,14H,13,15-16H2,(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604961
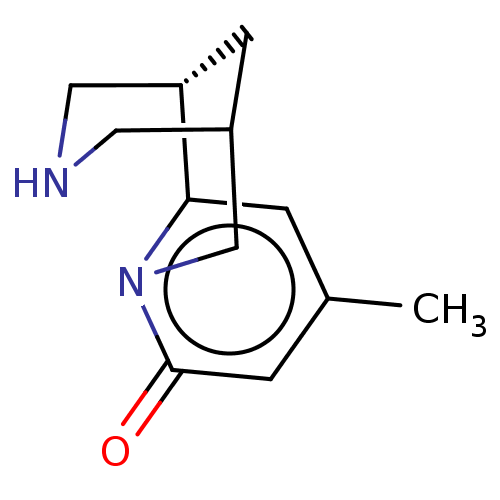
(US11667638, Example 110) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604899
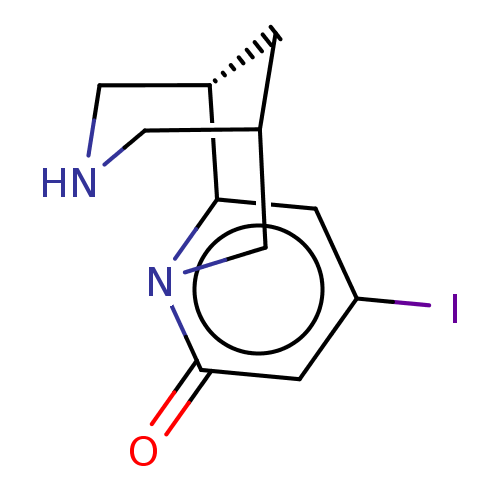
(US11667638, Example 82) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604952
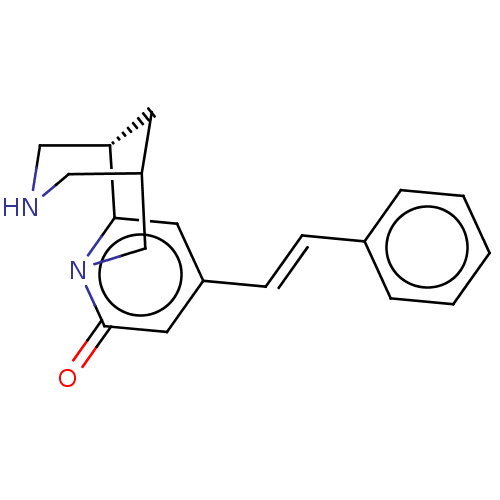
(US11667638, Example 114)Show SMILES O=c1cc(\C=C\c2ccccc2)cc2[C@@H]3CNCC(C3)Cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50013550
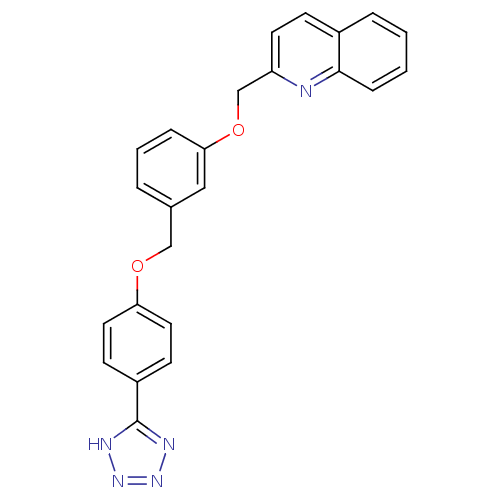
(2-{3-[4-(1H-Tetrazol-5-yl)-phenoxymethyl]-phenoxym...)Show SMILES C(Oc1ccc(cc1)-c1nnn[nH]1)c1cccc(OCc2ccc3ccccc3n2)c1 Show InChI InChI=1S/C24H19N5O2/c1-2-7-23-18(5-1)8-11-20(25-23)16-31-22-6-3-4-17(14-22)15-30-21-12-9-19(10-13-21)24-26-28-29-27-24/h1-14H,15-16H2,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604960
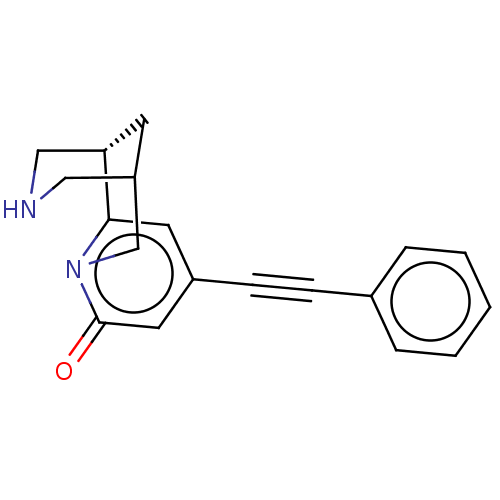
(US11667638, Example 129)Show SMILES O=c1cc(cc2[C@@H]3CNCC(C3)Cn12)C#Cc1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50197404

(2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...)Show SMILES CC(C)(Oc1c(Cl)cc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.19,17.28,21.21,wU:16.16,wD:23.29,TLB:16:17:24:20.21.22,15:16:24.19.20:22,THB:18:17:24.19.20:22,16:21:24:25.18.17,TEB:18:19:22:25.17.16,20:21:25:24.19.18,(24.03,-36.5,;24.84,-37.8,;25.66,-39.11,;26.17,-37.02,;27.51,-37.78,;28.83,-37,;28.81,-35.46,;30.17,-37.75,;30.18,-39.3,;31.52,-40.06,;28.85,-40.08,;27.52,-39.32,;26.19,-40.1,;23.56,-38.65,;23.65,-40.19,;22.18,-37.97,;20.9,-38.82,;20.89,-40.35,;19.87,-41.62,;18.47,-41.06,;18.46,-39.47,;19.5,-38.24,;18.15,-38.72,;18.16,-40.2,;16.97,-41.48,;19.49,-40.69,;16.67,-39.79,;15.17,-39.39,;17.07,-38.31,;16.26,-41.28,)| Show InChI InChI=1S/C20H25Cl2FN2O4S/c1-19(2,29-17-14(21)5-13(23)6-15(17)22)18(26)25-16-11-3-10-4-12(16)9-20(7-10,8-11)30(24,27)28/h5-6,10-12,16H,3-4,7-9H2,1-2H3,(H,25,26)(H2,24,27,28)/t10?,11?,12?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50006799
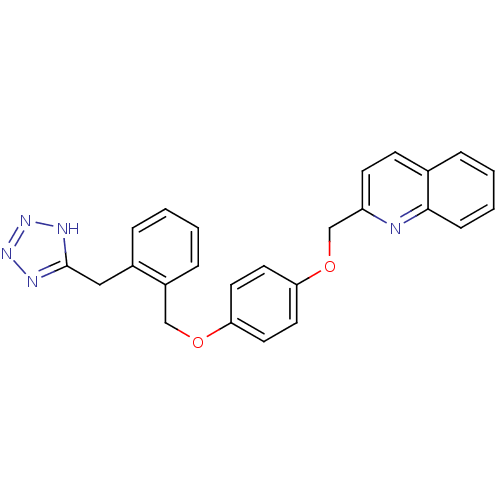
(2-{4-[2-(1H-Tetrazol-5-ylmethyl)-benzyloxy]-phenox...)Show SMILES C(Oc1ccc(OCc2ccccc2Cc2nnn[nH]2)cc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C25H21N5O2/c1-2-7-20(19(6-1)15-25-27-29-30-28-25)16-31-22-11-13-23(14-12-22)32-17-21-10-9-18-5-3-4-8-24(18)26-21/h1-14H,15-17H2,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50006799

(2-{4-[2-(1H-Tetrazol-5-ylmethyl)-benzyloxy]-phenox...)Show SMILES C(Oc1ccc(OCc2ccccc2Cc2nnn[nH]2)cc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C25H21N5O2/c1-2-7-20(19(6-1)15-25-27-29-30-28-25)16-31-22-11-13-23(14-12-22)32-17-21-10-9-18-5-3-4-8-24(18)26-21/h1-14H,15-17H2,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) |
J Med Chem 33: 1194-200 (1990)
BindingDB Entry DOI: 10.7270/Q2PK0F4V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50202094

(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat 11beta-HSD1 |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50202094

(CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)CN1CC[C@@H](C1)C(F)(F)F)C(C3)C2 |r,wU:7.8,wD:1.0,15.18,TLB:4:3:23:6.5.7,4:5:2.3.22:23,THB:7:5:2:22.21.23,7:21:2:6.4.5,8:7:2.3.22:23,(11.97,-27.52,;13.48,-28.24,;12.23,-29.46,;13.75,-29.11,;15.13,-29.73,;16.2,-28.5,;14.79,-28.79,;16.28,-26.98,;17.6,-26.18,;18.94,-26.93,;18.97,-28.47,;20.23,-26.07,;21.58,-26.81,;22.97,-26.15,;24.03,-27.27,;23.29,-28.62,;21.78,-28.33,;23.95,-30.01,;24.71,-31.34,;22.59,-30.72,;25.32,-29.31,;14.91,-26.34,;13.82,-27.52,;13.54,-26.75,)| Show InChI InChI=1S/C17H25F3N2O2/c18-17(19,20)13-1-2-22(8-13)9-14(23)21-15-11-3-10-4-12(15)7-16(24,5-10)6-11/h10-13,15,24H,1-9H2,(H,21,23)/t10?,11?,12?,13-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in E. coli by SPA |
J Med Chem 50: 149-64 (2007)
Article DOI: 10.1021/jm0609364
BindingDB Entry DOI: 10.7270/Q2Z60NQN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197410
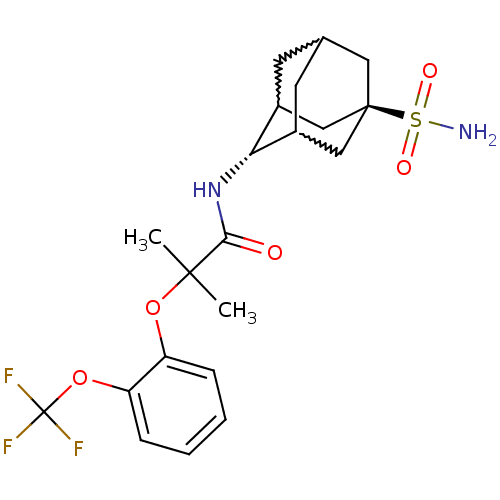
(2-methyl-N-(5-sulfamoyl-adamantan-2-yl)-2-(2-trifl...)Show SMILES CC(C)(Oc1ccccc1OC(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:19.30,23.23,21.22,wU:18.18,wD:25.31,TLB:18:19:26:22.23.24,17:18:26.21.22:24,THB:20:21:24:27.19.18,20:19:26.21.22:24,18:23:26:27.20.19,TEB:22:21:27:23.24.18,22:23:27:26.21.20,(22.5,-25.65,;23.31,-26.95,;24.12,-28.26,;24.64,-26.17,;25.98,-26.93,;25.98,-28.47,;27.32,-29.23,;28.65,-28.45,;28.64,-26.9,;27.3,-26.15,;27.28,-24.61,;28.6,-23.82,;29.93,-23.04,;27.82,-22.5,;29.39,-25.15,;22.03,-27.8,;22.12,-29.34,;20.65,-27.12,;19.36,-27.97,;19.35,-29.5,;18.34,-30.77,;16.93,-30.21,;16.93,-28.62,;17.97,-27.39,;16.62,-27.87,;16.63,-29.35,;15.43,-30.63,;17.96,-29.84,;15.14,-28.94,;13.64,-28.54,;15.54,-27.46,;14.73,-30.43,)| Show InChI InChI=1S/C21H27F3N2O5S/c1-19(2,30-15-5-3-4-6-16(15)31-21(22,23)24)18(27)26-17-13-7-12-8-14(17)11-20(9-12,10-13)32(25,28)29/h3-6,12-14,17H,7-11H2,1-2H3,(H,26,27)(H2,25,28,29)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197403
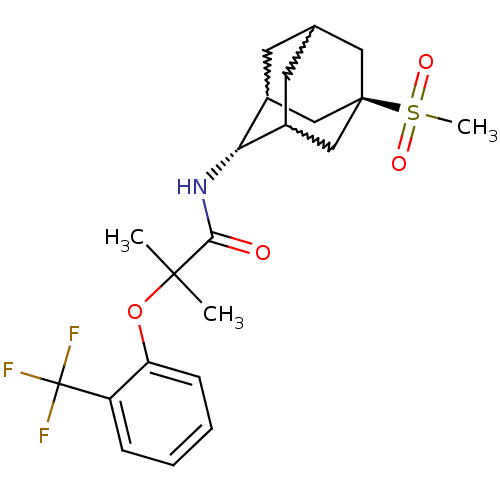
(CHEMBL393167 | N-(5-methanesulfonyl-adamantan-2-yl...)Show SMILES CC(C)(Oc1ccccc1C(F)(F)F)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(C)(=O)=O |w:20.20,18.29,22.22,wU:17.17,wD:24.30,TLB:17:18:25:21.22.23,16:17:25.20.21:23,THB:19:18:25.20.21:23,17:22:25:26.19.18,TEB:19:20:23:26.18.17,21:22:26:25.20.19,(22.98,-26.47,;23.79,-27.78,;24.61,-29.08,;25.12,-27,;26.46,-27.76,;26.47,-29.29,;27.8,-30.05,;29.13,-29.27,;29.12,-27.73,;27.78,-26.97,;27.76,-25.43,;27.75,-23.89,;26.22,-25.45,;29.3,-25.43,;22.51,-28.63,;22.6,-30.16,;21.13,-27.94,;19.85,-28.79,;19.84,-30.32,;18.82,-31.6,;17.42,-31.03,;17.41,-29.44,;18.45,-28.21,;17.1,-28.69,;17.11,-30.18,;15.92,-31.45,;18.44,-30.67,;15.62,-29.77,;14.12,-29.36,;16.02,-28.28,;15.21,-31.25,)| Show InChI InChI=1S/C22H28F3NO4S/c1-20(2,30-17-7-5-4-6-16(17)22(23,24)25)19(27)26-18-14-8-13-9-15(18)12-21(10-13,11-14)31(3,28)29/h4-7,13-15,18H,8-12H2,1-3H3,(H,26,27)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50197416
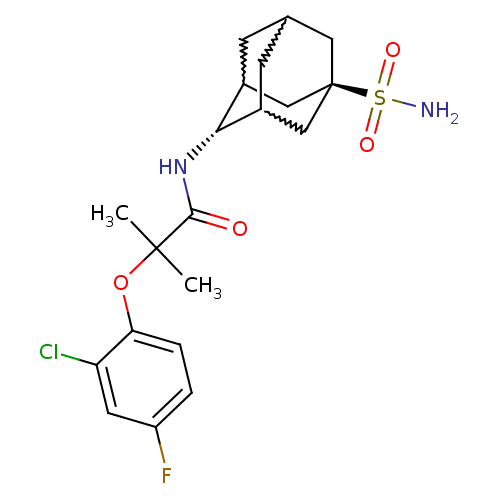
(2-(2-chloro-4-fluoro-phenoxy)-2-methyl-N-(5-sulfam...)Show SMILES CC(C)(Oc1ccc(F)cc1Cl)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)S(N)(=O)=O |w:18.18,16.27,20.20,wU:15.15,wD:22.28,TLB:15:16:23:19.20.21,14:15:23.18.19:21,THB:17:16:23.18.19:21,15:20:23:24.17.16,TEB:17:18:21:24.16.15,19:20:24:23.18.17,(2.86,-37.78,;3.68,-39.09,;4.49,-40.39,;5.01,-38.31,;6.35,-39.07,;6.35,-40.6,;7.69,-41.36,;9.02,-40.58,;10.36,-41.34,;9,-39.03,;7.67,-38.28,;7.65,-36.74,;2.4,-39.94,;2.49,-41.47,;1.02,-39.25,;-.27,-40.1,;-.28,-41.63,;-1.29,-42.91,;-2.7,-42.34,;-2.7,-40.75,;-1.66,-39.52,;-3.01,-40,;-3,-41.49,;-4.2,-42.76,;-1.67,-41.98,;-4.5,-41.07,;-5.99,-40.67,;-4.09,-39.59,;-4.9,-42.56,)| Show InChI InChI=1S/C20H26ClFN2O4S/c1-19(2,28-16-4-3-14(22)7-15(16)21)18(25)24-17-12-5-11-6-13(17)10-20(8-11,9-12)29(23,26)27/h3-4,7,11-13,17H,5-6,8-10H2,1-2H3,(H,24,25)(H2,23,26,27)/t11?,12?,13?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol |
Bioorg Med Chem Lett 17: 527-32 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.008
BindingDB Entry DOI: 10.7270/Q2BZ65PP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604958
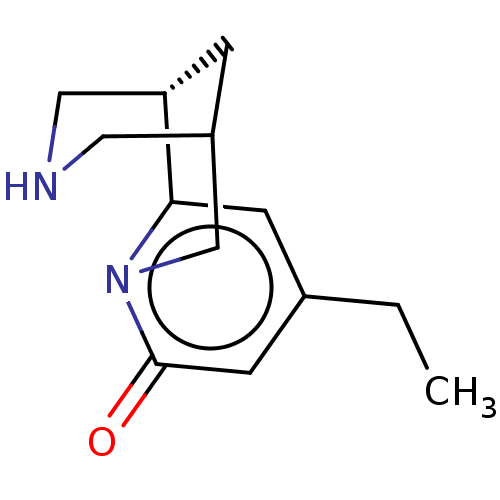
(US11667638, Example 118) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM604920
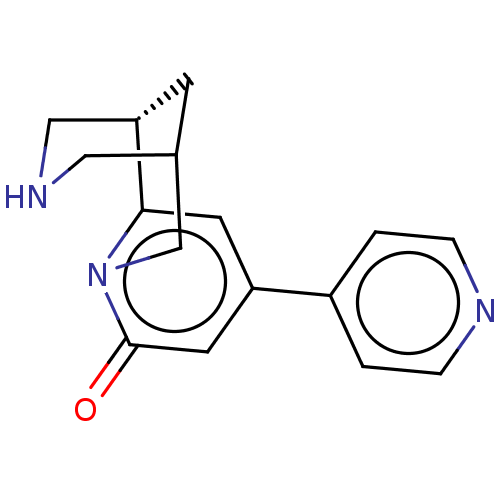
(US11667638, Example 103)Show SMILES O=c1cc(cc2[C@@H]3CNCC(C3)Cn12)-c1ccncc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VM4H7F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data