 Found 43 hits with Last Name = 'campbell' and Initial = 'sj'
Found 43 hits with Last Name = 'campbell' and Initial = 'sj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380359
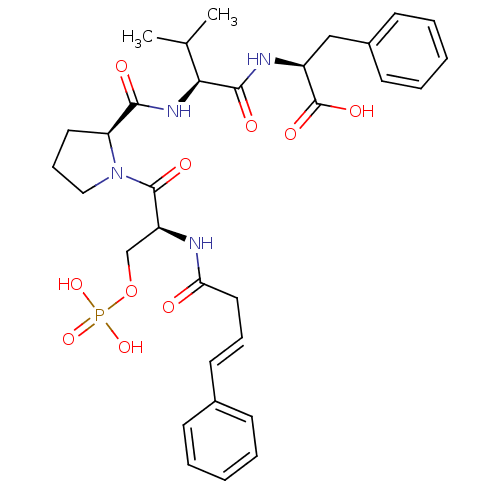
(CHEMBL2017822)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)C\C=C\c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C32H41N4O10P/c1-21(2)28(30(39)34-24(32(41)42)19-23-13-7-4-8-14-23)35-29(38)26-16-10-18-36(26)31(40)25(20-46-47(43,44)45)33-27(37)17-9-15-22-11-5-3-6-12-22/h3-9,11-15,21,24-26,28H,10,16-20H2,1-2H3,(H,33,37)(H,34,39)(H,35,38)(H,41,42)(H2,43,44,45)/b15-9+/t24-,25-,26-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380358
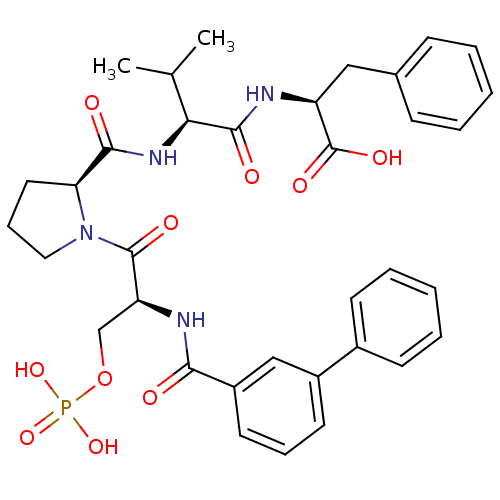
(CHEMBL2017821)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)c1cccc(c1)-c1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C35H41N4O10P/c1-22(2)30(33(42)36-27(35(44)45)19-23-11-5-3-6-12-23)38-32(41)29-17-10-18-39(29)34(43)28(21-49-50(46,47)48)37-31(40)26-16-9-15-25(20-26)24-13-7-4-8-14-24/h3-9,11-16,20,22,27-30H,10,17-19,21H2,1-2H3,(H,36,42)(H,37,40)(H,38,41)(H,44,45)(H2,46,47,48)/t27-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380353
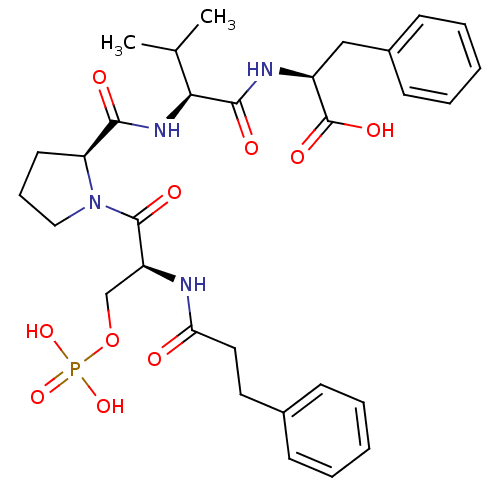
(CHEMBL2017816)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)CCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H41N4O10P/c1-20(2)27(29(38)33-23(31(40)41)18-22-12-7-4-8-13-22)34-28(37)25-14-9-17-35(25)30(39)24(19-45-46(42,43)44)32-26(36)16-15-21-10-5-3-6-11-21/h3-8,10-13,20,23-25,27H,9,14-19H2,1-2H3,(H,32,36)(H,33,38)(H,34,37)(H,40,41)(H2,42,43,44)/t23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380352

(CHEMBL2017815)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C37H46N5O11P/c1-22(2)32(35(46)39-29(37(48)49)19-24-10-5-4-6-11-24)41-34(45)31-14-9-17-42(31)36(47)30(21-53-54(50,51)52)40-33(44)28(38-23(3)43)20-25-15-16-26-12-7-8-13-27(26)18-25/h4-8,10-13,15-16,18,22,28-32H,9,14,17,19-21H2,1-3H3,(H,38,43)(H,39,46)(H,40,44)(H,41,45)(H,48,49)(H2,50,51,52)/t28-,29-,30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50345641
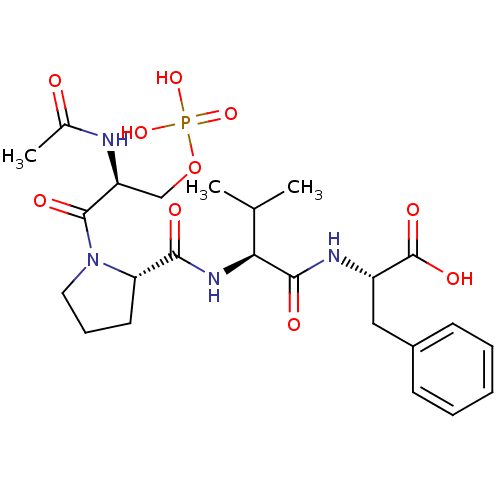
((S)-2-((S)-2-((S)-1-((S)-2-acetamido-3-(phosphonoo...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H35N4O10P/c1-14(2)20(22(31)26-17(24(33)34)12-16-8-5-4-6-9-16)27-21(30)19-10-7-11-28(19)23(32)18(25-15(3)29)13-38-39(35,36)37/h4-6,8-9,14,17-20H,7,10-13H2,1-3H3,(H,25,29)(H,26,31)(H,27,30)(H,33,34)(H2,35,36,37)/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50345640

((S)-2-((2S,3R)-2-((S)-1-((S)-2-acetamido-3-(phosph...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C23H33N4O11P/c1-13(28)19(21(31)25-16(23(33)34)11-15-7-4-3-5-8-15)26-20(30)18-9-6-10-27(18)22(32)17(24-14(2)29)12-38-39(35,36)37/h3-5,7-8,13,16-19,28H,6,9-12H2,1-2H3,(H,24,29)(H,25,31)(H,26,30)(H,33,34)(H2,35,36,37)/t13-,16+,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380354

(CHEMBL2017817)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)CCCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C32H43N4O10P/c1-21(2)28(30(39)34-24(32(41)42)19-23-13-7-4-8-14-23)35-29(38)26-16-10-18-36(26)31(40)25(20-46-47(43,44)45)33-27(37)17-9-15-22-11-5-3-6-12-22/h3-8,11-14,21,24-26,28H,9-10,15-20H2,1-2H3,(H,33,37)(H,34,39)(H,35,38)(H,41,42)(H2,43,44,45)/t24-,25-,26-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380351
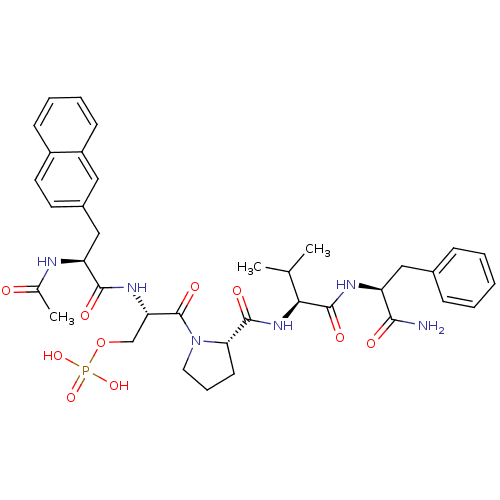
(CHEMBL2017814)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C37H47N6O10P/c1-22(2)32(36(48)40-28(33(38)45)19-24-10-5-4-6-11-24)42-35(47)31-14-9-17-43(31)37(49)30(21-53-54(50,51)52)41-34(46)29(39-23(3)44)20-25-15-16-26-12-7-8-13-27(26)18-25/h4-8,10-13,15-16,18,22,28-32H,9,14,17,19-21H2,1-3H3,(H2,38,45)(H,39,44)(H,40,48)(H,41,46)(H,42,47)(H2,50,51,52)/t28-,29-,30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380349
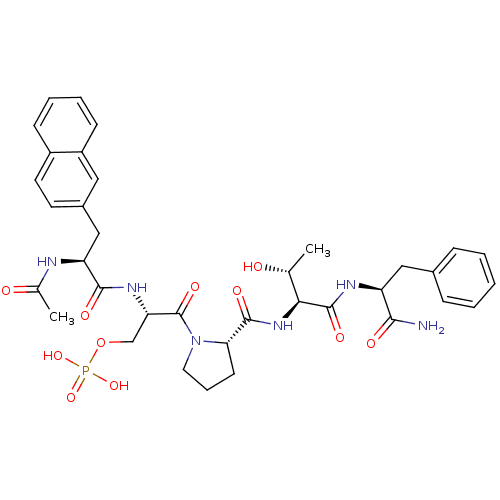
(CHEMBL2017812)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H45N6O11P/c1-21(43)31(35(48)39-27(32(37)45)18-23-9-4-3-5-10-23)41-34(47)30-13-8-16-42(30)36(49)29(20-53-54(50,51)52)40-33(46)28(38-22(2)44)19-24-14-15-25-11-6-7-12-26(25)17-24/h3-7,9-12,14-15,17,21,27-31,43H,8,13,16,18-20H2,1-2H3,(H2,37,45)(H,38,44)(H,39,48)(H,40,46)(H,41,47)(H2,50,51,52)/t21-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50345623
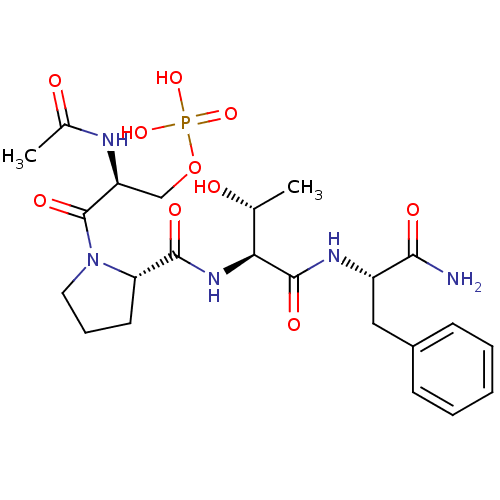
((S)-2-acetamido-3-((S)-2-((2S,3R)-1-((S)-1-amino-1...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C23H34N5O10P/c1-13(29)19(22(33)26-16(20(24)31)11-15-7-4-3-5-8-15)27-21(32)18-9-6-10-28(18)23(34)17(25-14(2)30)12-38-39(35,36)37/h3-5,7-8,13,16-19,29H,6,9-12H2,1-2H3,(H2,24,31)(H,25,30)(H,26,33)(H,27,32)(H2,35,36,37)/t13-,16+,17+,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50345629
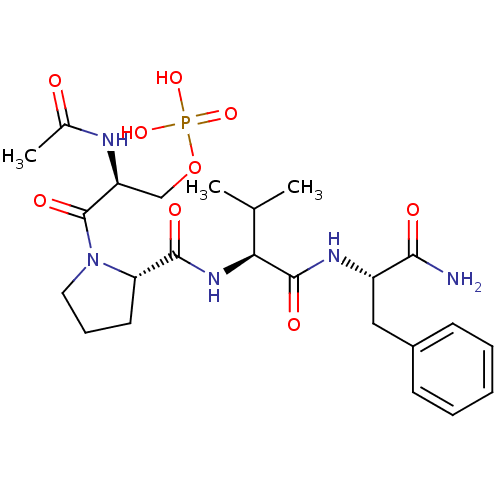
((S)-2-acetamido-3-((S)-2-((S)-1-((S)-1-amino-1-oxo...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C24H36N5O9P/c1-14(2)20(23(33)27-17(21(25)31)12-16-8-5-4-6-9-16)28-22(32)19-10-7-11-29(19)24(34)18(26-15(3)30)13-38-39(35,36)37/h4-6,8-9,14,17-20H,7,10-13H2,1-3H3,(H2,25,31)(H,26,30)(H,27,33)(H,28,32)(H2,35,36,37)/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380357
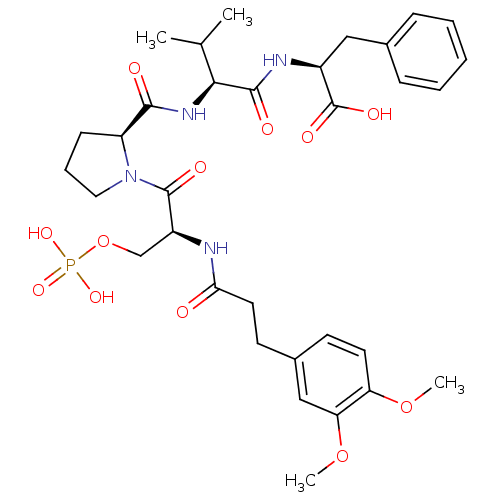
(CHEMBL2017820)Show SMILES COc1ccc(CCC(=O)N[C@@H](COP(O)(O)=O)C(=O)N2CCC[C@H]2C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2ccccc2)C(O)=O)cc1OC |r| Show InChI InChI=1S/C33H45N4O12P/c1-20(2)29(31(40)35-23(33(42)43)17-21-9-6-5-7-10-21)36-30(39)25-11-8-16-37(25)32(41)24(19-49-50(44,45)46)34-28(38)15-13-22-12-14-26(47-3)27(18-22)48-4/h5-7,9-10,12,14,18,20,23-25,29H,8,11,13,15-17,19H2,1-4H3,(H,34,38)(H,35,40)(H,36,39)(H,42,43)(H2,44,45,46)/t23-,24-,25-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380355

(CHEMBL2017818)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)CCCCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C33H45N4O10P/c1-22(2)29(31(40)35-25(33(42)43)20-24-15-7-4-8-16-24)36-30(39)27-17-11-19-37(27)32(41)26(21-47-48(44,45)46)34-28(38)18-10-9-14-23-12-5-3-6-13-23/h3-8,12-13,15-16,22,25-27,29H,9-11,14,17-21H2,1-2H3,(H,34,38)(H,35,40)(H,36,39)(H,42,43)(H2,44,45,46)/t25-,26-,27-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380356
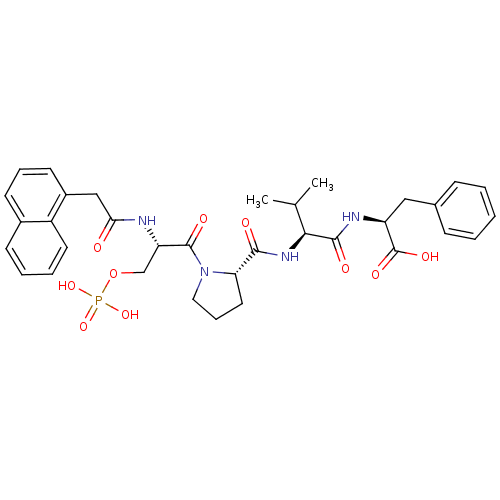
(CHEMBL2017819)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)Cc1cccc2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C34H41N4O10P/c1-21(2)30(32(41)36-26(34(43)44)18-22-10-4-3-5-11-22)37-31(40)28-16-9-17-38(28)33(42)27(20-48-49(45,46)47)35-29(39)19-24-14-8-13-23-12-6-7-15-25(23)24/h3-8,10-15,21,26-28,30H,9,16-20H2,1-2H3,(H,35,39)(H,36,41)(H,37,40)(H,43,44)(H2,45,46,47)/t26-,27-,28-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Breast cancer type 1 susceptibility protein
(Homo sapiens (Human)) | BDBM50380350
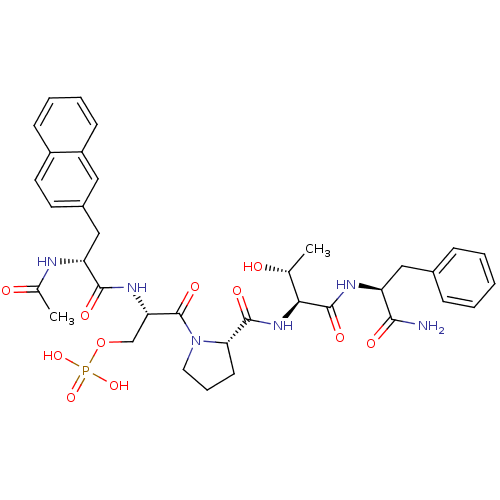
(CHEMBL2017813)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](COP(O)(O)=O)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C36H45N6O11P/c1-21(43)31(35(48)39-27(32(37)45)18-23-9-4-3-5-10-23)41-34(47)30-13-8-16-42(30)36(49)29(20-53-54(50,51)52)40-33(46)28(38-22(2)44)19-24-14-15-25-11-6-7-12-26(25)17-24/h3-7,9-12,14-15,17,21,27-31,43H,8,13,16,18-20H2,1-2H3,(H2,37,45)(H,38,44)(H,39,48)(H,40,46)(H,41,47)(H2,50,51,52)/t21-,27+,28-,29+,30+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BRCT domain of His-tagged BRCA1 after 1 min by competitive fluorescence polarization assay |
ACS Med Chem Lett 2: 764-767 (2011)
Article DOI: 10.1021/ml200147a
BindingDB Entry DOI: 10.7270/Q2WH2R0Q |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050527
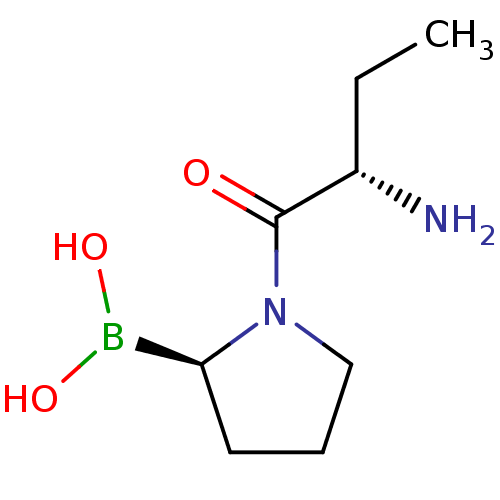
(Boronic acid derivative | CHEMBL66032 | US11096924...)Show InChI InChI=1S/C8H17BN2O3/c1-2-6(10)8(12)11-5-3-4-7(11)9(13)14/h6-7,13-14H,2-5,10H2,1H3/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Dipeptidylpeptidase II |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050521

((2-Dihydroxyborane-pyrrolidin-1-yl)-pyrrolidin-2-y...)Show InChI InChI=1S/C9H17BN2O3/c13-9(7-3-1-5-11-7)12-6-2-4-8(12)10(14)15/h7-8,11,14-15H,1-6H2/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050517
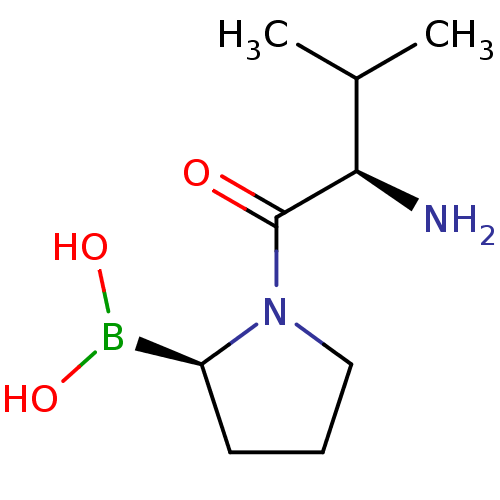
(Boronic acid derivative | CHEMBL305170 | N-alkyl G...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Dipeptidylpeptidase II |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50369128
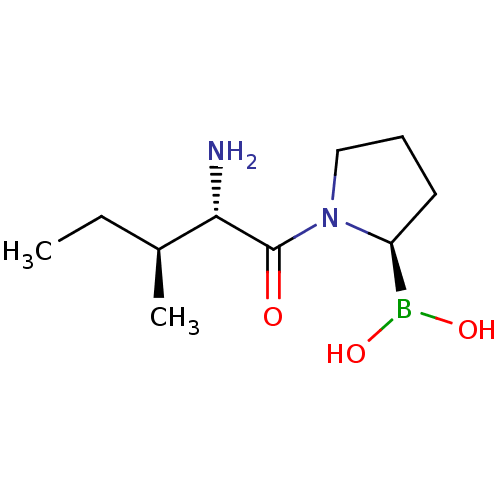
(CHEMBL1790483 | US11096924, DASH-inhibitors 4316 |...)Show InChI InChI=1S/C10H21BN2O3/c1-3-7(2)9(12)10(14)13-6-4-5-8(13)11(15)16/h7-9,15-16H,3-6,12H2,1-2H3/t7-,8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050514
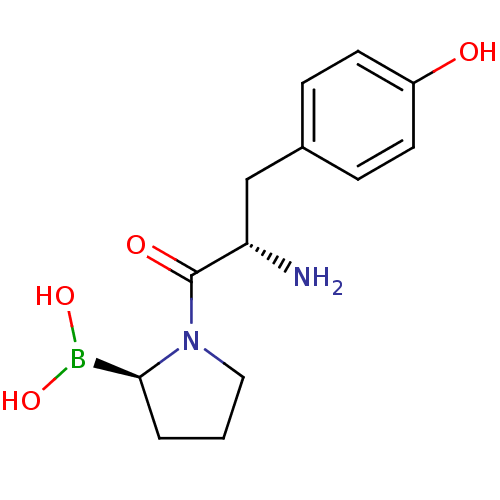
(Boronic acid derivative | CHEMBL304007)Show InChI InChI=1S/C13H19BN2O4/c15-11(8-9-3-5-10(17)6-4-9)13(18)16-7-1-2-12(16)14(19)20/h3-6,11-12,17,19-20H,1-2,7-8,15H2/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050528

(Boronic acid derivative | CHEMBL63698 | US11096924...)Show InChI InChI=1S/C10H21BN2O3/c1-7(2)6-8(12)10(14)13-5-3-4-9(13)11(15)16/h7-9,15-16H,3-6,12H2,1-2H3/t8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Dipeptidylpeptidase II |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050520

(Boronic acid derivative | CHEMBL63406 | US11096924...)Show InChI InChI=1S/C10H21BN2O3/c1-10(2,3)8(12)9(14)13-6-4-5-7(13)11(15)16/h7-8,15-16H,4-6,12H2,1-3H3/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050524
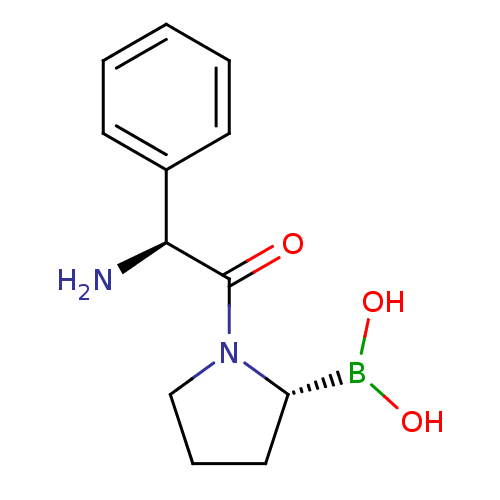
(Boronic acid derivative | CHEMBL291428)Show InChI InChI=1S/C12H17BN2O3/c14-11(9-5-2-1-3-6-9)12(16)15-8-4-7-10(15)13(17)18/h1-3,5-6,10-11,17-18H,4,7-8,14H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050515

(Boronic acid derivative | CHEMBL63652 | US11096924...)Show InChI InChI=1S/C13H19BN2O3/c15-11(9-10-5-2-1-3-6-10)13(17)16-8-4-7-12(16)14(18)19/h1-3,5-6,11-12,18-19H,4,7-9,15H2/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050516
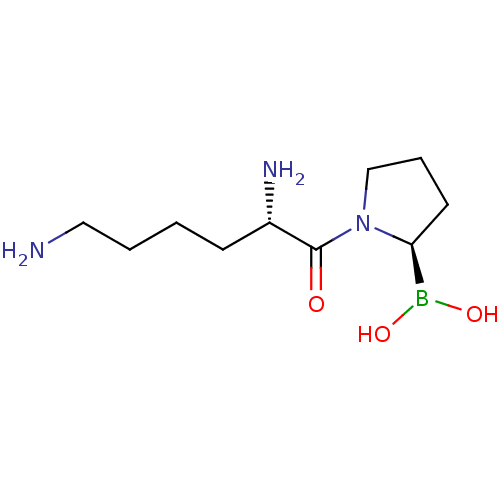
(Boronic acid derivative | CHEMBL63726 | US11096924...)Show InChI InChI=1S/C10H22BN3O3/c12-6-2-1-4-8(13)10(15)14-7-3-5-9(14)11(16)17/h8-9,16-17H,1-7,12-13H2/t8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50369129
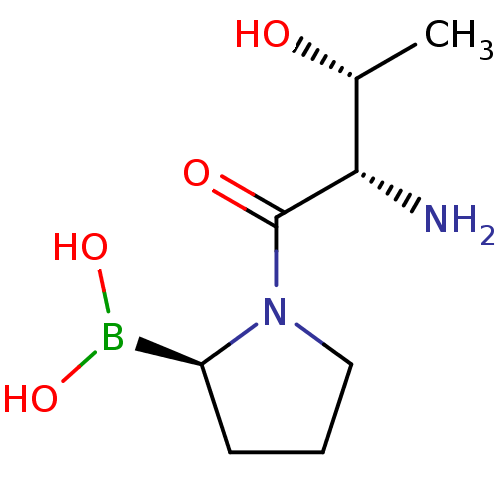
(CHEMBL1790478)Show InChI InChI=1S/C8H17BN2O4/c1-5(12)7(10)8(13)11-4-2-3-6(11)9(14)15/h5-7,12,14-15H,2-4,10H2,1H3/t5-,6+,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050526
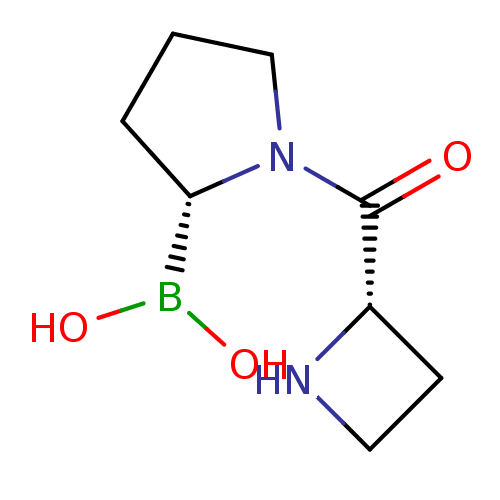
(Boronic acid derivative | CHEMBL292342)Show InChI InChI=1S/C8H15BN2O3/c12-8(6-3-4-10-6)11-5-1-2-7(11)9(13)14/h6-7,10,13-14H,1-5H2/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50369129

(CHEMBL1790478)Show InChI InChI=1S/C8H17BN2O4/c1-5(12)7(10)8(13)11-4-2-3-6(11)9(14)15/h5-7,12,14-15H,2-4,10H2,1H3/t5-,6+,7+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Dipeptidylpeptidase II |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050521

((2-Dihydroxyborane-pyrrolidin-1-yl)-pyrrolidin-2-y...)Show InChI InChI=1S/C9H17BN2O3/c13-9(7-3-1-5-11-7)12-6-2-4-8(12)10(14)15/h7-8,11,14-15H,1-6H2/t7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Dipeptidylpeptidase II |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50050525

((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...)Show InChI InChI=1S/C7H15BN2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6,12-13H,2-4,9H2,1H3/t5-,6-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Prolyl endopeptidase |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050512
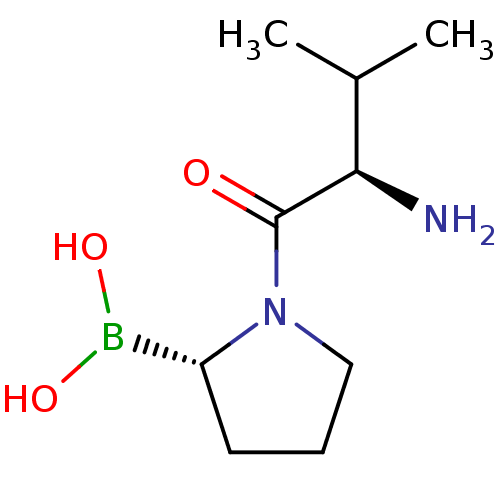
(Boronic acid derivative | CHEMBL65406)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50369129

(CHEMBL1790478)Show InChI InChI=1S/C8H17BN2O4/c1-5(12)7(10)8(13)11-4-2-3-6(11)9(14)15/h5-7,12,14-15H,2-4,10H2,1H3/t5-,6+,7+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Prolyl endopeptidase |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050511
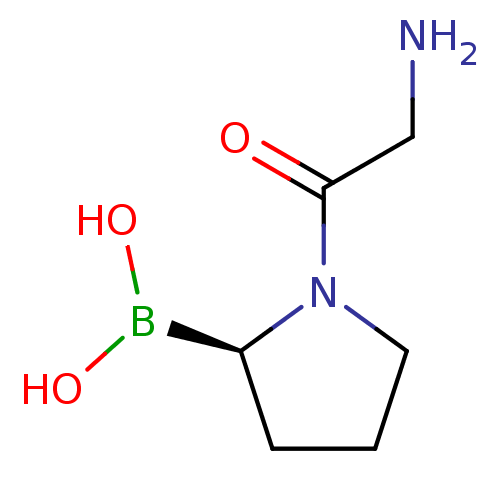
((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...)Show InChI InChI=1S/C6H13BN2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5,11-12H,1-4,8H2/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050519

(Boronic acid derivative | CHEMBL303131)Show InChI InChI=1S/C10H17BN4O3/c12-8(4-7-5-13-6-14-7)10(16)15-3-1-2-9(15)11(17)18/h5-6,8-9,17-18H,1-4,12H2,(H,13,14)/t8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50050512

(Boronic acid derivative | CHEMBL65406)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Dipeptidylpeptidase II |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50050513

((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Prolyl endopeptidase |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050523
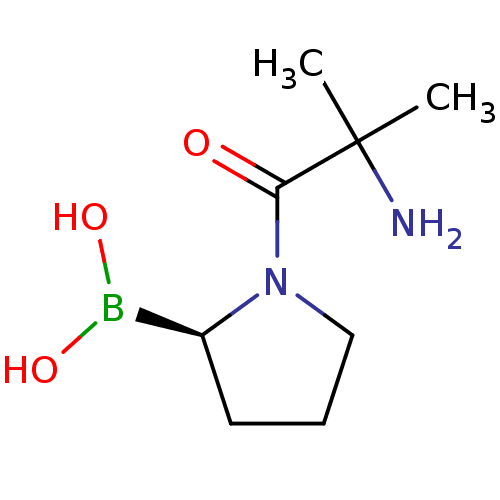
(Boronic acid derivative | CHEMBL66189)Show InChI InChI=1S/C8H17BN2O3/c1-8(2,10)7(12)11-5-3-4-6(11)9(13)14/h6,13-14H,3-5,10H2,1-2H3/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50050517

(Boronic acid derivative | CHEMBL305170 | N-alkyl G...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibition of Prolyl endopeptidase |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50050517

(Boronic acid derivative | CHEMBL305170 | N-alkyl G...)Show InChI InChI=1S/C9H19BN2O3/c1-6(2)8(11)9(13)12-5-3-4-7(12)10(14)15/h6-8,14-15H,3-5,11H2,1-2H3/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro for inhibition of Dipeptidylpeptidase IV. |
J Med Chem 39: 2087-94 (1996)
Article DOI: 10.1021/jm950732f
BindingDB Entry DOI: 10.7270/Q29Z95K5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data