

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM50548231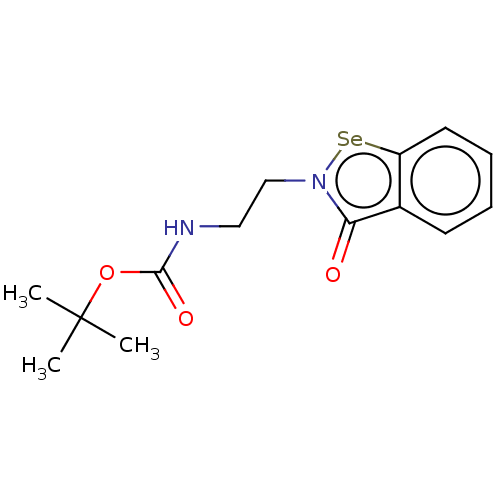 (CHEMBL4753599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type N-terminal His6-tagged Klebsiella pneumoniae NDM-1 expressed in Escherichia coli BL21 assessed as inhibition constant using n... | Citation and Details Article DOI: 10.1016/j.ejmech.2018.06.007 BindingDB Entry DOI: 10.7270/Q2SB49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151874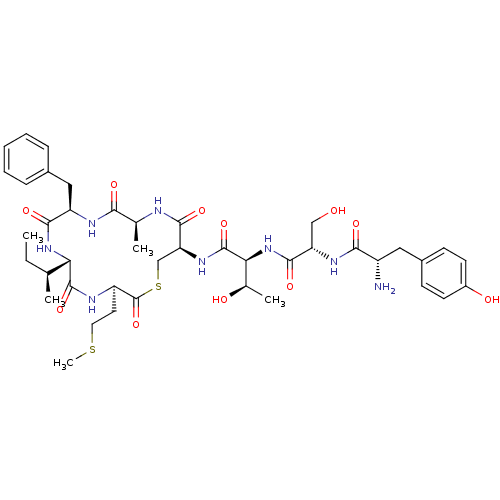 (CHEMBL362838 | Tyr-Ser-Thr-cyclo(Cys-Ala-Phe-Ile-M...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151871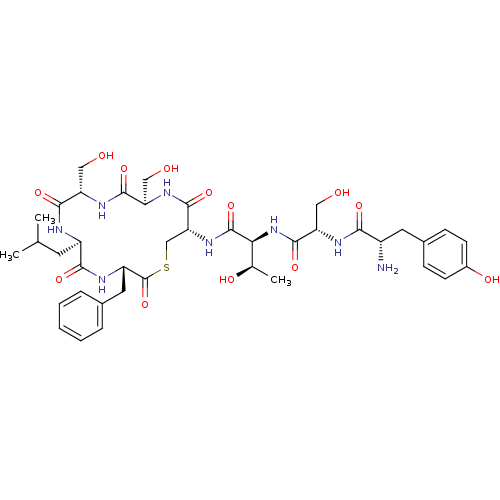 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151876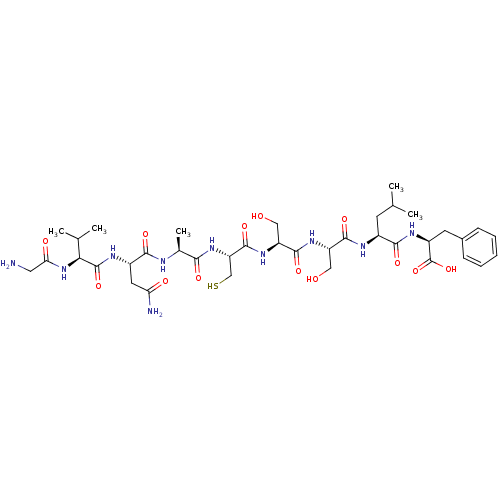 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C3 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151880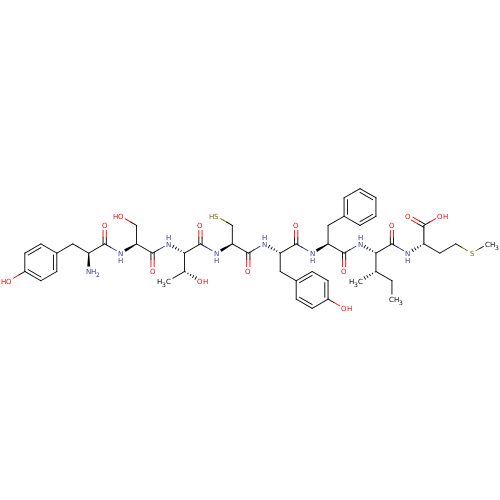 (CHEMBL426697 | YSTCYFIM) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C3 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117496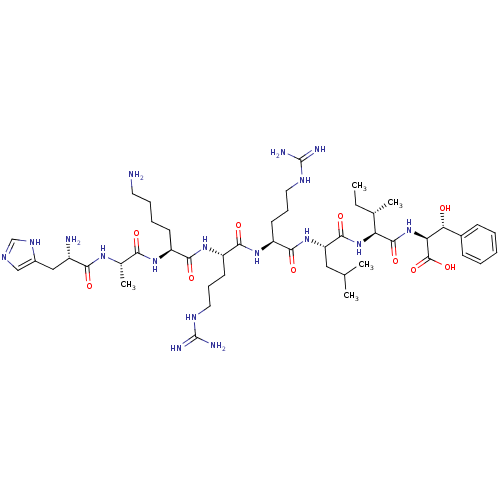 (CHEMBL429171 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-(L)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151873 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151876 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Antagonistic concentration for accessory gene regulator C1 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151880 (CHEMBL426697 | YSTCYFIM) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C2 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151877 (CHEMBL182114 | N-[9-Benzyl-6-sec-butyl-12-methyl-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151877 (CHEMBL182114 | N-[9-Benzyl-6-sec-butyl-12-methyl-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151879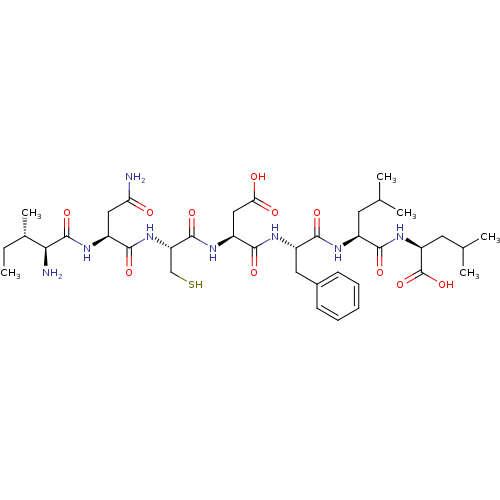 (2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-pentanoylamino)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C2 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151874 (CHEMBL362838 | Tyr-Ser-Thr-cyclo(Cys-Ala-Phe-Ile-M...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151871 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151871 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151883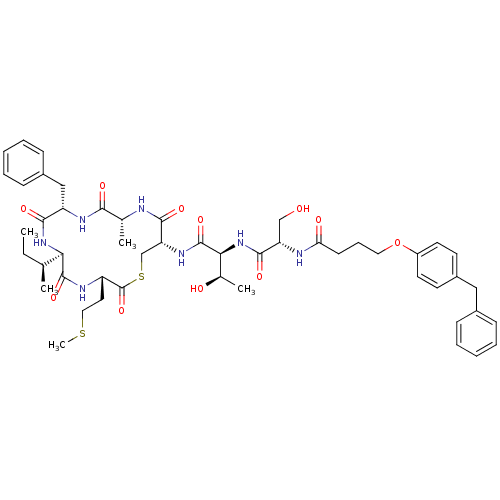 (CHEMBL276191 | N-[9-Benzyl-6-sec-butyl-12-methyl-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151874 (CHEMBL362838 | Tyr-Ser-Thr-cyclo(Cys-Ala-Phe-Ile-M...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151874 (CHEMBL362838 | Tyr-Ser-Thr-cyclo(Cys-Ala-Phe-Ile-M...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117492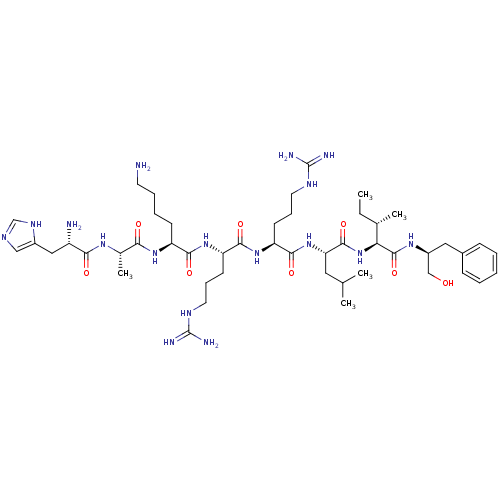 (CHEMBL267119 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-(L)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117488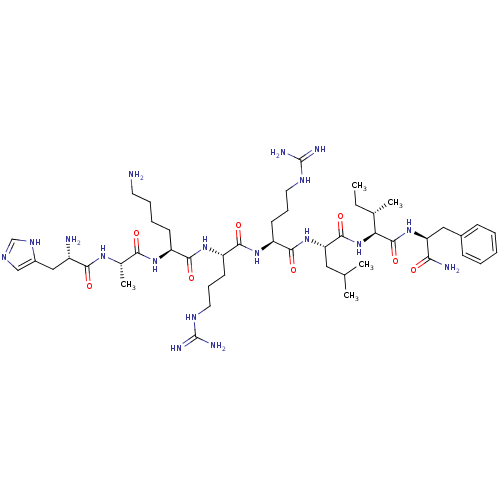 (CHEMBL217362 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-Phe-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117490 (CHEMBL384574 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-(Z)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151879 (2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-pentanoylamino)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Antagonistic concentration for accessory gene regulator C1 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151876 (CHEMBL182783 | GVNACSSLF) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C4 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151873 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151879 (2-{2-[2-(2-{2-[2-(2-Amino-3-methyl-pentanoylamino)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to antagonise accessory gene regulator C4 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117487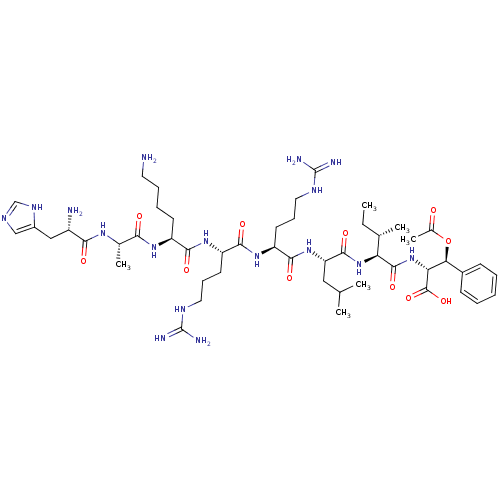 (CHEMBL216619 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-(D)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151871 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151878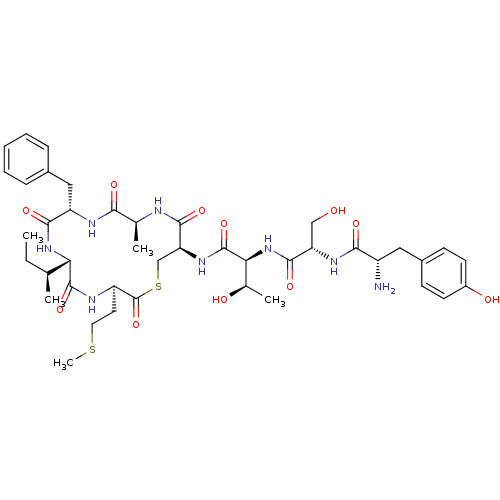 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151878 (2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151883 (CHEMBL276191 | N-[9-Benzyl-6-sec-butyl-12-methyl-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117499 (CHEMBL412632 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-(D)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151872 (2-{2-[4-(4-Benzoyl-phenoxy)-butyrylamino]-3-hydrox...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151872 (2-{2-[4-(4-Benzoyl-phenoxy)-butyrylamino]-3-hydrox...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117495 (CHEMBL385674 | H-His-Ala-Lys-Arg-Arg-Leu-Ile-(L)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117494 (CHEMBL267588 | H-His-Ser-Lys-Arg-Arg-Leu-Ile-(L)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117497 (CHEMBL266900 | H-His-Ser-Lys-Arg-Arg-Leu-Ile-(L)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117498 (CHEMBL415127 | H-His-Ser-Lys-Arg-Arg-Leu-Ile-(D)-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117491 (CHEMBL263837 | H-His-Ser-Lys-Arg-Arg-Leu-Ile-(Z)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50117493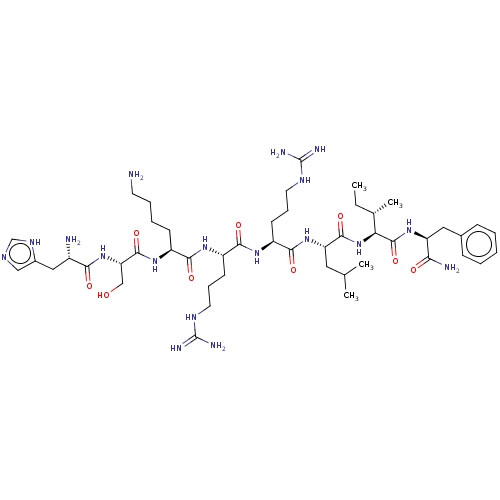 (CHEMBL2369934 | H-His-Ser-Lys-Arg-Arg-Leu-Ile-Phe-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description In vitro inhibition of CDK2-cyclin A kinase activity on retinoblastoma protein | Bioorg Med Chem Lett 12: 2501-5 (2002) BindingDB Entry DOI: 10.7270/Q2CC101N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM50548232 (CHEMBL4758313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Klebsiella pneumoniae NDM-1 | Citation and Details Article DOI: 10.1016/j.ejmech.2018.06.007 BindingDB Entry DOI: 10.7270/Q2SB49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151875 (CHEMBL369309 | N-(3-Benzyl-9,12-bis-hydroxymethyl-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151875 (CHEMBL369309 | N-(3-Benzyl-9,12-bis-hydroxymethyl-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Accessory gene regulator protein A (Staphylococcus aureus) | BDBM50351512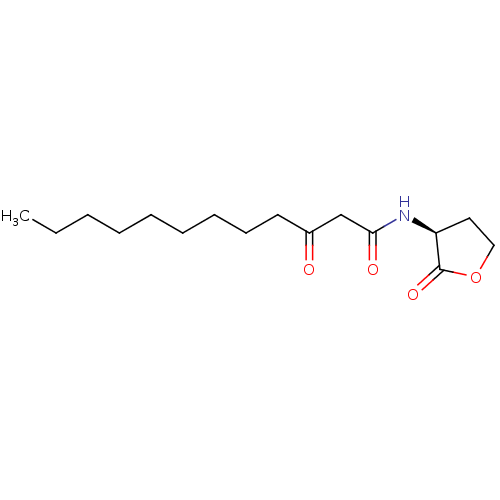 (CHEMBL8483) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus RN6390B Agr-mediated RNA3 promoter activation measured up to 6 hrs by Northern blot analysis | J Med Chem 56: 1389-404 (2013) Article DOI: 10.1021/jm3014635 BindingDB Entry DOI: 10.7270/Q2BV7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151880 (CHEMBL426697 | YSTCYFIM) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Antagonistic concentration for accessory gene regulator C1 of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensory histidine kinase DcuS (Staphylococcus aureus) | BDBM50151881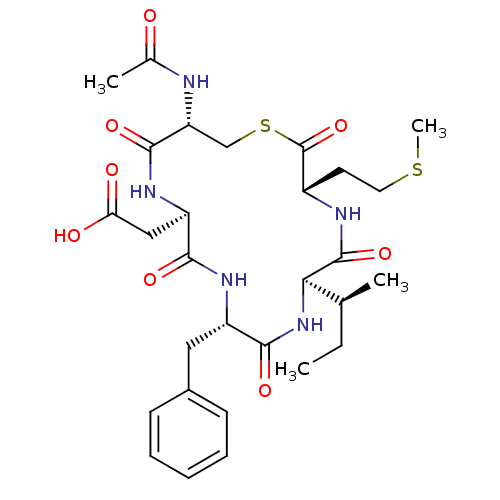 (CHEMBL362839 | Cyclo(Ac-Cys-Asp-Phe-Ile-Met)) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Concentration required to inhibit quorum sensor of Staphylococcus aureus | J Med Chem 47: 4633-41 (2004) Article DOI: 10.1021/jm0400754 BindingDB Entry DOI: 10.7270/Q2P26XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator MgrA (Staphylococcus aureus) | BDBM11979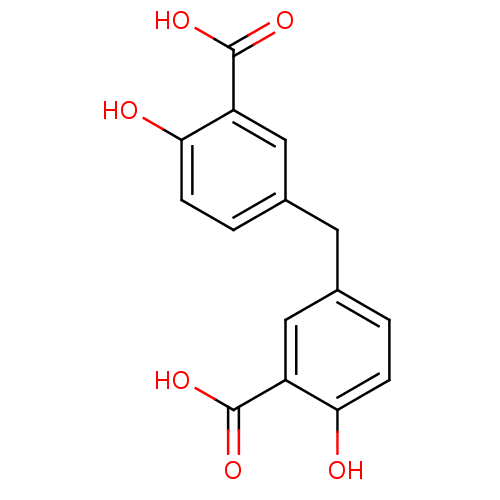 (5-[(3-carboxy-4-hydroxyphenyl)methyl]-2-hydroxyben...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Newman His6-tagged MgrA expressed in Escherichia coli BL21(DE3) using 5'-6-F-TAAACAACAAGTTGTCCAAA-3' as substrate... | J Med Chem 56: 1389-404 (2013) Article DOI: 10.1021/jm3014635 BindingDB Entry DOI: 10.7270/Q2BV7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM50548231 (CHEMBL4753599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type N-terminal His6-tagged Klebsiella pneumoniae NDM-1 expressed in Escherichia coli BL21 using nitrocefin as substrate measured ... | Citation and Details Article DOI: 10.1016/j.ejmech.2018.06.007 BindingDB Entry DOI: 10.7270/Q2SB49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator MgrA (Staphylococcus aureus) | BDBM81554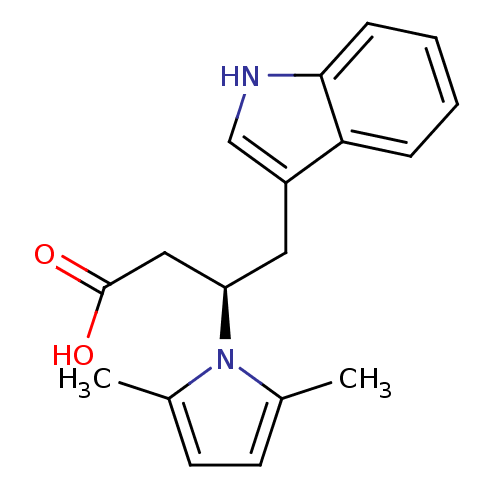 (MgrA inhibitor, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Newman His6-tagged MgrA expressed in Escherichia coli BL21(DE3) using 5'-6-F-TAAACAACAAGTTGTCCAAA-3' as substrate... | J Med Chem 56: 1389-404 (2013) Article DOI: 10.1021/jm3014635 BindingDB Entry DOI: 10.7270/Q2BV7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator MgrA (Staphylococcus aureus) | BDBM81553 (MgrA inhibitor, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Newman His6-tagged MgrA expressed in Escherichia coli BL21(DE3) using 5'-6-F-TAAACAACAAGTTGTCCAAA-3' as substrate... | J Med Chem 56: 1389-404 (2013) Article DOI: 10.1021/jm3014635 BindingDB Entry DOI: 10.7270/Q2BV7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HTH-type transcriptional regulator MgrA (Staphylococcus aureus) | BDBM81555 (MgrA inhibitor, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Newman His6-tagged MgrA expressed in Escherichia coli BL21(DE3) using 5'-6-F-TAAACAACAAGTTGTCCAAA-3' as substrate... | J Med Chem 56: 1389-404 (2013) Article DOI: 10.1021/jm3014635 BindingDB Entry DOI: 10.7270/Q2BV7J0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |