

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244370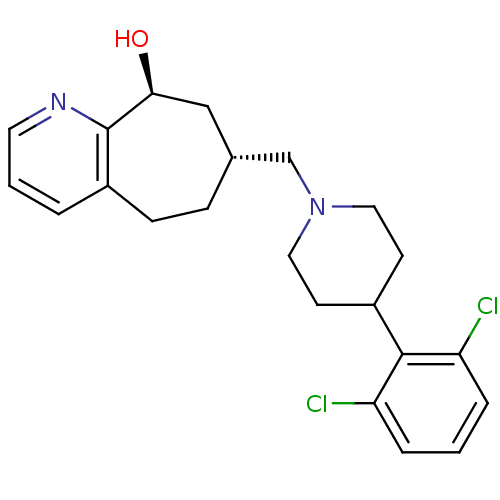 ((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244371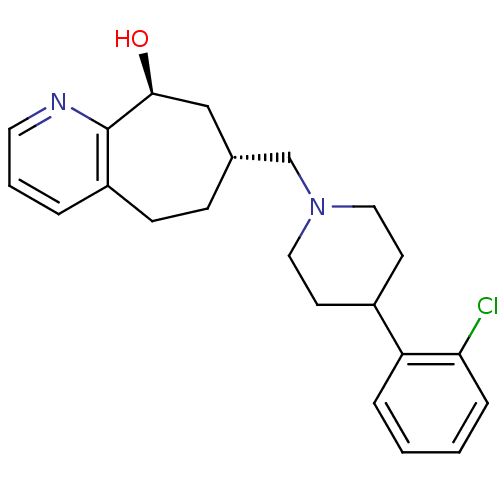 ((7R,9S)-7-((4-(2-chlorophenyl)piperidin-1-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9955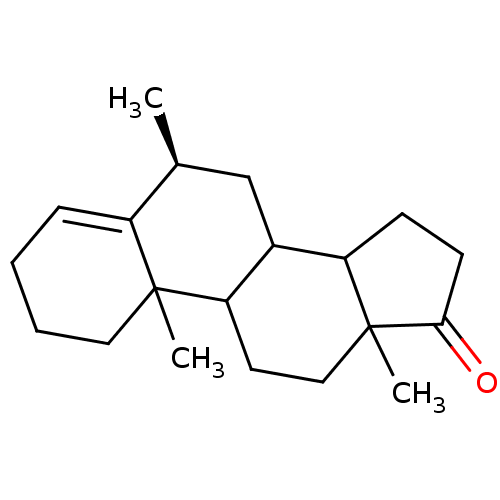 ((8S)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -50.5 | 37 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243726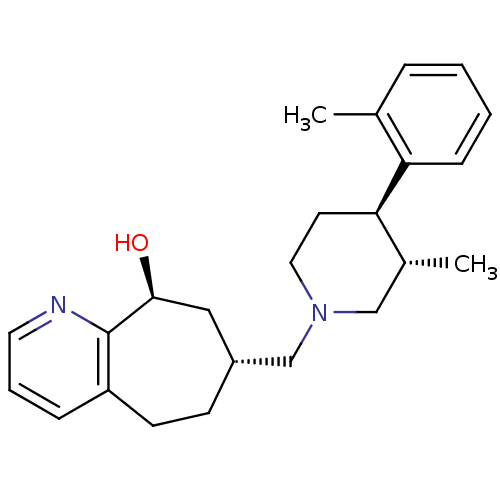 ((7R,9S)-7-(((3S,4R)-3-methyl-4-o-tolylpiperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243729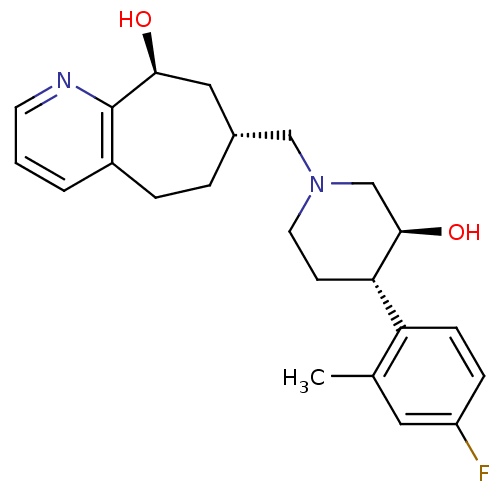 ((7R,9S)-7-(((3S,4S)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243727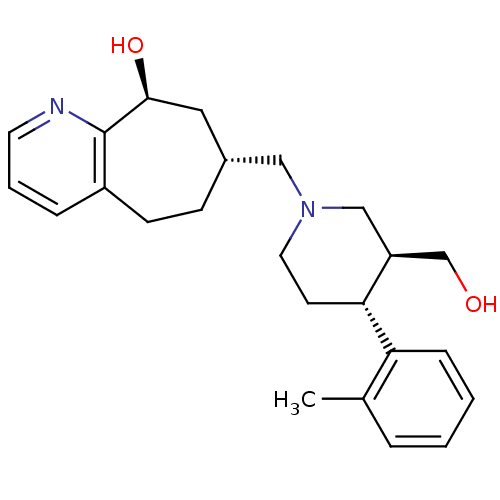 ((7R,9S)-7-(((3S,4R)-3-(hydroxymethyl)-4-o-tolylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243730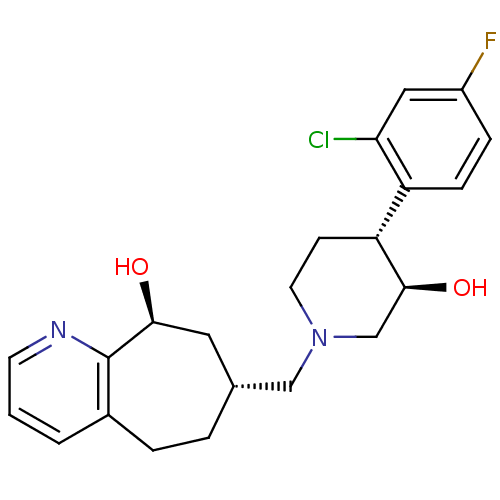 ((7R,9S)-7-(((3R,4R)-4-(2-chloro-4-fluorophenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9948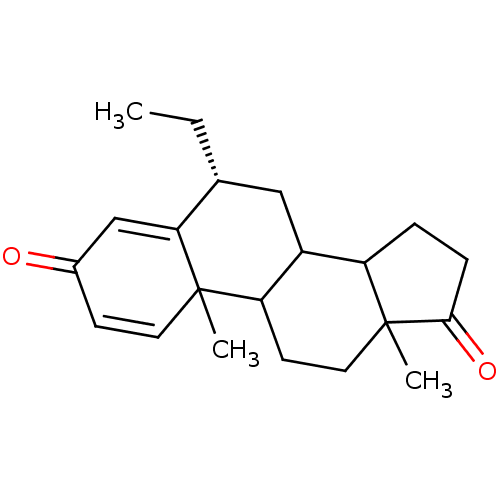 ((8R)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -49.4 | 54 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9951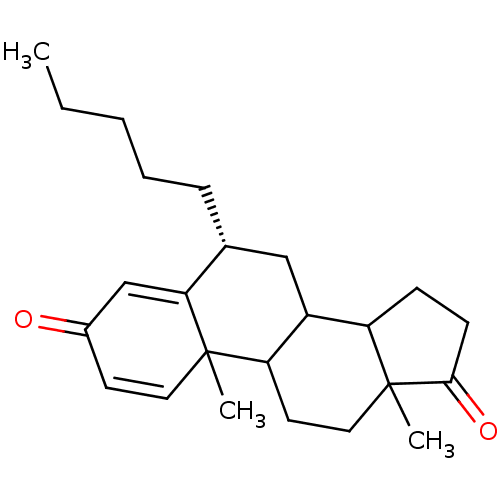 ((8R)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.3 | 54 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9960 ((8R)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -49.1 | 49 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9968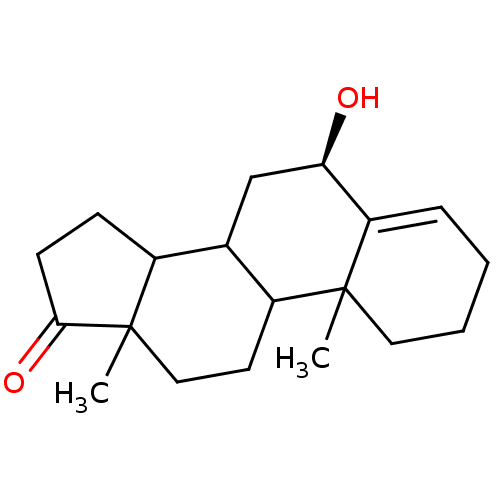 ((8R)-8-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -48.8 | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244297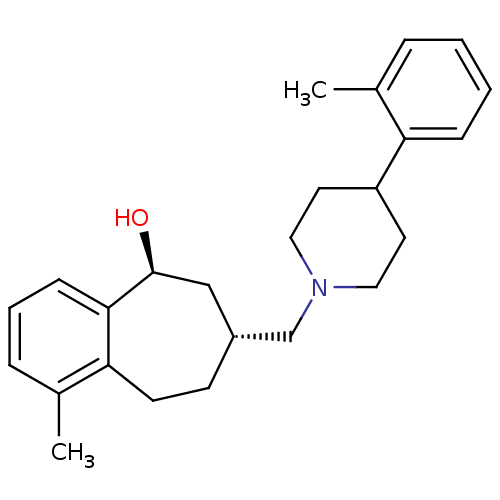 (CHEMBL513585 | cis-1-methyl-7-((4-o-tolylpiperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9981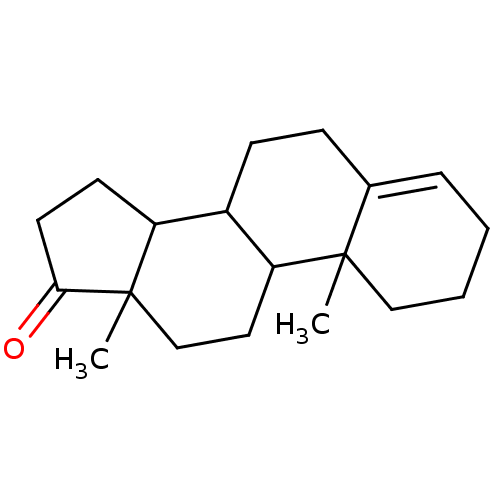 (2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9949 ((8R)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -48.4 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9953 ((8R)-8-heptyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.80 | -48.1 | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9952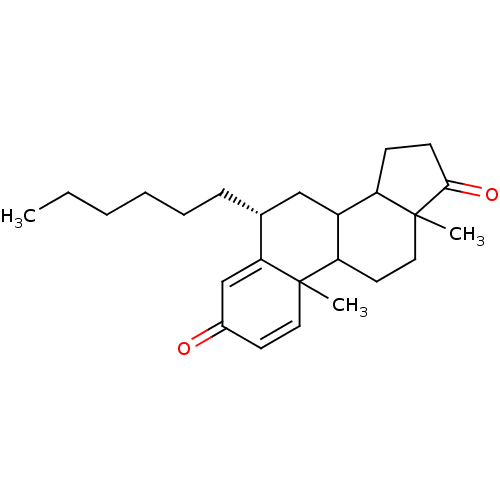 ((8R)-8-hexyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | 76 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243728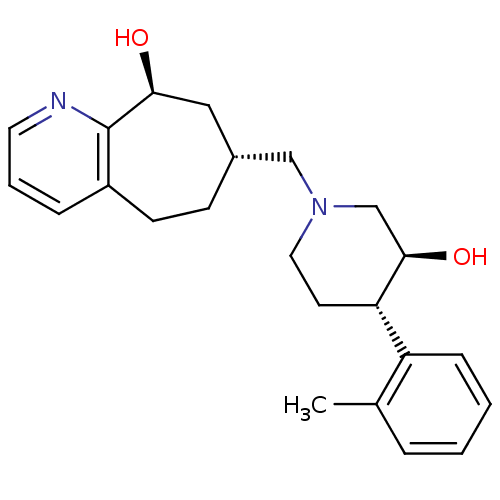 ((7R,9S)-7-(((3S,4S)-3-hydroxy-4-o-tolylpiperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9944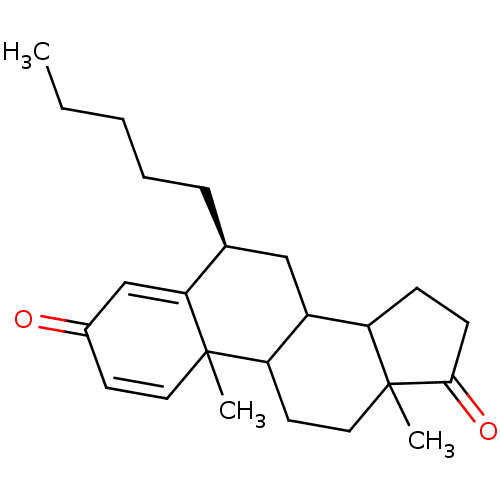 ((8S)-2,15-dimethyl-8-pentyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244336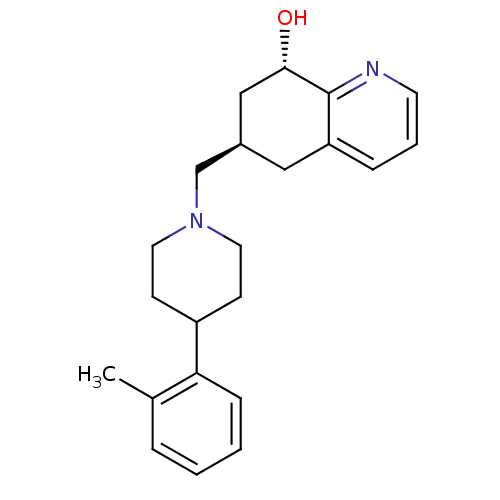 ((6R,8S)-6-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243887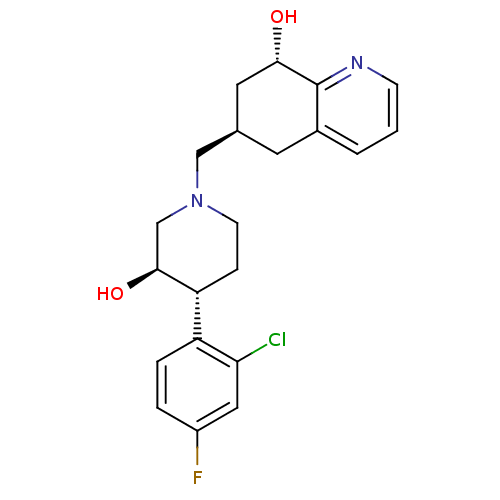 ((-)-(3R,4R)-4-(2-Chloro-4-fluorophenyl)-3-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9967 ((8S)-8-methoxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.0 | 120 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9970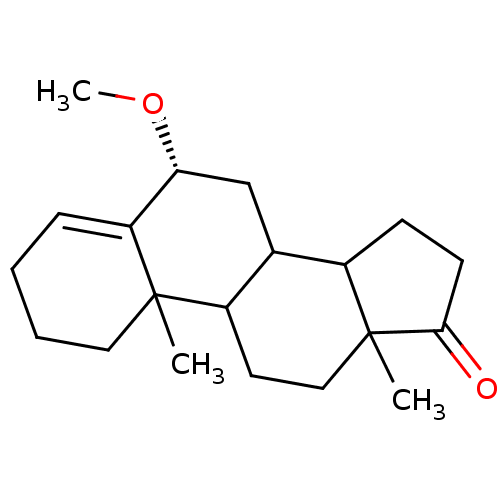 ((8R)-8-methoxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50332807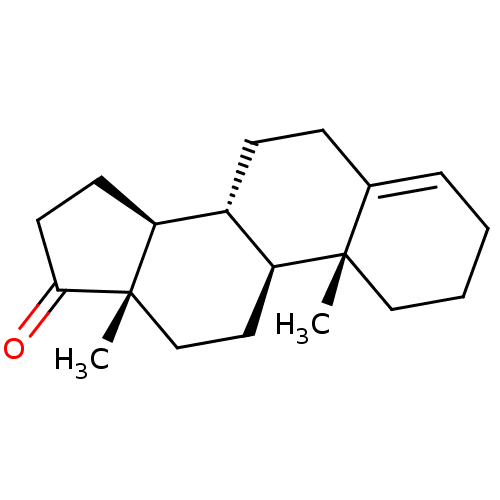 ((8R,9S,10R,13S,14S)-10,13-dimethyl-2,3,7,8,9,10,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description The binding affinity was determined on Cytochrome P450 19A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9956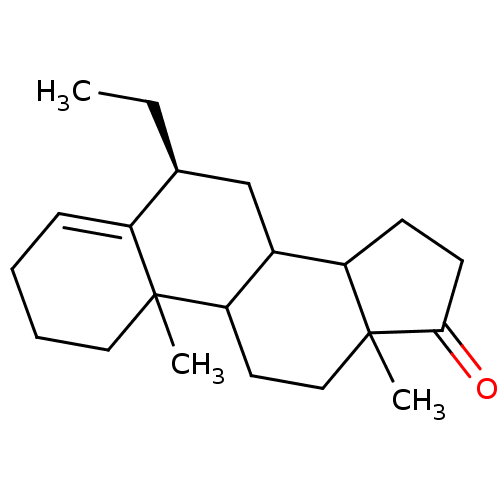 ((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -46.6 | 110 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9950 ((8R)-8-butyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -46.6 | 180 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9943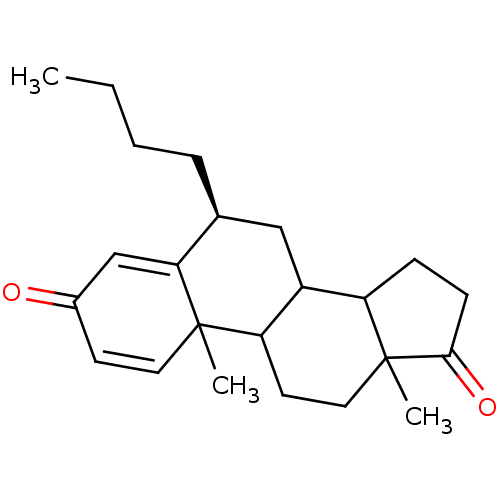 ((8S)-8-butyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -46.3 | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334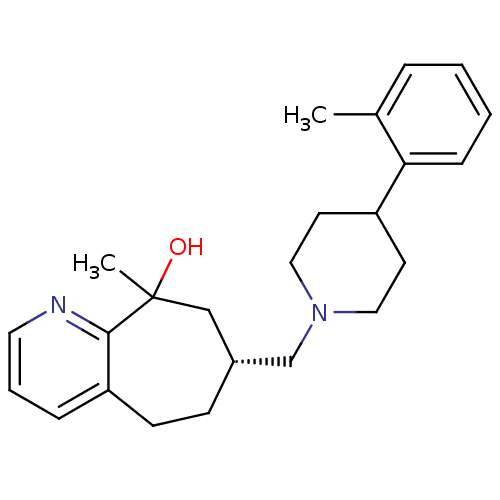 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244334 ((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9966 ((8S)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0^{2,7}....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | 160 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295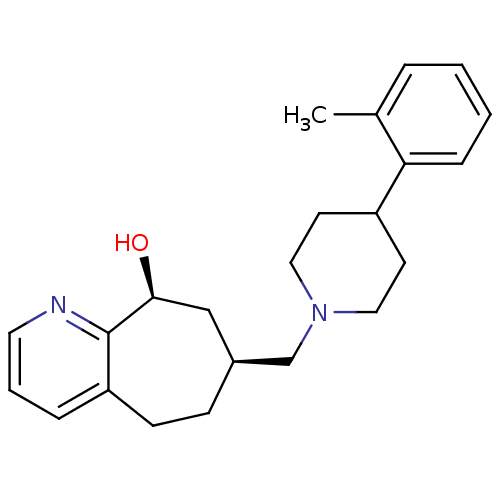 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244295 ((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9941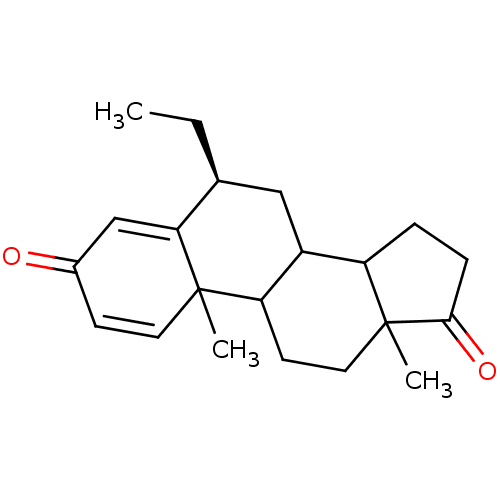 ((8S)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | 250 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9946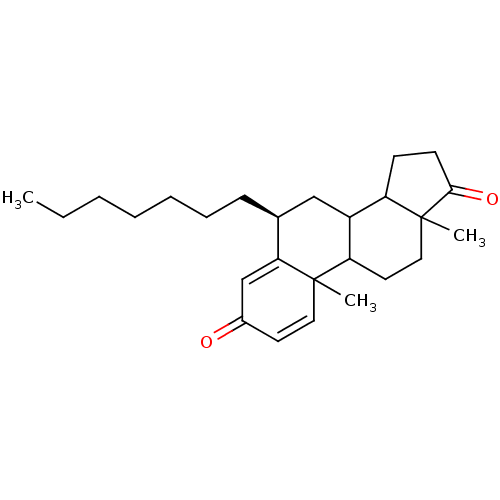 ((8S)-8-heptyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | 250 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8592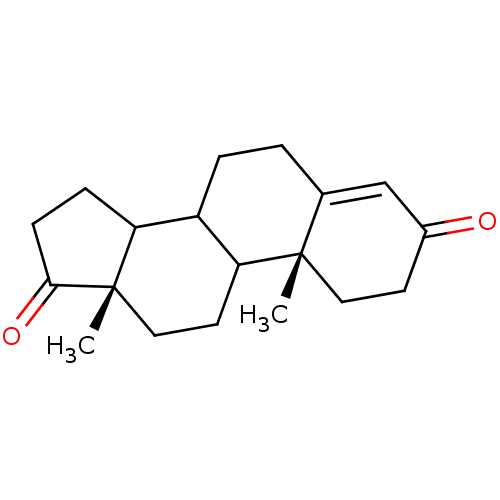 ((2R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | -45.7 | 300 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025428 (10,13-Dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dode...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Binding affinity for aromatase cytochrome P45019A1 by analysis of Dixon plot | J Med Chem 37: 2198-205 (1994) BindingDB Entry DOI: 10.7270/Q22F7MG3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244372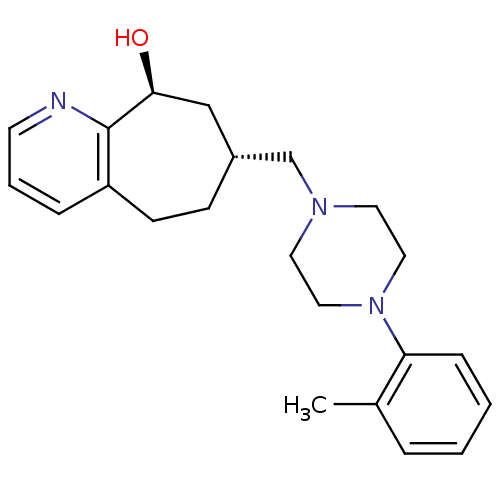 ((7R,9S)-7-((4-o-tolylpiperazin-1-yl)methyl)-6,7,8,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9965 ((8S)-8-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | 190 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50243886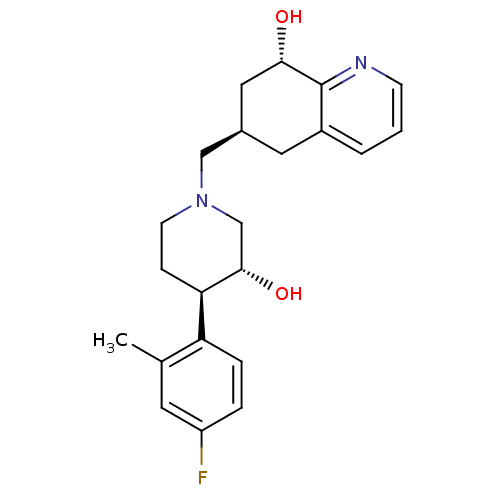 ((6R,8S)-6-(((3R,4R)-4-(4-fluoro-2-methylphenyl)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9942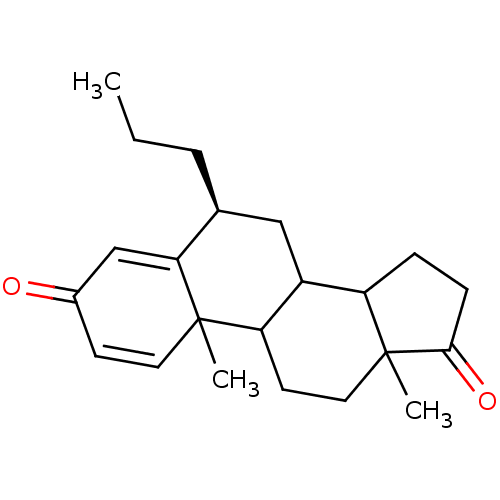 ((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -45.5 | 270 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9969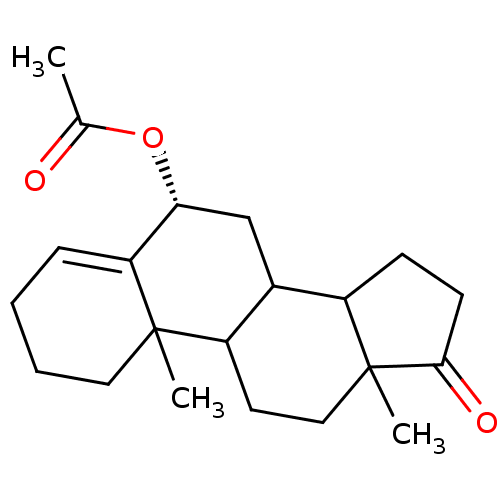 ((8R)-2,15-dimethyl-14-oxotetracyclo[8.7.0.0^{2,7}....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9945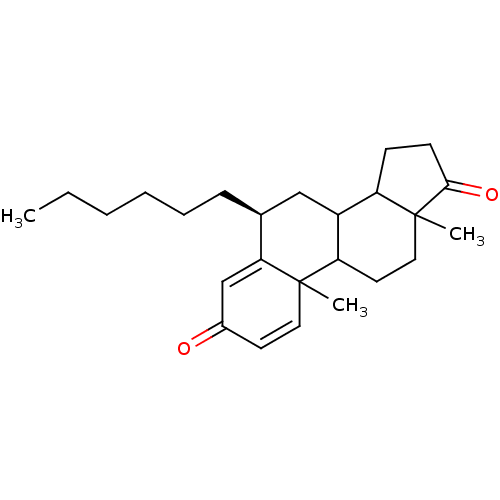 ((8S)-8-hexyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -45.1 | 280 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244335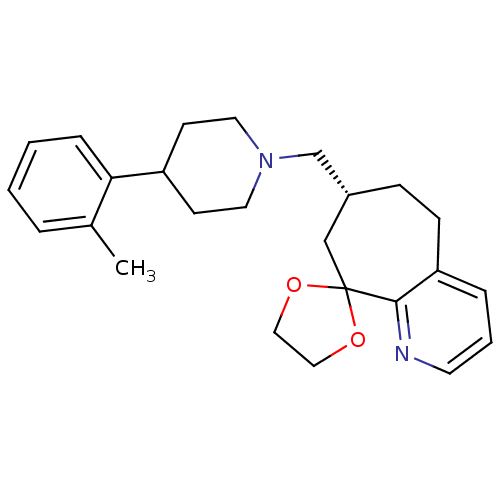 ((3R)-3-{[4-(2-methylphenyl)piperidin-1-yl]methyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9957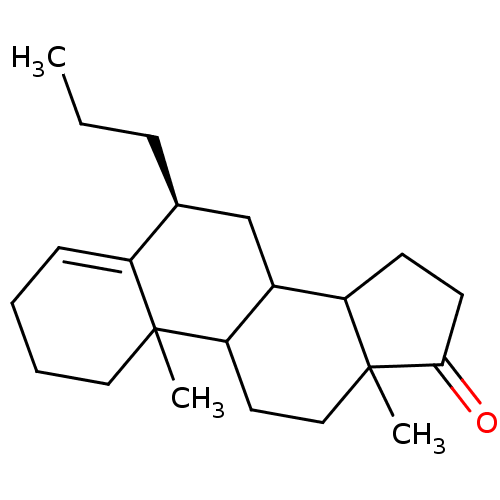 ((8S)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -44.7 | 230 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244373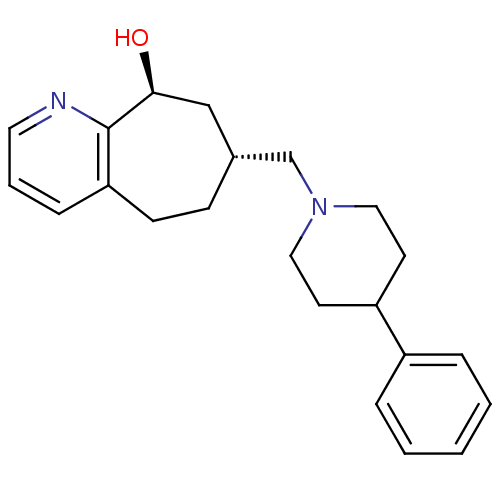 ((7R,9S)-7-((4-phenylpiperidin-1-yl)methyl)-6,7,8,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9961 ((8R)-8-ethyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -43.7 | 320 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9947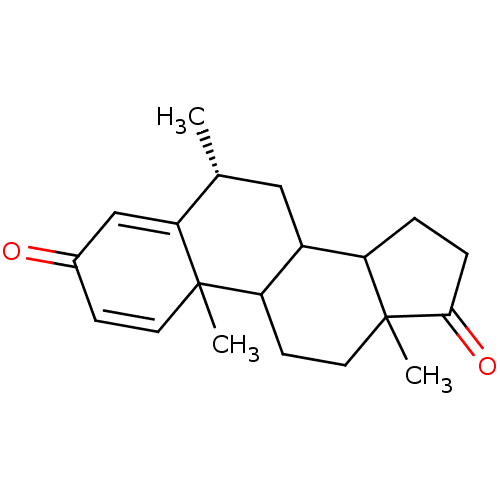 ((8R)-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -43.7 | 520 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9954 (1,4-Androsten-3,17-dione | 2,15-dimethyltetracyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | -43.7 | 530 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 1033-8 (1996) Article DOI: 10.1021/jm950720u BindingDB Entry DOI: 10.7270/Q21V5C6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9982 ((14S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244333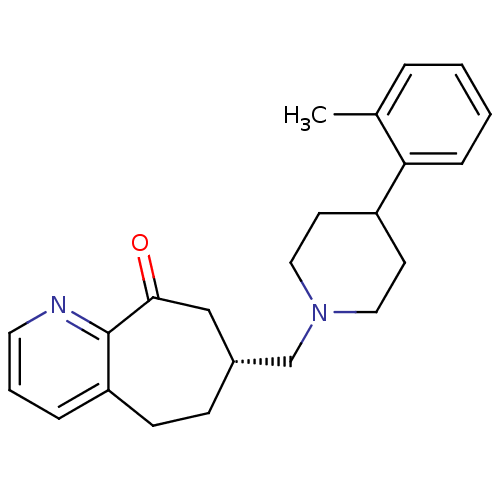 ((R)-7-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9962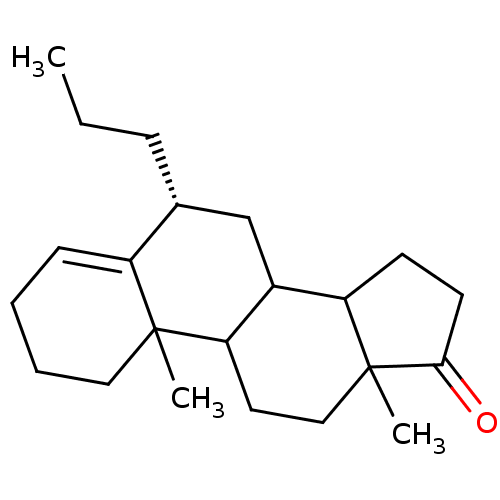 ((8R)-2,15-dimethyl-8-propyltetracyclo[8.7.0.0^{2,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | -43.3 | 440 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku College of Pharmacy | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 39: 2245-52 (1996) Article DOI: 10.1021/jm960047o BindingDB Entry DOI: 10.7270/Q2X34VPV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 354 total ) | Next | Last >> |