 Found 266 hits with Last Name = 'cichy-knight' and Initial = 'm'
Found 266 hits with Last Name = 'cichy-knight' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064027
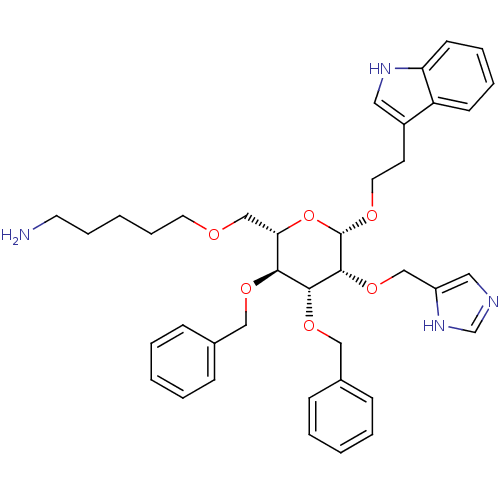
(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064027

(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064025
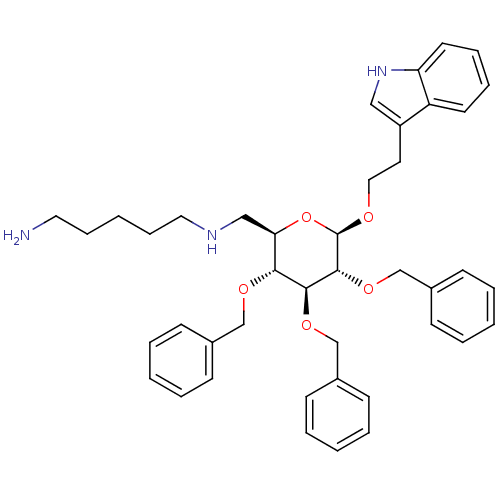
(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064027

(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064027

(5-{(2S,3S,4R,5R,6S)-3,4-Bis-benzyloxy-5-(1H-imidaz...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2cnc[nH]2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H48N4O6/c40-19-10-3-11-20-44-27-35-36(46-24-29-12-4-1-5-13-29)37(47-25-30-14-6-2-7-15-30)38(48-26-32-23-41-28-43-32)39(49-35)45-21-18-31-22-42-34-17-9-8-16-33(31)34/h1-2,4-9,12-17,22-23,28,35-39,42H,3,10-11,18-21,24-27,40H2,(H,41,43)/t35-,36-,37+,38+,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051567
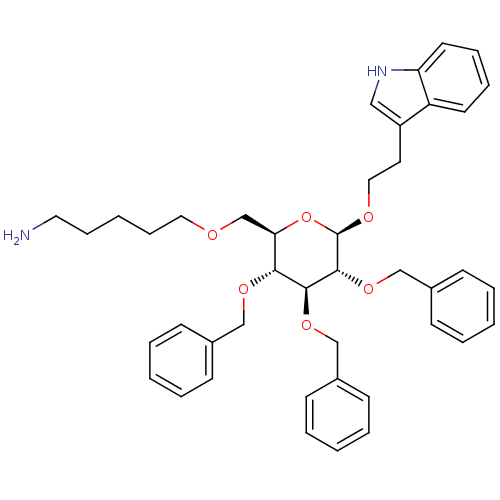
(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50051578
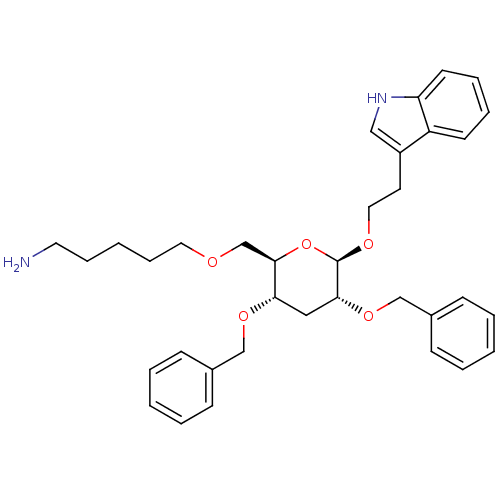
(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064024
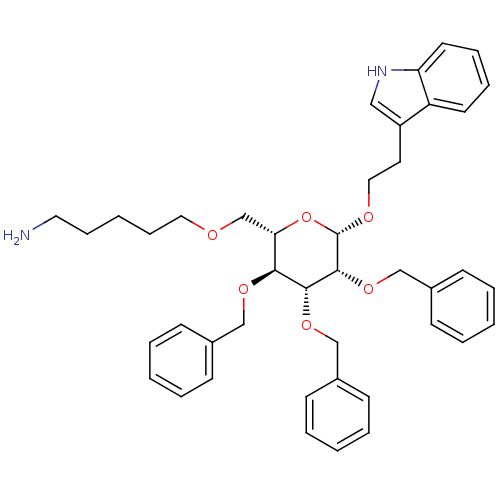
(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064026
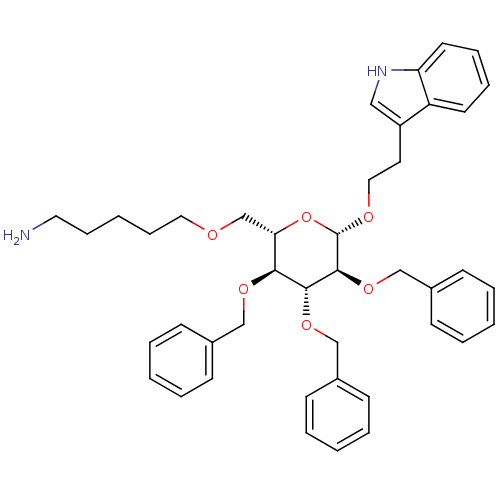
(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064025

(CHEMBL282129 | N*1*-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES NCCCCCNC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H51N3O5/c43-24-13-4-14-25-44-28-38-39(47-29-32-15-5-1-6-16-32)40(48-30-33-17-7-2-8-18-33)41(49-31-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-45-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44-45H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50064029
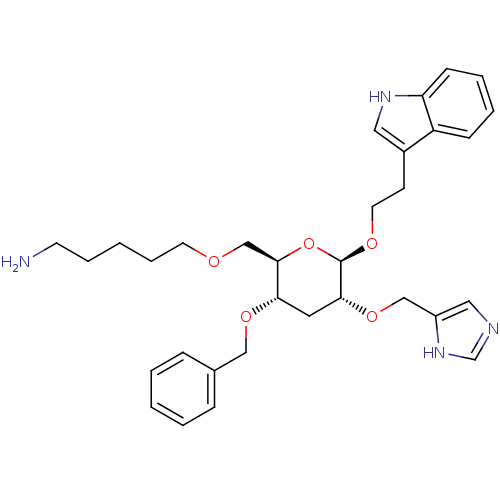
(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR4. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064024

(5-{(2S,3S,4R,5R,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41+,42-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50051567

(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064030

(5-((2R,3R,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-methoxy...)Show SMILES CO[C@@H]1O[C@H](COCCCCCN)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C33H43NO6/c1-35-33-32(39-24-28-18-10-4-11-19-28)31(38-23-27-16-8-3-9-17-27)30(37-22-26-14-6-2-7-15-26)29(40-33)25-36-21-13-5-12-20-34/h2-4,6-11,14-19,29-33H,5,12-13,20-25,34H2,1H3/t29-,30-,31+,32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR2 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50051578

(5-{(2R,3S,5R,6R)-3,5-Bis-benzyloxy-6-[2-(1H-indol-...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1ccccc1 Show InChI InChI=1S/C35H44N2O5/c36-19-10-3-11-20-38-26-34-32(40-24-27-12-4-1-5-13-27)22-33(41-25-28-14-6-2-7-15-28)35(42-34)39-21-18-29-23-37-31-17-9-8-16-30(29)31/h1-2,4-9,12-17,23,32-35,37H,3,10-11,18-22,24-26,36H2/t32-,33+,34+,35+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR5. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50064026

(5-{(2S,3S,4R,5S,6S)-3,4,5-Tris-benzyloxy-6-[2-(1H-...)Show SMILES NCCCCCOC[C@@H]1O[C@H](OCCc2c[nH]c3ccccc23)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C42H50N2O6/c43-24-13-4-14-25-45-31-38-39(47-28-32-15-5-1-6-16-32)40(48-29-33-17-7-2-8-18-33)41(49-30-34-19-9-3-10-20-34)42(50-38)46-26-23-35-27-44-37-22-12-11-21-36(35)37/h1-3,5-12,15-22,27,38-42,44H,4,13-14,23-26,28-31,43H2/t38-,39-,40+,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR3. |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064029

(5-{(2R,3S,5R,6R)-3-Benzyloxy-5-(1H-imidazol-4-ylme...)Show SMILES NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@@H](C[C@@H]1OCc1ccccc1)OCc1cnc[nH]1 Show InChI InChI=1S/C32H42N4O5/c33-14-7-2-8-15-37-22-31-29(39-20-24-9-3-1-4-10-24)17-30(40-21-26-19-34-23-36-26)32(41-31)38-16-13-25-18-35-28-12-6-5-11-27(25)28/h1,3-6,9-12,18-19,23,29-32,35H,2,7-8,13-17,20-22,33H2,(H,34,36)/t29-,30+,31+,32+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity for human receptor subtype hSSTR1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50064035
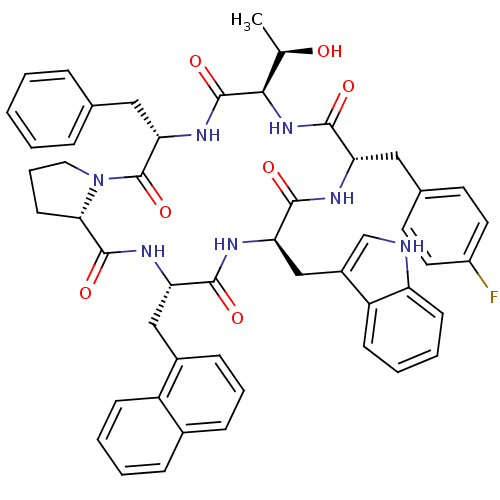
((5S,8R,11S,14R,17S,19aS)-5-Benzyl-11-(4-fluoro-ben...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C51H52FN7O7/c1-30(60)45-50(65)57-43(26-31-11-3-2-4-12-31)51(66)59-24-10-19-44(59)49(64)56-41(27-34-15-9-14-33-13-5-6-16-37(33)34)46(61)55-42(28-35-29-53-39-18-8-7-17-38(35)39)47(62)54-40(48(63)58-45)25-32-20-22-36(52)23-21-32/h2-9,11-18,20-23,29-30,40-45,53,60H,10,19,24-28H2,1H3,(H,54,62)(H,55,61)(H,56,64)(H,57,65)(H,58,63)/t30-,40+,41+,42-,43+,44+,45-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity was measured for Tachykinin receptor 1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50407836
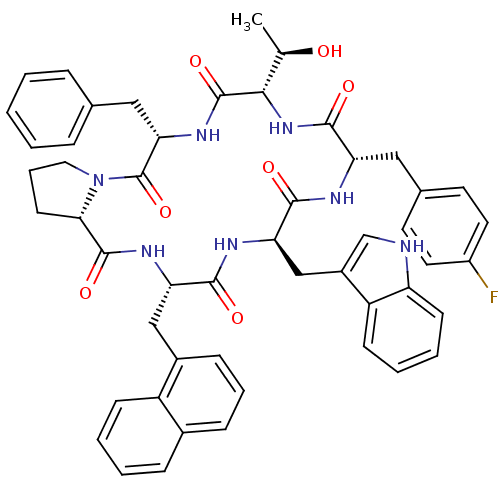
(CHEMBL2112240)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C51H52FN7O7/c1-30(60)45-50(65)57-43(26-31-11-3-2-4-12-31)51(66)59-24-10-19-44(59)49(64)56-41(27-34-15-9-14-33-13-5-6-16-37(33)34)46(61)55-42(28-35-29-53-39-18-8-7-17-38(35)39)47(62)54-40(48(63)58-45)25-32-20-22-36(52)23-21-32/h2-9,11-18,20-23,29-30,40-45,53,60H,10,19,24-28H2,1H3,(H,54,62)(H,55,61)(H,56,64)(H,57,65)(H,58,63)/t30-,40+,41+,42-,43+,44+,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Tachykinin receptor 1 |
J Med Chem 39: 2441-8 (1996)
Article DOI: 10.1021/jm960281e
BindingDB Entry DOI: 10.7270/Q2P84CJT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50407838

(CHEMBL2112243)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C51H53N7O7/c1-31(59)45-50(64)56-43(27-33-16-6-3-7-17-33)51(65)58-25-13-24-44(58)49(63)55-41(28-35-20-12-19-34-18-8-9-21-37(34)35)46(60)54-42(29-36-30-52-39-23-11-10-22-38(36)39)47(61)53-40(48(62)57-45)26-32-14-4-2-5-15-32/h2-12,14-23,30-31,40-45,52,59H,13,24-29H2,1H3,(H,53,61)(H,54,60)(H,55,63)(H,56,64)(H,57,62)/t31-,40+,41+,42-,43+,44+,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Tachykinin receptor 1 |
J Med Chem 39: 2441-8 (1996)
Article DOI: 10.1021/jm960281e
BindingDB Entry DOI: 10.7270/Q2P84CJT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50064032
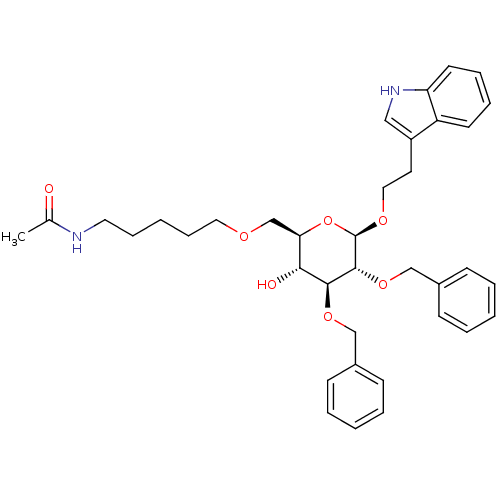
(CHEMBL29161 | N-(5-{(2R,3R,4S,5R,6R)-4,5-Bis-benzy...)Show SMILES CC(=O)NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1O Show InChI InChI=1S/C37H46N2O7/c1-27(40)38-20-11-4-12-21-42-26-33-34(41)35(44-24-28-13-5-2-6-14-28)36(45-25-29-15-7-3-8-16-29)37(46-33)43-22-19-30-23-39-32-18-10-9-17-31(30)32/h2-3,5-10,13-18,23,33-37,39,41H,4,11-12,19-22,24-26H2,1H3,(H,38,40)/t33-,34-,35+,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity was measured for Tachykinin receptor 1 |
J Med Chem 41: 1382-91 (1998)
Article DOI: 10.1021/jm9800346
BindingDB Entry DOI: 10.7270/Q28S4P2Q |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50407835
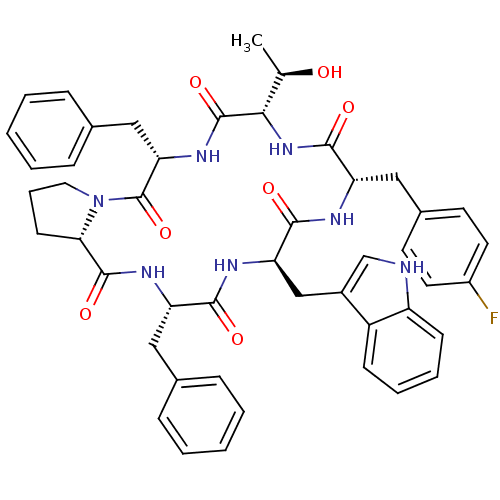
(CHEMBL2112242)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C47H50FN7O7/c1-28(56)41-46(61)53-39(25-30-13-6-3-7-14-30)47(62)55-22-10-17-40(55)45(60)52-36(23-29-11-4-2-5-12-29)42(57)51-38(26-32-27-49-35-16-9-8-15-34(32)35)43(58)50-37(44(59)54-41)24-31-18-20-33(48)21-19-31/h2-9,11-16,18-21,27-28,36-41,49,56H,10,17,22-26H2,1H3,(H,50,58)(H,51,57)(H,52,60)(H,53,61)(H,54,59)/t28-,36+,37+,38-,39+,40+,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Tachykinin receptor 1 |
J Med Chem 39: 2441-8 (1996)
Article DOI: 10.1021/jm960281e
BindingDB Entry DOI: 10.7270/Q2P84CJT |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50407839
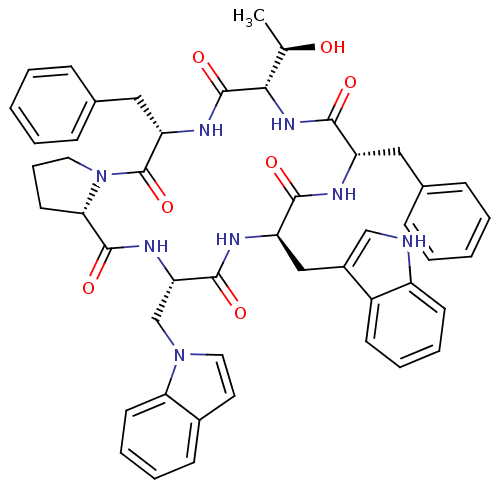
(CHEMBL2112244)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cn2ccc3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C49H52N8O7/c1-30(58)43-48(63)53-39(26-32-15-6-3-7-16-32)49(64)57-23-12-21-42(57)47(62)54-40(29-56-24-22-33-17-8-11-20-41(33)56)46(61)52-38(27-34-28-50-36-19-10-9-18-35(34)36)44(59)51-37(45(60)55-43)25-31-13-4-2-5-14-31/h2-11,13-20,22,24,28,30,37-40,42-43,50,58H,12,21,23,25-27,29H2,1H3,(H,51,59)(H,52,61)(H,53,63)(H,54,62)(H,55,60)/t30-,37+,38-,39+,40+,42+,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Tachykinin receptor 1 |
J Med Chem 39: 2441-8 (1996)
Article DOI: 10.1021/jm960281e
BindingDB Entry DOI: 10.7270/Q2P84CJT |
More data for this
Ligand-Target Pair | |
Lipoprotein lipase
(Rattus norvegicus) | BDBM50254432
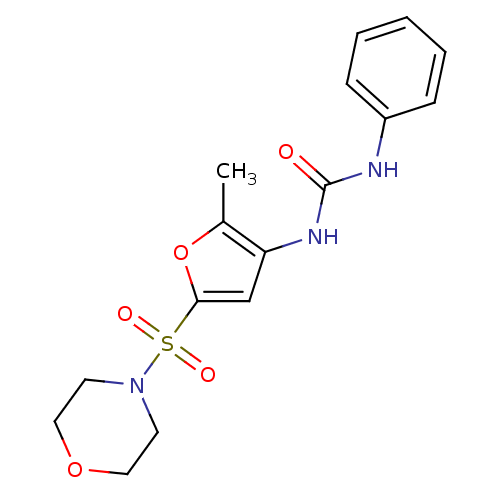
(1-(2-methyl-5-(morpholinosulfonyl)furan-3-yl)-3-ph...)Show InChI InChI=1S/C16H19N3O5S/c1-12-14(18-16(20)17-13-5-3-2-4-6-13)11-15(24-12)25(21,22)19-7-9-23-10-8-19/h2-6,11H,7-10H2,1H3,(H2,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Lipoprotein lipase from adipose tissue of rat |
Bioorg Med Chem Lett 19: 27-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.033
BindingDB Entry DOI: 10.7270/Q2BP02NZ |
More data for this
Ligand-Target Pair | |
Lipoprotein lipase
(Rattus norvegicus) | BDBM50254434
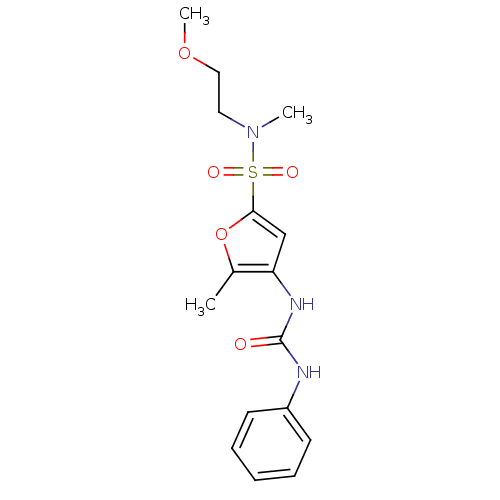
(5-Methyl-4-(3-phenyl-ureido)-furan-2-sulfonic acid...)Show SMILES COCCN(C)S(=O)(=O)c1cc(NC(=O)Nc2ccccc2)c(C)o1 Show InChI InChI=1S/C16H21N3O5S/c1-12-14(18-16(20)17-13-7-5-4-6-8-13)11-15(24-12)25(21,22)19(2)9-10-23-3/h4-8,11H,9-10H2,1-3H3,(H2,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Lipoprotein lipase from adipose tissue of rat |
Bioorg Med Chem Lett 19: 27-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.033
BindingDB Entry DOI: 10.7270/Q2BP02NZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50407840
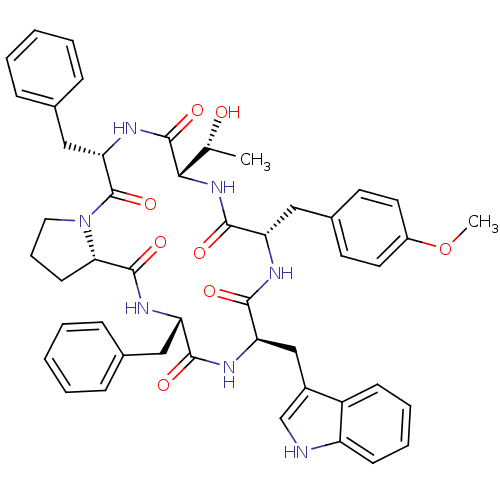
(CHEMBL2112241)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)NC(=O)[C@@H](NC2=O)[C@@H](C)O)cc1 Show InChI InChI=1S/C48H53N7O8/c1-29(56)42-47(61)53-40(26-31-14-7-4-8-15-31)48(62)55-23-11-18-41(55)46(60)52-37(24-30-12-5-3-6-13-30)43(57)51-39(27-33-28-49-36-17-10-9-16-35(33)36)44(58)50-38(45(59)54-42)25-32-19-21-34(63-2)22-20-32/h3-10,12-17,19-22,28-29,37-42,49,56H,11,18,23-27H2,1-2H3,(H,50,58)(H,51,57)(H,52,60)(H,53,61)(H,54,59)/t29-,37+,38+,39-,40+,41+,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of Tachykinin receptor 1 |
J Med Chem 39: 2441-8 (1996)
Article DOI: 10.1021/jm960281e
BindingDB Entry DOI: 10.7270/Q2P84CJT |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM50254343
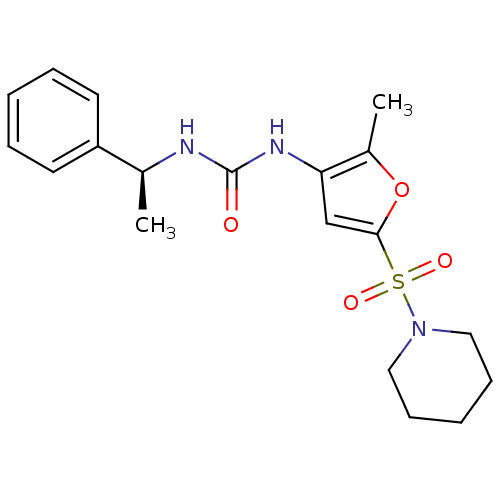
((S)-1-(2-methyl-5-(piperidin-1-ylsulfonyl)furan-3-...)Show SMILES C[C@H](NC(=O)Nc1cc(oc1C)S(=O)(=O)N1CCCCC1)c1ccccc1 |r| Show InChI InChI=1S/C19H25N3O4S/c1-14(16-9-5-3-6-10-16)20-19(23)21-17-13-18(26-15(17)2)27(24,25)22-11-7-4-8-12-22/h3,5-6,9-10,13-14H,4,7-8,11-12H2,1-2H3,(H2,20,21,23)/t14-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of endothelial lipase (unknown origin) |
Bioorg Med Chem Lett 19: 27-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.033
BindingDB Entry DOI: 10.7270/Q2BP02NZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50051573
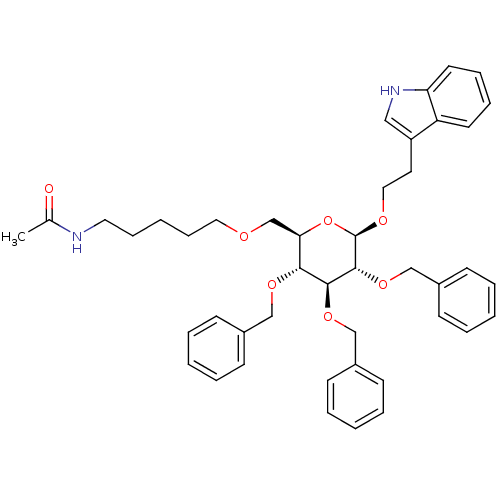
(CHEMBL420221 | N-(5-{(2R,3R,4S,5R,6R)-3,4,5-Tris-b...)Show SMILES CC(=O)NCCCCCOC[C@H]1O[C@@H](OCCc2c[nH]c3ccccc23)[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 Show InChI InChI=1S/C44H52N2O7/c1-33(47)45-25-14-5-15-26-48-32-40-41(50-29-34-16-6-2-7-17-34)42(51-30-35-18-8-3-9-19-35)43(52-31-36-20-10-4-11-21-36)44(53-40)49-27-24-37-28-46-39-23-13-12-22-38(37)39/h2-4,6-13,16-23,28,40-44,46H,5,14-15,24-27,29-32H2,1H3,(H,45,47)/t40-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity towards Tachykinin receptor 1 |
J Med Chem 39: 2441-8 (1996)
Article DOI: 10.1021/jm960281e
BindingDB Entry DOI: 10.7270/Q2P84CJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data