

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121729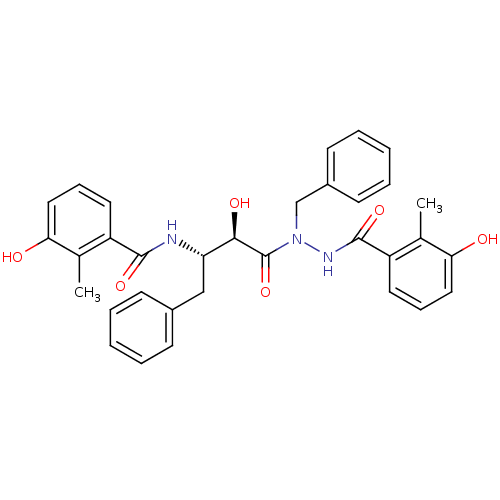 (CHEMBL368169 | KNI-1167 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121730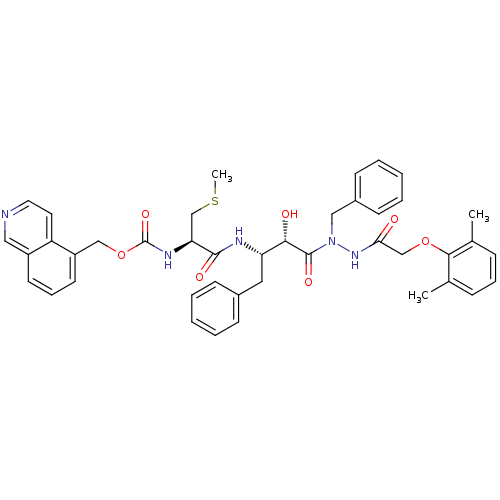 (CHEMBL366433 | {1-[(S)-3-{N-Benzyl-N'-[2-(2,6-dime...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121732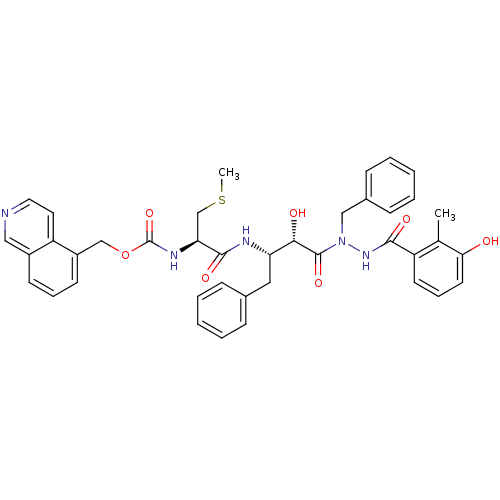 (CHEMBL172850 | {1-[(S)-3-[N-Benzyl-N'-(3-hydroxy-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984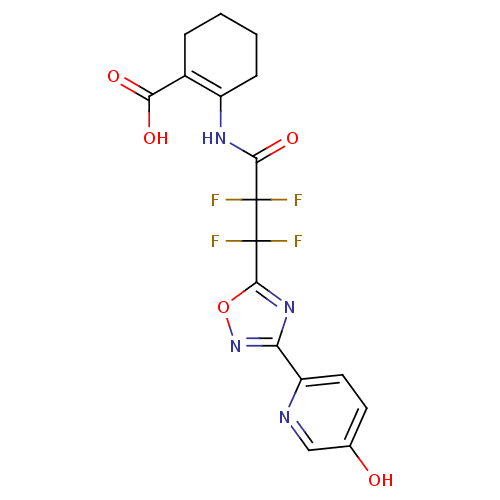 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121735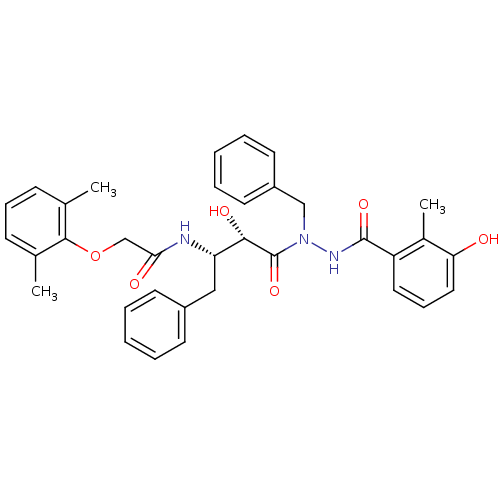 (CHEMBL367679 | KNI-1277 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977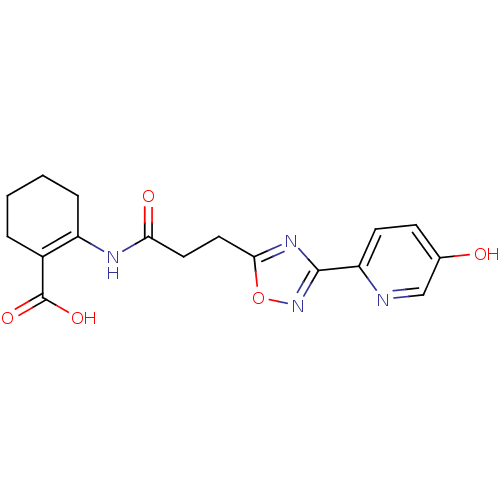 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533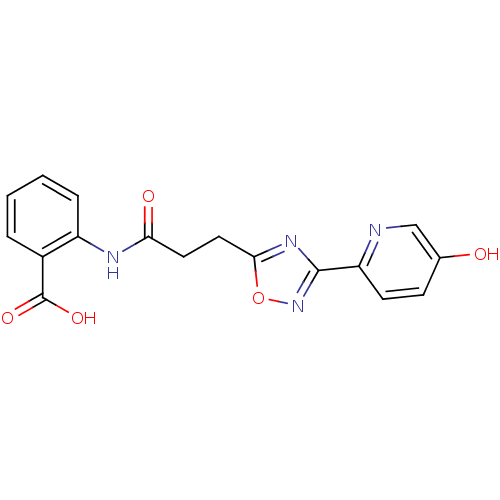 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976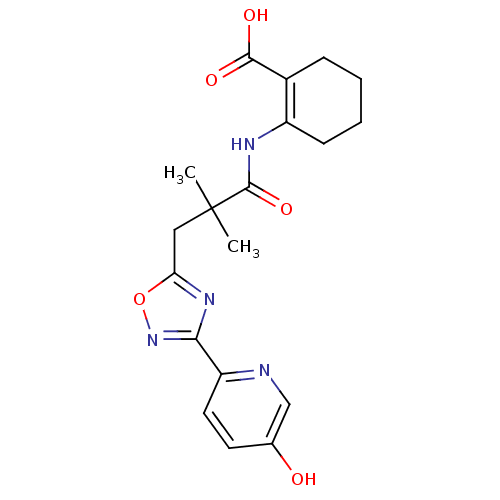 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121733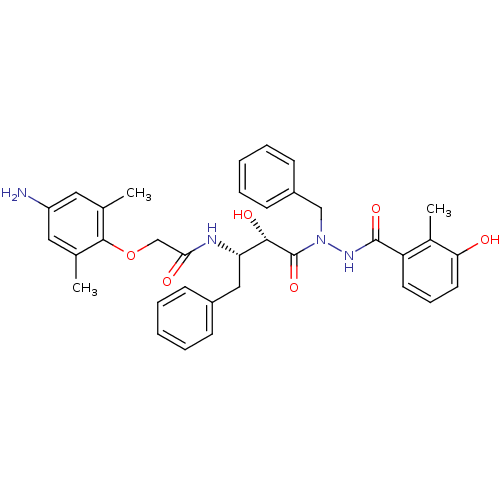 (2-(4-Amino-2,6-dimethyl-phenoxy)-N-[(S)-3-[N-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121728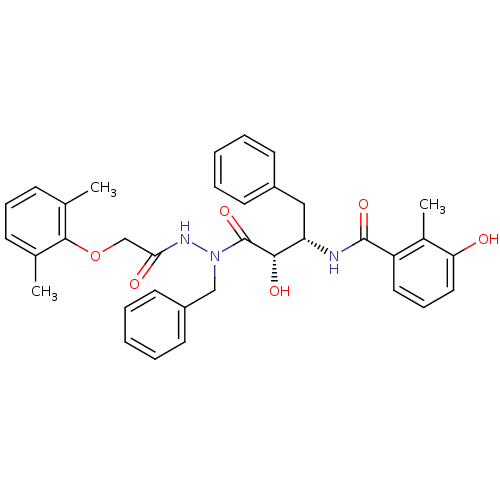 (CHEMBL172012 | KNI-1279 | N-[(S)-3-{N-Benzyl-N'-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50238632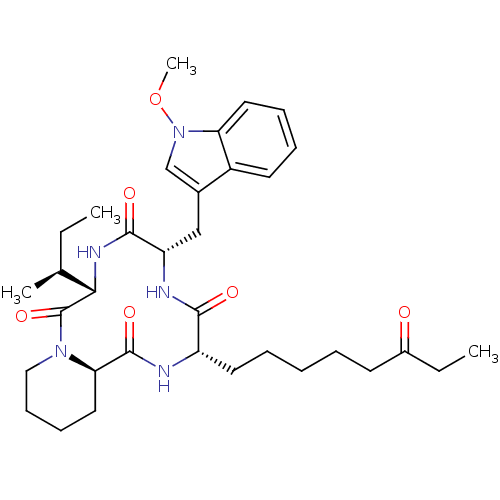 ((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366737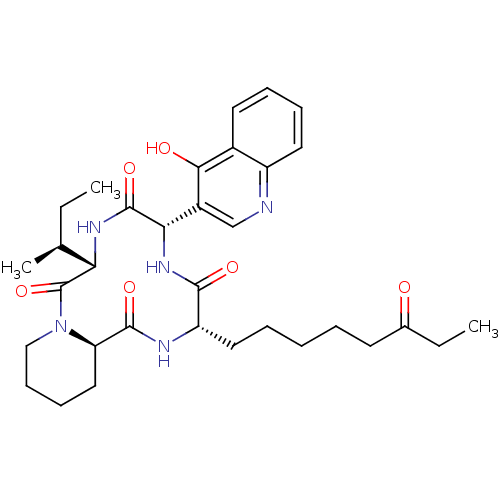 (CHEMBL1793971) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979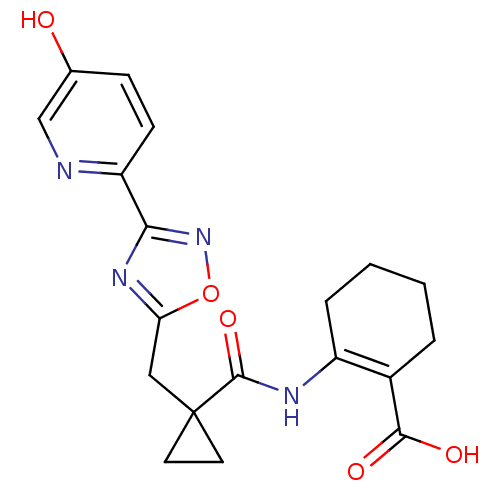 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121734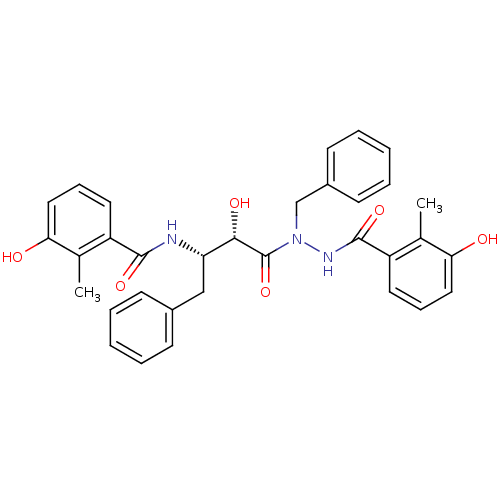 (CHEMBL177917 | KNI-1276 | N-[(S)-3-[N-Benzyl-N'-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366726 (CHEMBL1793982) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981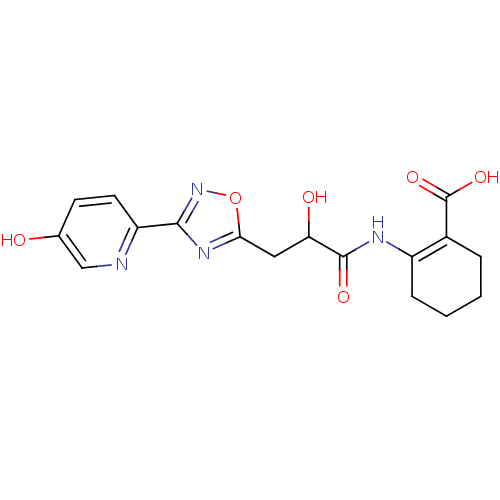 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982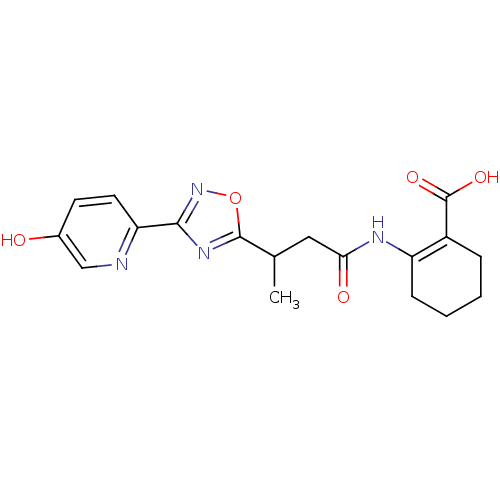 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366733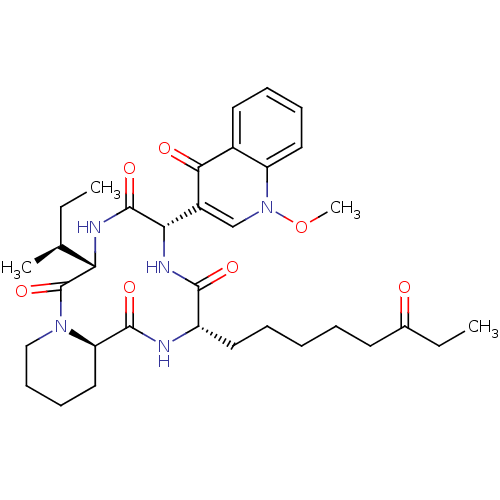 (CHEMBL1793973) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366749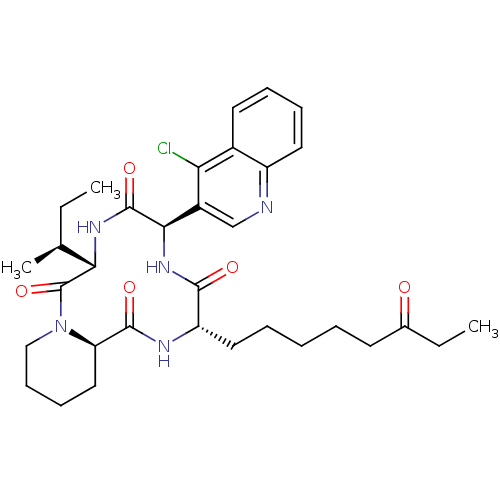 (CHEMBL1793983) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells | J Med Chem 51: 5101-8 (2008) Article DOI: 10.1021/jm800258p BindingDB Entry DOI: 10.7270/Q2CF9PWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366736 (CHEMBL1793972) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366727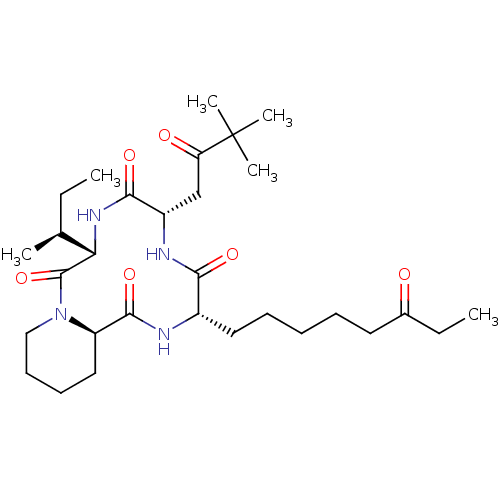 (CHEMBL1793981) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983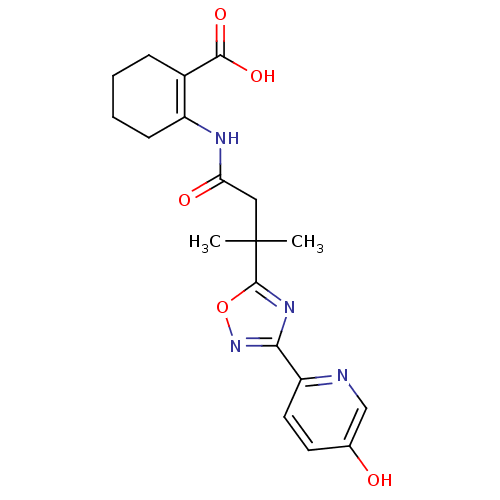 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366729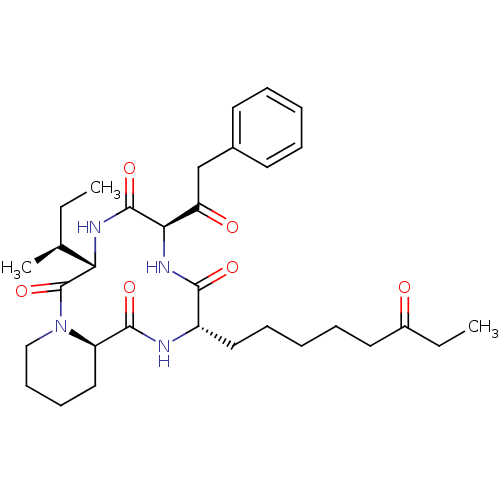 (CHEMBL1793969) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366752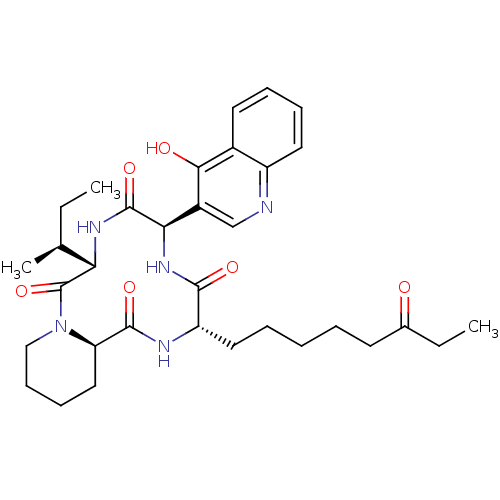 (CHEMBL1793970) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980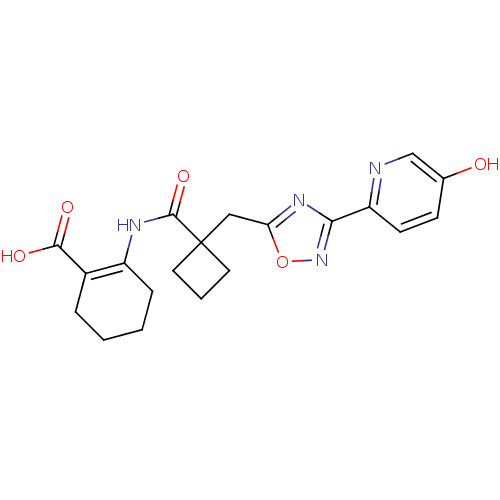 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366728 (CHEMBL1793978) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50273099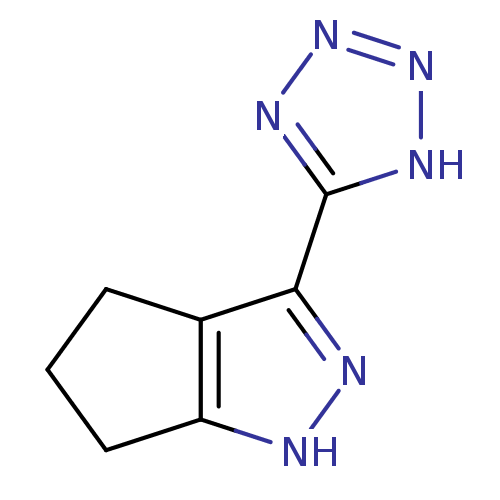 (3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells | J Med Chem 51: 5101-8 (2008) Article DOI: 10.1021/jm800258p BindingDB Entry DOI: 10.7270/Q2CF9PWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Cryptosporidium parvum) | BDBM50366750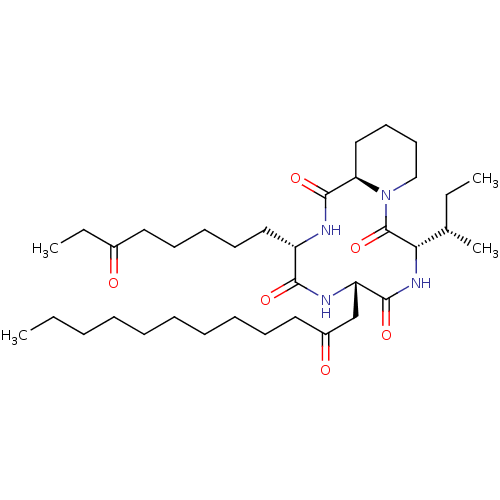 (CHEMBL1793987) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa | Bioorg Med Chem Lett 11: 113-7 (2001) BindingDB Entry DOI: 10.7270/Q2M045ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121731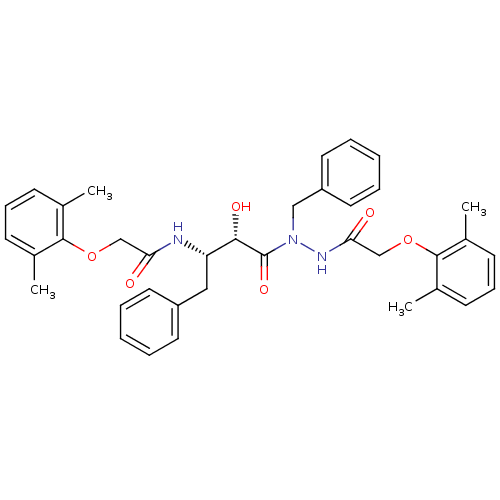 (CHEMBL177918 | KNI-1278 | N-[(S)-3-{N-Benzyl-N'-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | Bioorg Med Chem Lett 13: 93-6 (2002) BindingDB Entry DOI: 10.7270/Q2N29W9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1791 total ) | Next | Last >> |