 Found 4527 hits with Last Name = 'cowen' and Initial = 's'
Found 4527 hits with Last Name = 'cowen' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50107639

(3-{2-[2-(4-Chloro-2-fluoro-benzoylamino)-3-m-tolyl...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(Cl)cc2F)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H25ClFN3O5/c1-17-4-2-5-18(10-17)12-25(33-26(34)23-9-8-21(29)13-24(23)30)27(35)32-22(14-31)16-38-15-19-6-3-7-20(11-19)28(36)37/h2-11,13,22,25H,12,15-16H2,1H3,(H,32,35)(H,33,34)(H,36,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379339
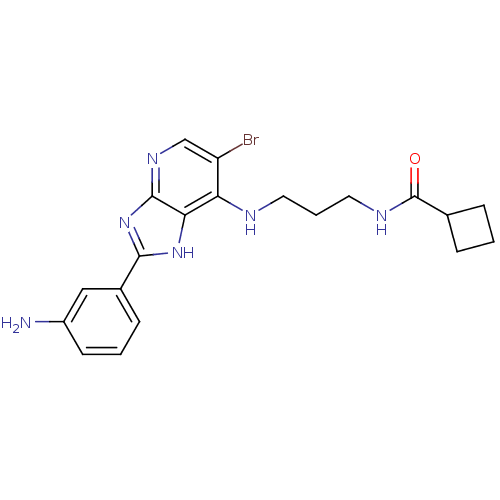
(CHEMBL2011940)Show SMILES Nc1cccc(c1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C20H23BrN6O/c21-15-11-25-19-17(26-18(27-19)13-6-2-7-14(22)10-13)16(15)23-8-3-9-24-20(28)12-4-1-5-12/h2,6-7,10-12H,1,3-5,8-9,22H2,(H,24,28)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379363
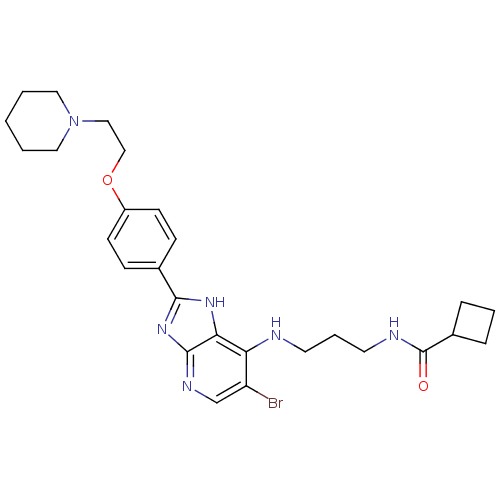
(CHEMBL2011941)Show SMILES Brc1cnc2nc([nH]c2c1NCCCNC(=O)C1CCC1)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C27H35BrN6O2/c28-22-18-31-26-24(23(22)29-12-5-13-30-27(35)20-6-4-7-20)32-25(33-26)19-8-10-21(11-9-19)36-17-16-34-14-2-1-3-15-34/h8-11,18,20H,1-7,12-17H2,(H,30,35)(H2,29,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379357
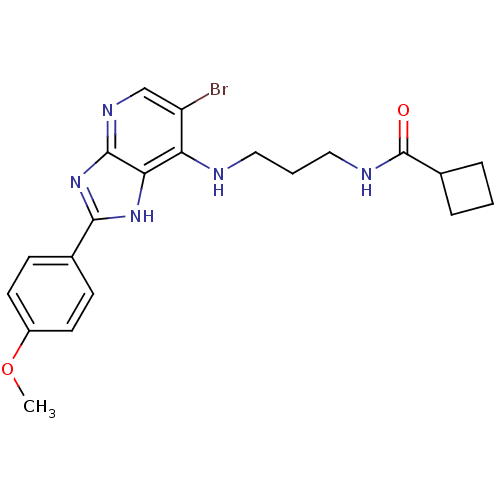
(CHEMBL2011933)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C21H24BrN5O2/c1-29-15-8-6-13(7-9-15)19-26-18-17(16(22)12-25-20(18)27-19)23-10-3-11-24-21(28)14-4-2-5-14/h6-9,12,14H,2-5,10-11H2,1H3,(H,24,28)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length TBK1 (unknown origin) using CK1tide as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 1138-43 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.123
BindingDB Entry DOI: 10.7270/Q20V8F87 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
(Homo sapiens (Human)) | BDBM50379339

(CHEMBL2011940)Show SMILES Nc1cccc(c1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C20H23BrN6O/c21-15-11-25-19-17(26-18(27-19)13-6-2-7-14(22)10-13)16(15)23-8-3-9-24-20(28)12-4-1-5-12/h2,6-7,10-12H,1,3-5,8-9,22H2,(H,24,28)(H2,23,25,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Ikkepsilon using 5FAM-AKELDQGSLCTpSFVGTLQ-NH2 as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50379339

(CHEMBL2011940)Show SMILES Nc1cccc(c1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C20H23BrN6O/c21-15-11-25-19-17(26-18(27-19)13-6-2-7-14(22)10-13)16(15)23-8-3-9-24-20(28)12-4-1-5-12/h2,6-7,10-12H,1,3-5,8-9,22H2,(H,24,28)(H2,23,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379356
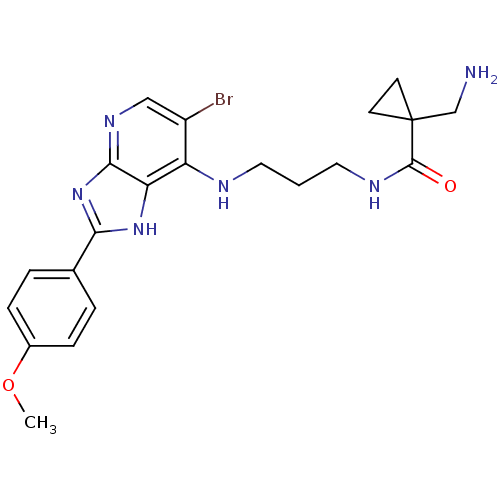
(CHEMBL2011932)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3(CN)CC3)c2[nH]1 Show InChI InChI=1S/C21H25BrN6O2/c1-30-14-5-3-13(4-6-14)18-27-17-16(15(22)11-26-19(17)28-18)24-9-2-10-25-20(29)21(12-23)7-8-21/h3-6,11H,2,7-10,12,23H2,1H3,(H,25,29)(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379364
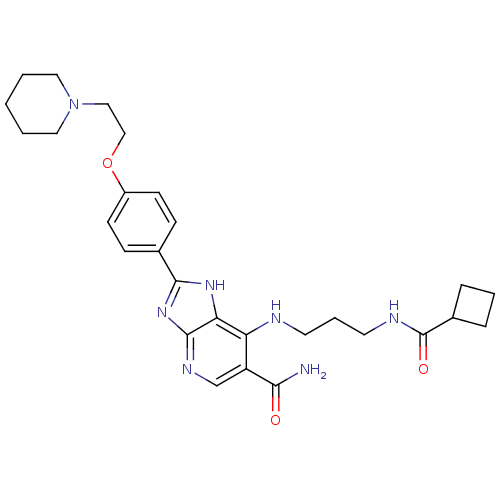
(CHEMBL2011942)Show SMILES NC(=O)c1cnc2nc([nH]c2c1NCCCNC(=O)C1CCC1)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H37N7O3/c29-25(36)22-18-32-27-24(23(22)30-12-5-13-31-28(37)20-6-4-7-20)33-26(34-27)19-8-10-21(11-9-19)38-17-16-35-14-2-1-3-15-35/h8-11,18,20H,1-7,12-17H2,(H2,29,36)(H,31,37)(H2,30,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379357

(CHEMBL2011933)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C21H24BrN5O2/c1-29-15-8-6-13(7-9-15)19-26-18-17(16(22)12-25-20(18)27-19)23-10-3-11-24-21(28)14-4-2-5-14/h6-9,12,14H,2-5,10-11H2,1H3,(H,24,28)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135541
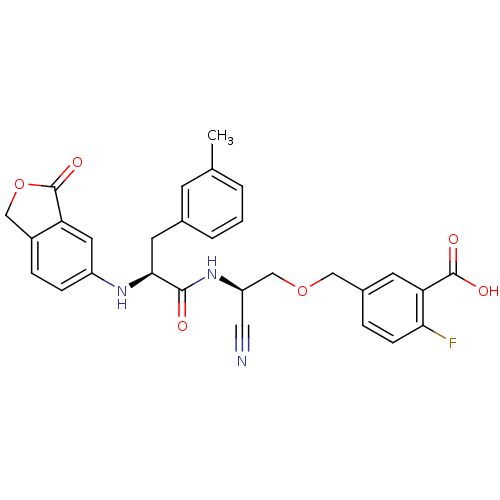
(5-{(R)-2-Cyano-2-[(S)-2-(3-oxo-1,3-dihydro-isobenz...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3COC(=O)c3c2)C(=O)N[C@@H](COCc2ccc(F)c(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C29H26FN3O6/c1-17-3-2-4-18(9-17)11-26(32-21-7-6-20-15-39-29(37)23(20)12-21)27(34)33-22(13-31)16-38-14-19-5-8-25(30)24(10-19)28(35)36/h2-10,12,22,26,32H,11,14-16H2,1H3,(H,33,34)(H,35,36)/t22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135543

(3-{(R)-2-Cyano-2-[(S)-2-(2-methyl-1,3-dioxo-2,3-di...)Show SMILES CN1C(=O)c2ccc(N[C@@H](Cc3cccc(C)c3)C(=O)N[C@@H](COCc3cccc(c3)C(O)=O)C#N)cc2C1=O Show InChI InChI=1S/C30H28N4O6/c1-18-5-3-6-19(11-18)13-26(32-22-9-10-24-25(14-22)29(37)34(2)28(24)36)27(35)33-23(15-31)17-40-16-20-7-4-8-21(12-20)30(38)39/h3-12,14,23,26,32H,13,16-17H2,1-2H3,(H,33,35)(H,38,39)/t23-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379358
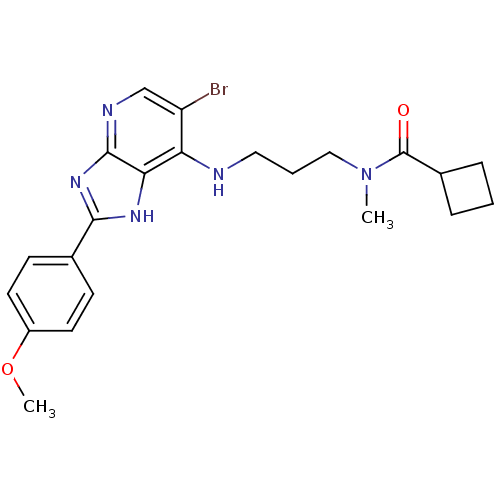
(CHEMBL2011934)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCN(C)C(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C22H26BrN5O2/c1-28(22(29)15-5-3-6-15)12-4-11-24-18-17(23)13-25-21-19(18)26-20(27-21)14-7-9-16(30-2)10-8-14/h7-10,13,15H,3-6,11-12H2,1-2H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
(Homo sapiens (Human)) | BDBM50379356

(CHEMBL2011932)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3(CN)CC3)c2[nH]1 Show InChI InChI=1S/C21H25BrN6O2/c1-30-14-5-3-13(4-6-14)18-27-17-16(15(22)11-26-19(17)28-18)24-9-2-10-25-20(29)21(12-23)7-8-21/h3-6,11H,2,7-10,12,23H2,1H3,(H,25,29)(H2,24,26,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Ikkepsilon using 5FAM-AKELDQGSLCTpSFVGTLQ-NH2 as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107633
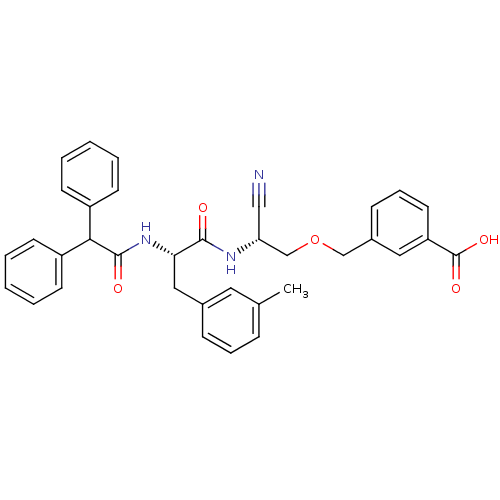
(3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C35H33N3O5/c1-24-10-8-11-25(18-24)20-31(38-34(40)32(27-13-4-2-5-14-27)28-15-6-3-7-16-28)33(39)37-30(21-36)23-43-22-26-12-9-17-29(19-26)35(41)42/h2-19,30-32H,20,22-23H2,1H3,(H,37,39)(H,38,40)(H,41,42)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135533

((S)-N-[(R)-Cyano-(3-tetrazol-1-yl-benzyloxymethyl)...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3COC(=O)c3c2)C(=O)N[C@@H](COCc2cccc(c2)-n2cnnn2)C#N)c1 Show InChI InChI=1S/C29H27N7O4/c1-19-4-2-5-20(10-19)12-27(32-23-9-8-22-16-40-29(38)26(22)13-23)28(37)33-24(14-30)17-39-15-21-6-3-7-25(11-21)36-18-31-34-35-36/h2-11,13,18,24,27,32H,12,15-17H2,1H3,(H,33,37)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50256750
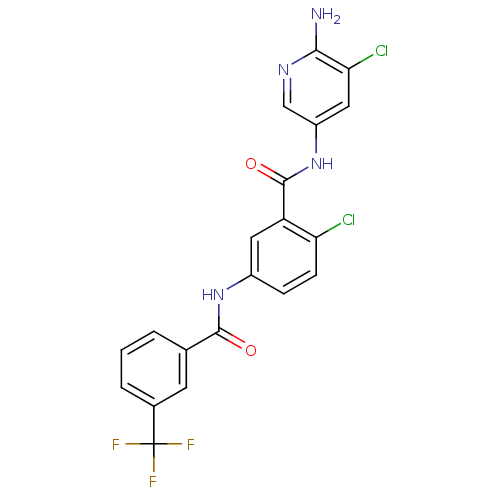
(CHEMBL475817 | N-(6-amino-5-chloropyridin-3-yl)-2-...)Show SMILES Nc1ncc(NC(=O)c2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2Cl)cc1Cl Show InChI InChI=1S/C20H13Cl2F3N4O2/c21-15-5-4-12(28-18(30)10-2-1-3-11(6-10)20(23,24)25)7-14(15)19(31)29-13-8-16(22)17(26)27-9-13/h1-9H,(H2,26,27)(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha (unknown origin) |
Bioorg Med Chem Lett 19: 1026-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.10.053
BindingDB Entry DOI: 10.7270/Q2222TMP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107633

(3-[2-Cyano-2-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C35H33N3O5/c1-24-10-8-11-25(18-24)20-31(38-34(40)32(27-13-4-2-5-14-27)28-15-6-3-7-16-28)33(39)37-30(21-36)23-43-22-26-12-9-17-29(19-26)35(41)42/h2-19,30-32H,20,22-23H2,1H3,(H,37,39)(H,38,40)(H,41,42)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50256750

(CHEMBL475817 | N-(6-amino-5-chloropyridin-3-yl)-2-...)Show SMILES Nc1ncc(NC(=O)c2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2Cl)cc1Cl Show InChI InChI=1S/C20H13Cl2F3N4O2/c21-15-5-4-12(28-18(30)10-2-1-3-11(6-10)20(23,24)25)7-14(15)19(31)29-13-8-16(22)17(26)27-9-13/h1-9H,(H2,26,27)(H,28,30)(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R (unknown origin) |
Bioorg Med Chem Lett 19: 1026-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.10.053
BindingDB Entry DOI: 10.7270/Q2222TMP |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135537

(3-{(R)-2-Cyano-2-[(S)-2-(3-oxo-indan-5-ylamino)-3-...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3CCC(=O)c3c2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C30H29N3O5/c1-19-4-2-5-20(12-19)14-27(32-24-10-8-22-9-11-28(34)26(22)15-24)29(35)33-25(16-31)18-38-17-21-6-3-7-23(13-21)30(36)37/h2-8,10,12-13,15,25,27,32H,9,11,14,17-18H2,1H3,(H,33,35)(H,36,37)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135535
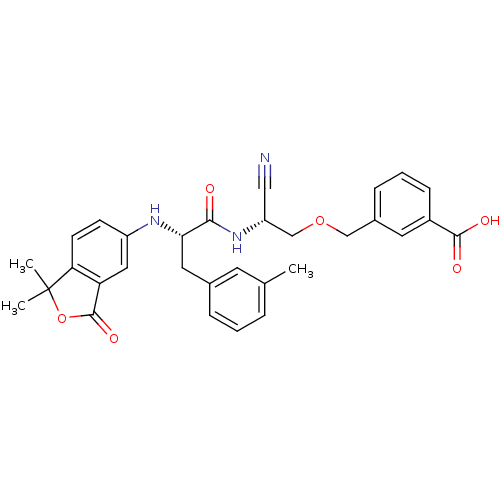
(3-{(R)-2-Cyano-2-[(S)-2-(1,1-dimethyl-3-oxo-1,3-di...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3c(c2)C(=O)OC3(C)C)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C31H31N3O6/c1-19-6-4-7-20(12-19)14-27(33-23-10-11-26-25(15-23)30(38)40-31(26,2)3)28(35)34-24(16-32)18-39-17-21-8-5-9-22(13-21)29(36)37/h4-13,15,24,27,33H,14,17-18H2,1-3H3,(H,34,35)(H,36,37)/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107646

(3-[4-Cyano-4-(2-diphenylacetylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](CC#Cc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C36H31N3O4/c1-25-11-8-14-27(21-25)23-32(39-35(41)33(28-15-4-2-5-16-28)29-17-6-3-7-18-29)34(40)38-31(24-37)20-10-13-26-12-9-19-30(22-26)36(42)43/h2-9,11-12,14-19,21-22,31-33H,20,23H2,1H3,(H,38,40)(H,39,41)(H,42,43)/t31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50107623
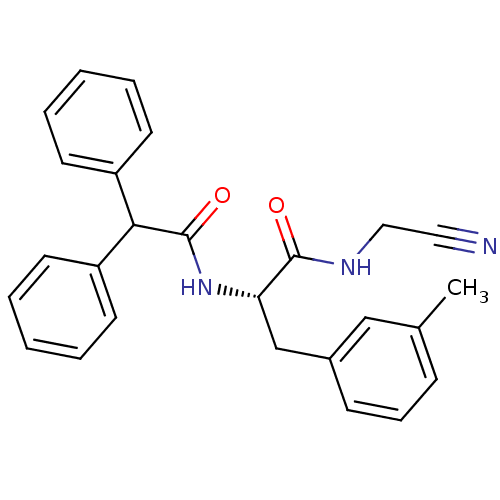
(CHEMBL140756 | N-Cyanomethyl-2-diphenylacetylamino...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)c1 Show InChI InChI=1S/C26H25N3O2/c1-19-9-8-10-20(17-19)18-23(25(30)28-16-15-27)29-26(31)24(21-11-4-2-5-12-21)22-13-6-3-7-14-22/h2-14,17,23-24H,16,18H2,1H3,(H,28,30)(H,29,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity of the compound against recombinant human cathepsin L (cat L) expressed in baculovirus |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379357

(CHEMBL2011933)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C21H24BrN5O2/c1-29-15-8-6-13(7-9-15)19-26-18-17(16(22)12-25-20(18)27-19)23-10-3-11-24-21(28)14-4-2-5-14/h6-9,12,14H,2-5,10-11H2,1H3,(H,24,28)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 in HEK293 cells after 4.5 hrs by ISRE-luciferase reporter gene assay in presence of poly I:C |
Bioorg Med Chem Lett 24: 1138-43 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.123
BindingDB Entry DOI: 10.7270/Q20V8F87 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135546
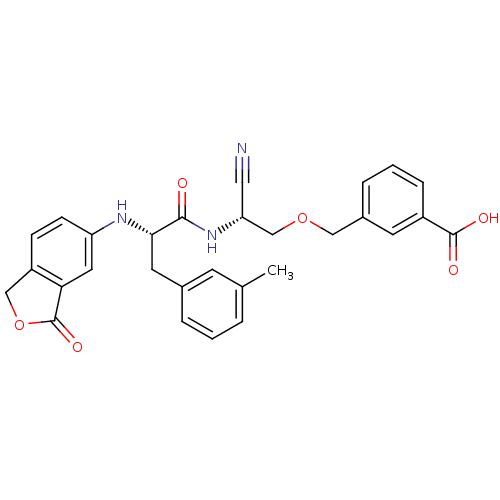
(3-{(R)-2-Cyano-2-[(S)-2-(3-oxo-1,3-dihydro-isobenz...)Show SMILES Cc1cccc(C[C@H](Nc2ccc3COC(=O)c3c2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C29H27N3O6/c1-18-4-2-5-19(10-18)12-26(31-23-9-8-22-16-38-29(36)25(22)13-23)27(33)32-24(14-30)17-37-15-20-6-3-7-21(11-20)28(34)35/h2-11,13,24,26,31H,12,15-17H2,1H3,(H,32,33)(H,34,35)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107626

(3-{(R)-2-Cyano-2-[(S)-2-(2,4-difluoro-benzoylamino...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(F)cc2F)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H25F2N3O5/c1-17-4-2-5-18(10-17)12-25(33-26(34)23-9-8-21(29)13-24(23)30)27(35)32-22(14-31)16-38-15-19-6-3-7-20(11-19)28(36)37/h2-11,13,22,25H,12,15-16H2,1H3,(H,32,35)(H,33,34)(H,36,37)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50448992
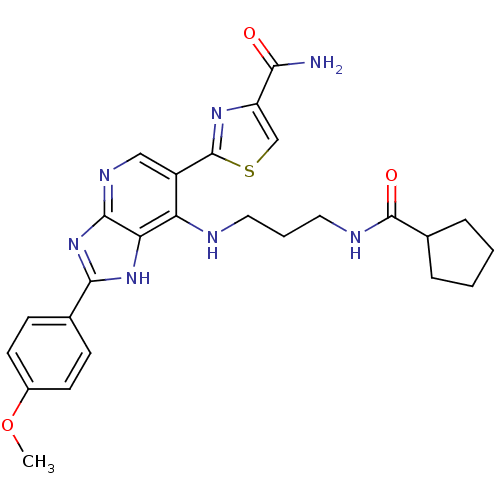
(CHEMBL3125721)Show SMILES COc1ccc(cc1)-c1nc2ncc(-c3nc(cs3)C(N)=O)c(NCCCNC(=O)C3CCCC3)c2[nH]1 Show InChI InChI=1S/C26H29N7O3S/c1-36-17-9-7-15(8-10-17)23-32-21-20(28-11-4-12-29-25(35)16-5-2-3-6-16)18(13-30-24(21)33-23)26-31-19(14-37-26)22(27)34/h7-10,13-14,16H,2-6,11-12H2,1H3,(H2,27,34)(H,29,35)(H2,28,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 in HEK293 cells after 4.5 hrs by ISRE-luciferase reporter gene assay in presence of poly I:C |
Bioorg Med Chem Lett 24: 1138-43 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.123
BindingDB Entry DOI: 10.7270/Q20V8F87 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50379358

(CHEMBL2011934)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCN(C)C(=O)C3CCC3)c2[nH]1 Show InChI InChI=1S/C22H26BrN5O2/c1-28(22(29)15-5-3-6-15)12-4-11-24-18-17(23)13-25-21-19(18)26-20(27-21)14-7-9-16(30-2)10-8-14/h7-10,13,15H,3-6,11-12H2,1-2H3,(H2,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379354

(CHEMBL2011930)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C(C)(C)C)c2[nH]1 Show InChI InChI=1S/C21H26BrN5O2/c1-21(2,3)20(28)24-11-5-10-23-16-15(22)12-25-19-17(16)26-18(27-19)13-6-8-14(29-4)9-7-13/h6-9,12H,5,10-11H2,1-4H3,(H,24,28)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379359
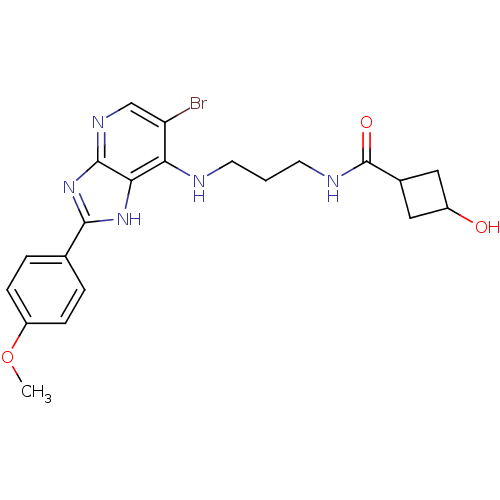
(CHEMBL2011935)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CC(O)C3)c2[nH]1 |(36.6,-30.43,;35.82,-29.1,;34.28,-29.11,;33.52,-30.45,;31.98,-30.46,;31.2,-29.13,;31.96,-27.8,;33.49,-27.78,;29.66,-29.14,;28.76,-30.39,;27.29,-29.92,;25.95,-30.7,;24.62,-29.93,;24.62,-28.39,;23.29,-27.62,;25.95,-27.62,;25.95,-26.08,;27.28,-25.3,;27.27,-23.76,;28.61,-23,;28.61,-21.46,;29.95,-20.69,;31.28,-21.46,;29.95,-19.15,;31.04,-18.07,;29.95,-16.98,;29.96,-15.44,;28.86,-18.06,;27.29,-28.38,;28.75,-27.9,)| Show InChI InChI=1S/C21H24BrN5O3/c1-30-15-5-3-12(4-6-15)19-26-18-17(16(22)11-25-20(18)27-19)23-7-2-8-24-21(29)13-9-14(28)10-13/h3-6,11,13-14,28H,2,7-10H2,1H3,(H,24,29)(H2,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379337
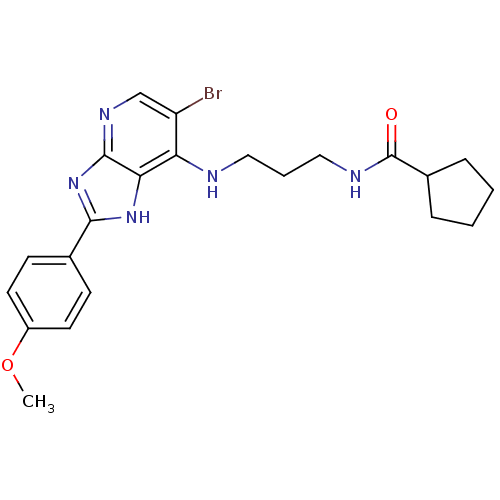
(CHEMBL2011936)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCCC3)c2[nH]1 Show InChI InChI=1S/C22H26BrN5O2/c1-30-16-9-7-14(8-10-16)20-27-19-18(17(23)13-26-21(19)28-20)24-11-4-12-25-22(29)15-5-2-3-6-15/h7-10,13,15H,2-6,11-12H2,1H3,(H,25,29)(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TBK1 in HEK293 cells after 4.5 hrs by ISRE-luciferase reporter gene assay in presence of poly I:C |
Bioorg Med Chem Lett 24: 1138-43 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.123
BindingDB Entry DOI: 10.7270/Q20V8F87 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135532
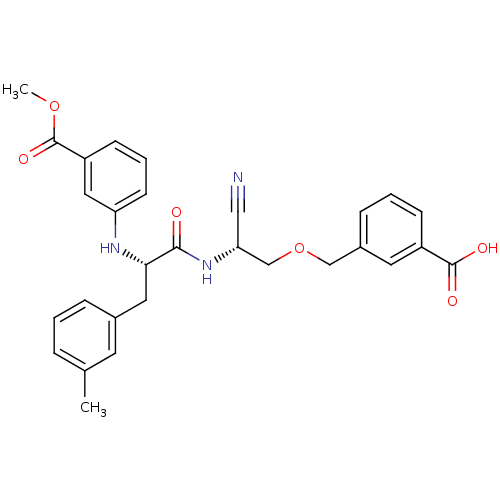
(3-{2-cyano-2-[1-(3-methyloxycarbonylanilino)-2-(3-...)Show SMILES COC(=O)c1cccc(N[C@@H](Cc2cccc(C)c2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C29H29N3O6/c1-19-6-3-7-20(12-19)14-26(31-24-11-5-10-23(15-24)29(36)37-2)27(33)32-25(16-30)18-38-17-21-8-4-9-22(13-21)28(34)35/h3-13,15,25-26,31H,14,17-18H2,1-2H3,(H,32,33)(H,34,35)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135544

(2-Chloro-5-[(R)-2-cyano-2-((S)-2-phenylamino-3-m-t...)Show SMILES Cc1cccc(C[C@H](Nc2ccccc2)C(=O)N[C@@H](COCc2ccc(Cl)c(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C27H26ClN3O4/c1-18-6-5-7-19(12-18)14-25(30-21-8-3-2-4-9-21)26(32)31-22(15-29)17-35-16-20-10-11-24(28)23(13-20)27(33)34/h2-13,22,25,30H,14,16-17H2,1H3,(H,31,32)(H,33,34)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50448992

(CHEMBL3125721)Show SMILES COc1ccc(cc1)-c1nc2ncc(-c3nc(cs3)C(N)=O)c(NCCCNC(=O)C3CCCC3)c2[nH]1 Show InChI InChI=1S/C26H29N7O3S/c1-36-17-9-7-15(8-10-17)23-32-21-20(28-11-4-12-29-25(35)16-5-2-3-6-16)18(13-30-24(21)33-23)26-31-19(14-37-26)22(27)34/h7-10,13-14,16H,2-6,11-12H2,1H3,(H2,27,34)(H,29,35)(H2,28,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length TBK1 (unknown origin) using CK1tide as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 1138-43 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.123
BindingDB Entry DOI: 10.7270/Q20V8F87 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379365
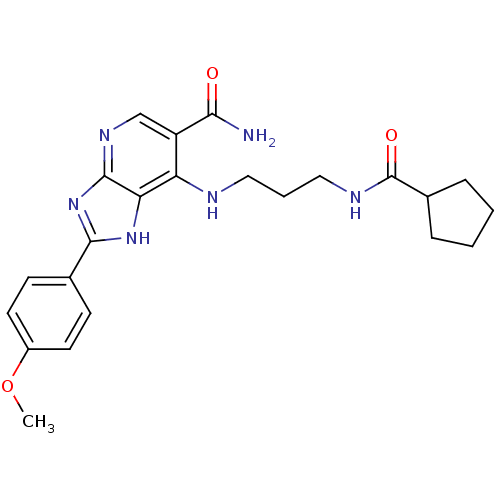
(CHEMBL2011943)Show SMILES COc1ccc(cc1)-c1nc2ncc(C(N)=O)c(NCCCNC(=O)C3CCCC3)c2[nH]1 Show InChI InChI=1S/C23H28N6O3/c1-32-16-9-7-14(8-10-16)21-28-19-18(17(20(24)30)13-27-22(19)29-21)25-11-4-12-26-23(31)15-5-2-3-6-15/h7-10,13,15H,2-6,11-12H2,1H3,(H2,24,30)(H,26,31)(H2,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
(Homo sapiens (Human)) | BDBM50379364

(CHEMBL2011942)Show SMILES NC(=O)c1cnc2nc([nH]c2c1NCCCNC(=O)C1CCC1)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H37N7O3/c29-25(36)22-18-32-27-24(23(22)30-12-5-13-31-28(37)20-6-4-7-20)33-26(34-27)19-8-10-21(11-9-19)38-17-16-35-14-2-1-3-15-35/h8-11,18,20H,1-7,12-17H2,(H2,29,36)(H,31,37)(H2,30,32,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Ikkepsilon using 5FAM-AKELDQGSLCTpSFVGTLQ-NH2 as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135547

(3-{(R)-2-Cyano-2-[(S)-2-(2-methyl-3-oxo-2,3-dihydr...)Show SMILES CN1Cc2ccc(N[C@@H](Cc3cccc(C)c3)C(=O)N[C@@H](COCc3cccc(c3)C(O)=O)C#N)cc2C1=O Show InChI InChI=1S/C30H30N4O5/c1-19-5-3-6-20(11-19)13-27(32-24-10-9-23-16-34(2)29(36)26(23)14-24)28(35)33-25(15-31)18-39-17-21-7-4-8-22(12-21)30(37)38/h3-12,14,25,27,32H,13,16-18H2,1-2H3,(H,33,35)(H,37,38)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107620

(3-[2-(2-Benzoylamino-3-m-tolyl-propionylamino)-2-c...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H27N3O5/c1-19-7-5-8-20(13-19)15-25(31-26(32)22-10-3-2-4-11-22)27(33)30-24(16-29)18-36-17-21-9-6-12-23(14-21)28(34)35/h2-14,24-25H,15,17-18H2,1H3,(H,30,33)(H,31,32)(H,34,35)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379337

(CHEMBL2011936)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCCC3)c2[nH]1 Show InChI InChI=1S/C22H26BrN5O2/c1-30-16-9-7-14(8-10-16)20-27-19-18(17(23)13-26-21(19)28-20)24-11-4-12-25-22(29)15-5-2-3-6-15/h7-10,13,15H,2-6,11-12H2,1H3,(H,25,29)(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length TBK1 (unknown origin) using CK1tide as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 24: 1138-43 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.123
BindingDB Entry DOI: 10.7270/Q20V8F87 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379337

(CHEMBL2011936)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CCCC3)c2[nH]1 Show InChI InChI=1S/C22H26BrN5O2/c1-30-16-9-7-14(8-10-16)20-27-19-18(17(23)13-26-21(19)28-20)24-11-4-12-25-22(29)15-5-2-3-6-15/h7-10,13,15H,2-6,11-12H2,1H3,(H,25,29)(H2,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50379353
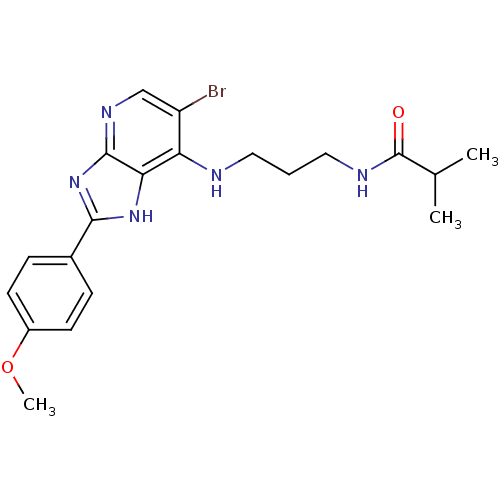
(CHEMBL2011929)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C(C)C)c2[nH]1 Show InChI InChI=1S/C20H24BrN5O2/c1-12(2)20(27)23-10-4-9-22-16-15(21)11-24-19-17(16)25-18(26-19)13-5-7-14(28-3)8-6-13/h5-8,11-12H,4,9-10H2,1-3H3,(H,23,27)(H2,22,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TBK1 using 5FAM-AhxKRRAL(ps)VASLPGL as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107638

(CHEMBL336436 | N-(Benzyloxymethyl-cyano-methyl)-2-...)Show SMILES Cc1cccc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)N[C@@H](COCc2ccccc2)C#N)c1 Show InChI InChI=1S/C34H33N3O3/c1-25-12-11-15-27(20-25)21-31(33(38)36-30(22-35)24-40-23-26-13-5-2-6-14-26)37-34(39)32(28-16-7-3-8-17-28)29-18-9-4-10-19-29/h2-20,30-32H,21,23-24H2,1H3,(H,36,38)(H,37,39)/t30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107650

(CHEMBL138661 | N-Cyanomethyl-3-(3,5-dimethyl-pheny...)Show SMILES Cc1cc(C)cc(C[C@H](NC(=O)C(c2ccccc2)c2ccccc2)C(=O)NCC#N)c1 Show InChI InChI=1S/C27H27N3O2/c1-19-15-20(2)17-21(16-19)18-24(26(31)29-14-13-28)30-27(32)25(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-12,15-17,24-25H,14,18H2,1-2H3,(H,29,31)(H,30,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
N-lysine methyltransferase SMYD2
(Homo sapiens (Human)) | BDBM50550528

(CHEMBL4756148)Show SMILES Clc1ccc(CCN2CC(C2)Oc2ccccc2-c2cncc(c2)C(=O)NCCCN2CCCC2)cc1Cl | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SMYD2 (unknown origin) |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2BG2SKF |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135534

(5-[(R)-2-Cyano-2-((S)-2-phenylamino-3-m-tolyl-prop...)Show SMILES Cc1cccc(C[C@H](Nc2ccccc2)C(=O)N[C@@H](COCc2ccc(F)c(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C27H26FN3O4/c1-18-6-5-7-19(12-18)14-25(30-21-8-3-2-4-9-21)26(32)31-22(15-29)17-35-16-20-10-11-24(28)23(13-20)27(33)34/h2-13,22,25,30H,14,16-17H2,1H3,(H,31,32)(H,33,34)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135545

(3-{(R)-2-Cyano-2-[(S)-2-(3-methanesulfonyl-phenyla...)Show SMILES Cc1cccc(C[C@H](Nc2cccc(c2)S(C)(=O)=O)C(=O)N[C@@H](COCc2cccc(c2)C(O)=O)C#N)c1 Show InChI InChI=1S/C28H29N3O6S/c1-19-6-3-7-20(12-19)14-26(30-23-10-5-11-25(15-23)38(2,35)36)27(32)31-24(16-29)18-37-17-21-8-4-9-22(13-21)28(33)34/h3-13,15,24,26,30H,14,17-18H2,1-2H3,(H,31,32)(H,33,34)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50379363

(CHEMBL2011941)Show SMILES Brc1cnc2nc([nH]c2c1NCCCNC(=O)C1CCC1)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C27H35BrN6O2/c28-22-18-31-26-24(23(22)29-12-5-13-30-27(35)20-6-4-7-20)32-25(33-26)19-8-10-21(11-9-19)36-17-16-34-14-2-1-3-15-34/h8-11,18,20H,1-7,12-17H2,(H,30,35)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50379359

(CHEMBL2011935)Show SMILES COc1ccc(cc1)-c1nc2ncc(Br)c(NCCCNC(=O)C3CC(O)C3)c2[nH]1 |(36.6,-30.43,;35.82,-29.1,;34.28,-29.11,;33.52,-30.45,;31.98,-30.46,;31.2,-29.13,;31.96,-27.8,;33.49,-27.78,;29.66,-29.14,;28.76,-30.39,;27.29,-29.92,;25.95,-30.7,;24.62,-29.93,;24.62,-28.39,;23.29,-27.62,;25.95,-27.62,;25.95,-26.08,;27.28,-25.3,;27.27,-23.76,;28.61,-23,;28.61,-21.46,;29.95,-20.69,;31.28,-21.46,;29.95,-19.15,;31.04,-18.07,;29.95,-16.98,;29.96,-15.44,;28.86,-18.06,;27.29,-28.38,;28.75,-27.9,)| Show InChI InChI=1S/C21H24BrN5O3/c1-30-15-5-3-12(4-6-15)19-26-18-17(16(22)11-25-20(18)27-19)23-7-2-8-24-21(29)13-9-14(28)10-13/h3-6,11,13-14,28H,2,7-10H2,1H3,(H,24,29)(H2,23,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50107647
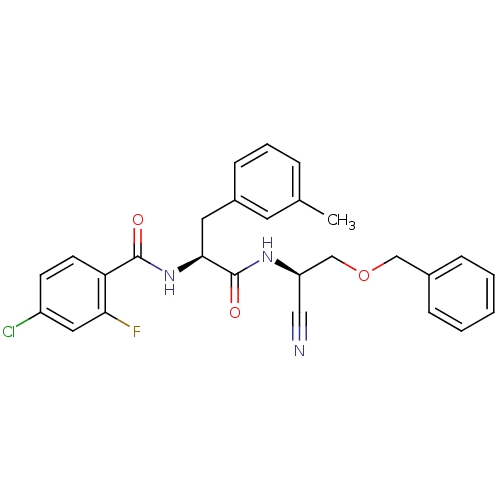
(CHEMBL140506 | N-{1-[(Benzyloxymethyl-cyano-methyl...)Show SMILES Cc1cccc(C[C@H](NC(=O)c2ccc(Cl)cc2F)C(=O)N[C@@H](COCc2ccccc2)C#N)c1 Show InChI InChI=1S/C27H25ClFN3O3/c1-18-6-5-9-20(12-18)13-25(32-26(33)23-11-10-21(28)14-24(23)29)27(34)31-22(15-30)17-35-16-19-7-3-2-4-8-19/h2-12,14,22,25H,13,16-17H2,1H3,(H,31,34)(H,32,33)/t22-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Inhibitiory activity against recombinant human cathepsin B (cat B) expressed in baculovirus. |
J Med Chem 44: 4524-34 (2001)
BindingDB Entry DOI: 10.7270/Q2GQ6X2J |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50135548

((S)-N-[(R)-Cyano-(3-tetrazol-1-yl-benzyloxymethyl)...)Show SMILES Cc1cccc(C[C@H](Nc2ccccc2)C(=O)N[C@@H](COCc2cccc(c2)-n2cnnn2)C#N)c1 Show InChI InChI=1S/C27H27N7O2/c1-20-7-5-8-21(13-20)15-26(30-23-10-3-2-4-11-23)27(35)31-24(16-28)18-36-17-22-9-6-12-25(14-22)34-19-29-32-33-34/h2-14,19,24,26,30H,15,17-18H2,1H3,(H,31,35)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibtitory activity against cathepsin B (catB) |
Bioorg Med Chem Lett 13: 4121-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BC3XZC |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
(Homo sapiens (Human)) | BDBM50379363

(CHEMBL2011941)Show SMILES Brc1cnc2nc([nH]c2c1NCCCNC(=O)C1CCC1)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C27H35BrN6O2/c28-22-18-31-26-24(23(22)29-12-5-13-30-27(35)20-6-4-7-20)32-25(33-26)19-8-10-21(11-9-19)36-17-16-34-14-2-1-3-15-34/h8-11,18,20H,1-7,12-17H2,(H,30,35)(H2,29,31,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Ikkepsilon using 5FAM-AKELDQGSLCTpSFVGTLQ-NH2 as substrate by microfluidic mobility shift assay |
Bioorg Med Chem Lett 22: 2063-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.018
BindingDB Entry DOI: 10.7270/Q2WS8V74 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data