

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50007002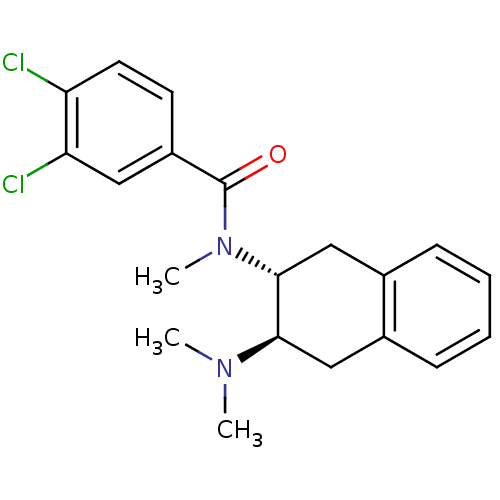 (3,4-Dichloro-N-(3-dimethylamino-1,2,3,4-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50229403 (CHEMBL2311130) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Compound was evaluated for time-dependent inactivation of Ribonucleotide diphosphate reductase (RDPR) in E. coli | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006996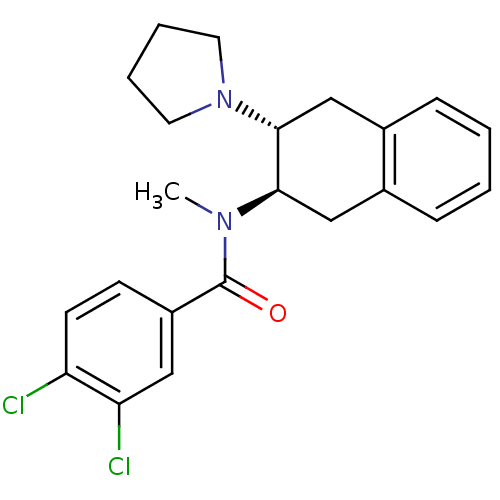 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007004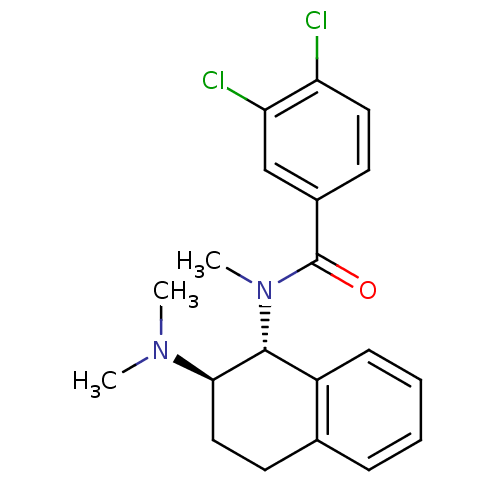 (3,4-Dichloro-N-(2-dimethylamino-1,2,3,4-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007001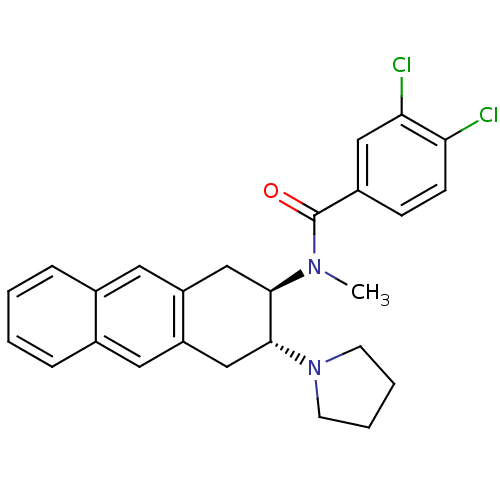 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006994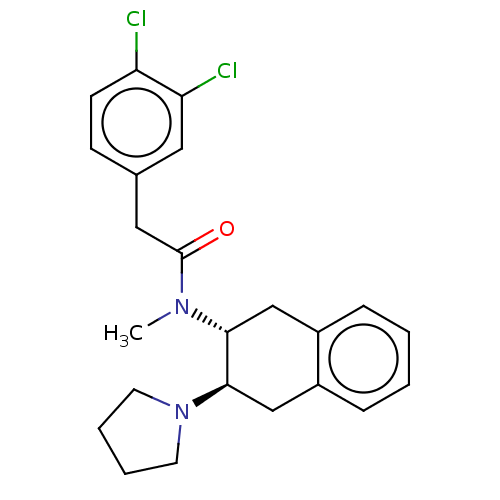 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007000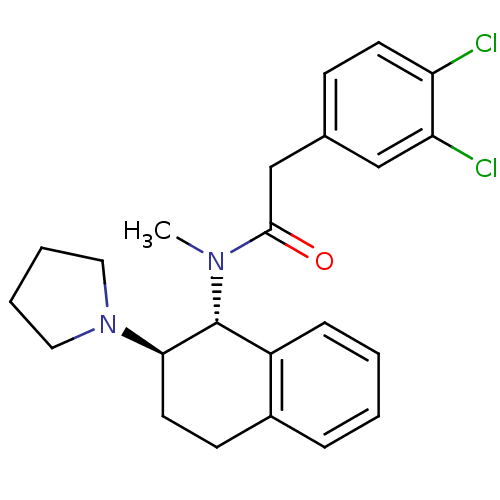 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006999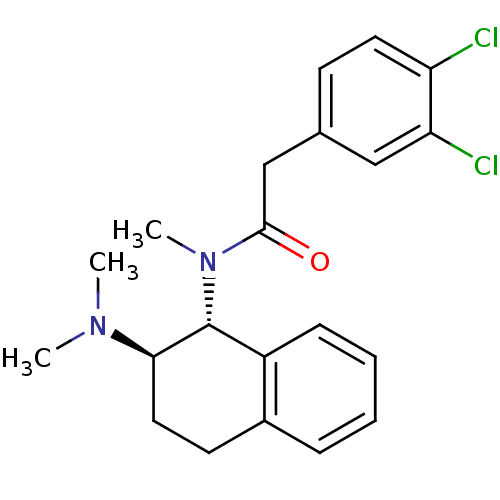 (2-(3,4-Dichloro-phenyl)-N-(2-dimethylamino-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006998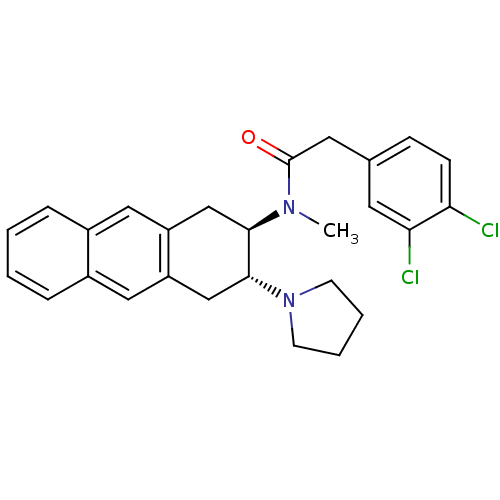 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006997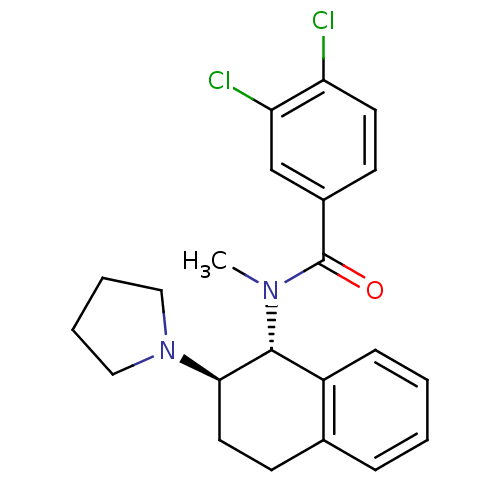 (3,4-Dichloro-N-methyl-N-(2-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287594 (Asunaprevir | BMS 650032 | BMS-650032) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287622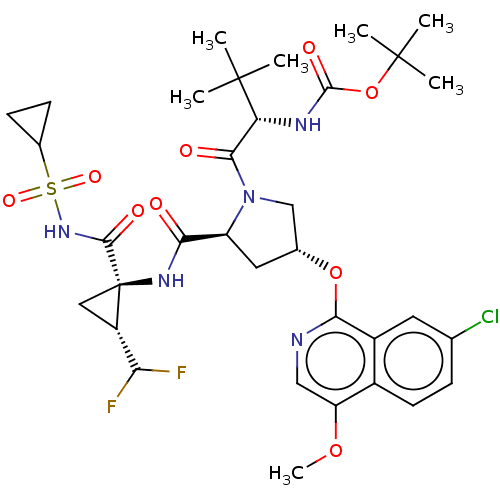 (CHEMBL4160876) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287595 (CHEMBL3921126) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006994 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287648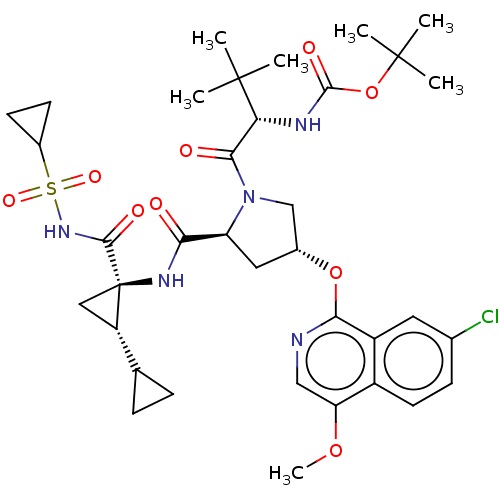 (CHEMBL3895075) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007000 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006999 (2-(3,4-Dichloro-phenyl)-N-(2-dimethylamino-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287621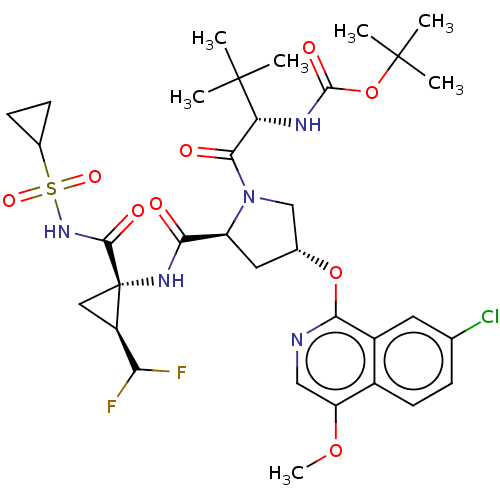 (CHEMBL4171493) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287586 (CHEMBL4176000) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287588 (CHEMBL4177477) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287685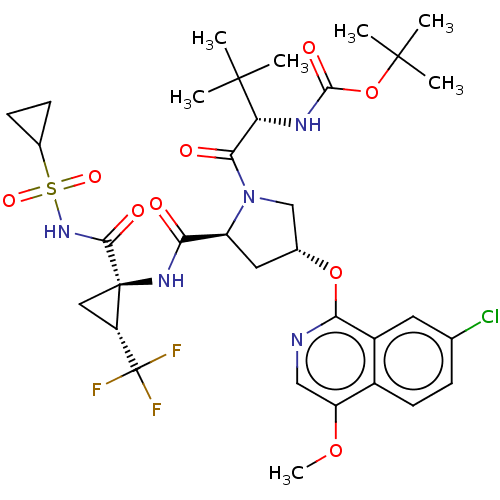 (CHEMBL4168149) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006996 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287587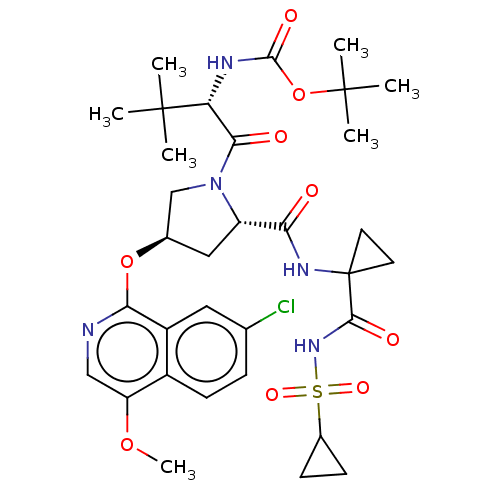 (CHEMBL4159744) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007002 (3,4-Dichloro-N-(3-dimethylamino-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007001 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006998 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287686 (CHEMBL4161286) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006997 (3,4-Dichloro-N-methyl-N-(2-pyrrolidin-1-yl-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287593 (CHEMBL4160158) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 976 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287592 (CHEMBL4169219) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291391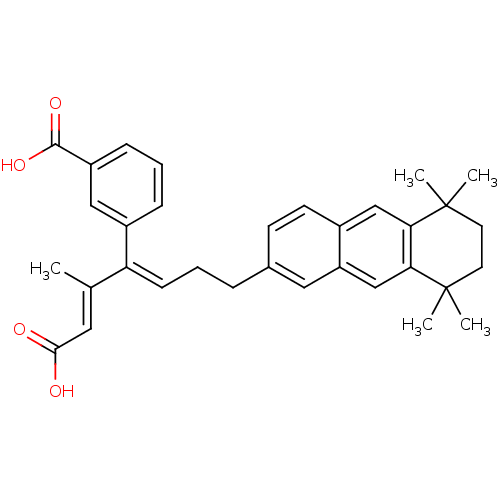 (3-[(E)-1-((E)-2-Carboxy-1-methyl-vinyl)-4-(5,5,8,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287651 (CHEMBL4170379) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007004 (3,4-Dichloro-N-(2-dimethylamino-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287641 (CHEMBL4168077) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287638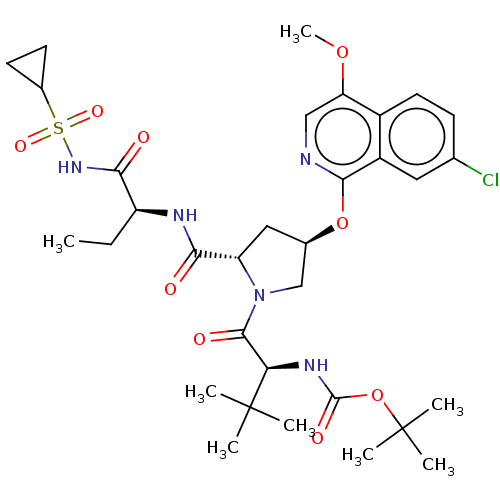 (CHEMBL4172603) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291389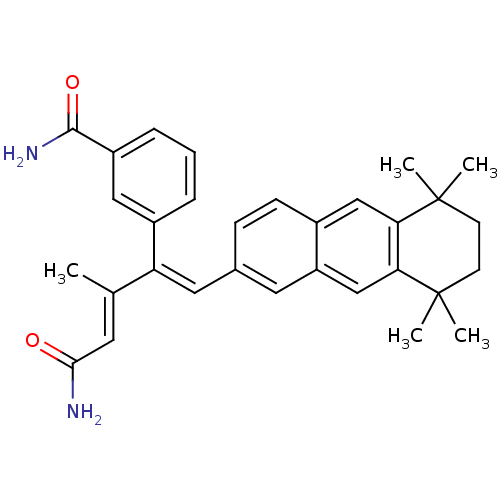 (3-{(E)-3-Carbamoyl-2-methyl-1-[1-(5,5,8,8-tetramet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291390 (3-{(E)-3-Carboxy-1-[1-(4-decyloxy-phenyl)-meth-(E)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287627 (CHEMBL4164707) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291388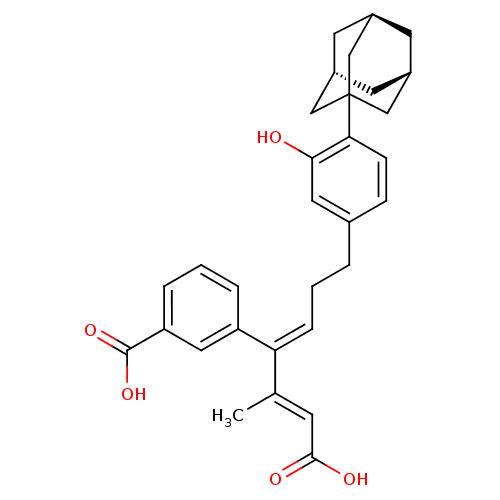 (3-[(E)-4-(4-Adamantan-1-yl-3-hydroxy-phenyl)-1-((E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291387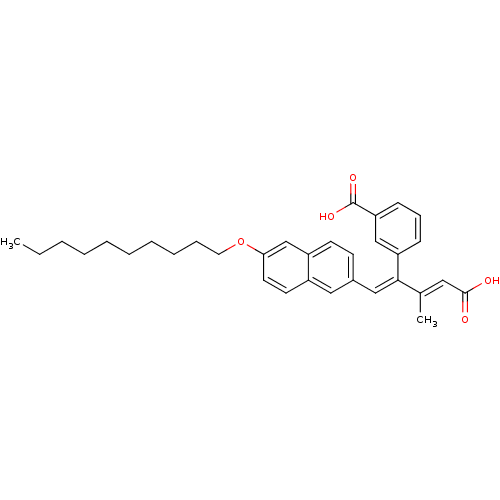 (3-{(E)-3-Carboxy-1-[1-(6-decyloxy-naphthalen-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291392 (3-{(E)-3-Carboxy-2-methyl-1-[1-(5,5,8,8-tetramethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50291386 (3-{(E)-3-Carboxy-2-methyl-1-[1-[6-(3-phenyl-propox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50287647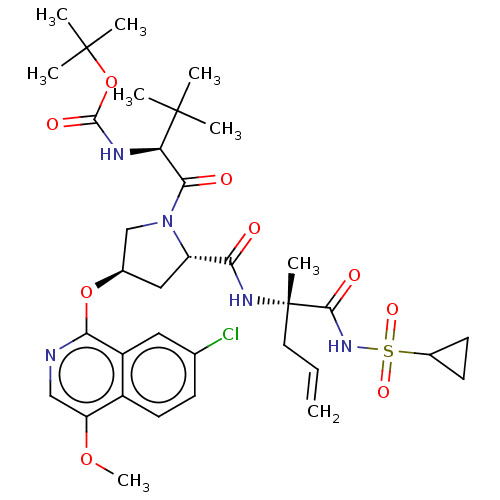 (CHEMBL4174861) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant full length HCV genotype 1a NS3/4A protease (1027 to 1711 residues) expressed in Escherichia coli strain BL21 (DE3) using R... | ACS Med Chem Lett 9: 143-148 (2018) Article DOI: 10.1021/acsmedchemlett.7b00503 BindingDB Entry DOI: 10.7270/Q2668GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50288286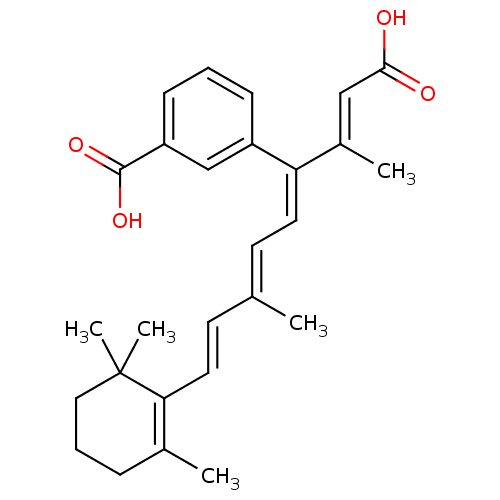 (3-[(1E,3E,5E)-1-((E)-2-Carboxy-1-methyl-vinyl)-4-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was measured for the inhibition of human platelet PLA2 (HP-PLA2) | Bioorg Med Chem Lett 7: 793-798 (1997) Article DOI: 10.1016/S0960-894X(97)00110-8 BindingDB Entry DOI: 10.7270/Q26D5T0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268398 (CHEMBL4061940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |