

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766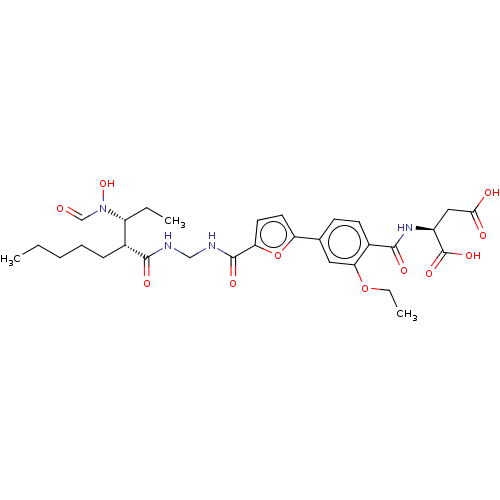 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770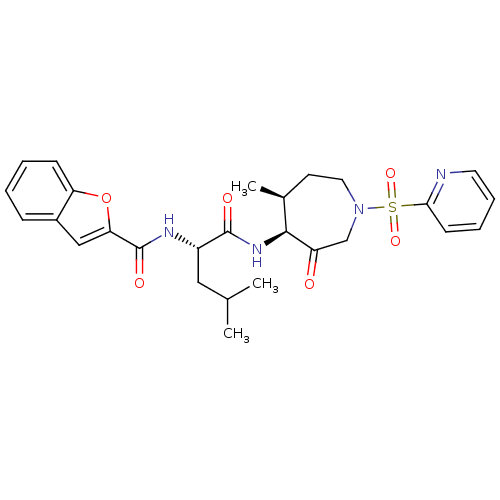 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50124984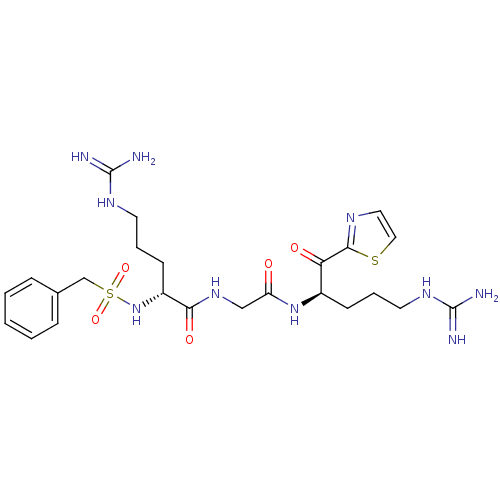 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards factor Xa | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771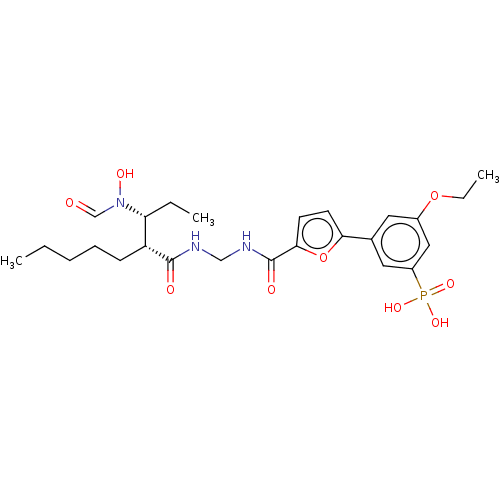 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394718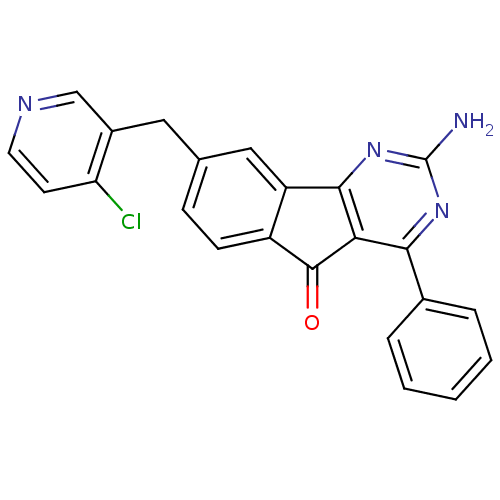 (CHEMBL2165801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50161342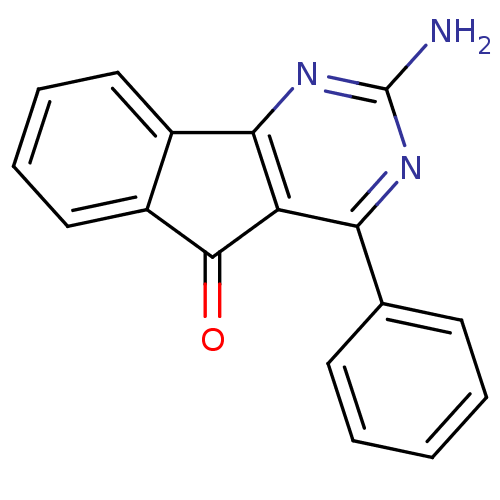 (2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317007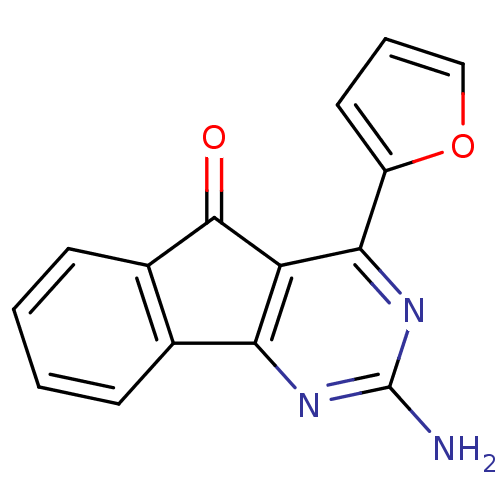 (2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775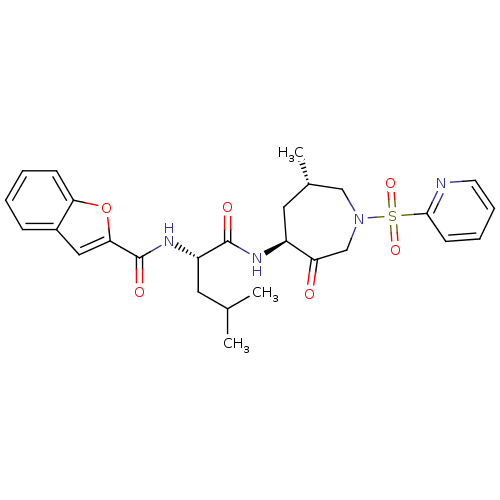 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50124975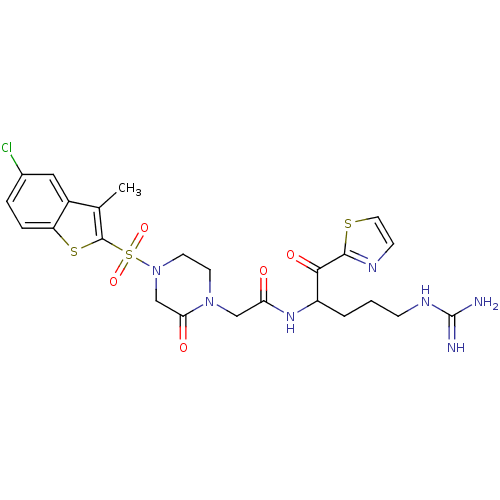 (2-[4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards factor Xa | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317008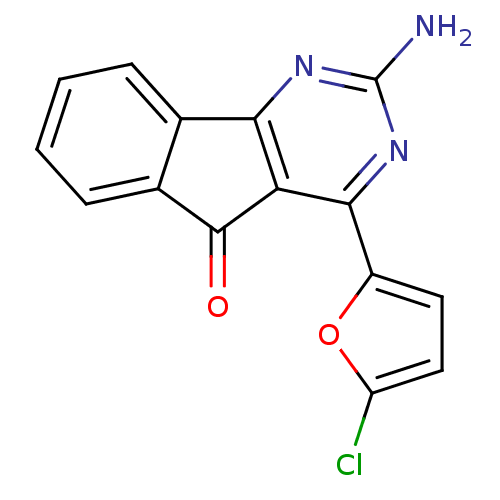 (2-amino-4-(5-chlorofuran-2-yl)-5H-indeno[1,2-d]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394722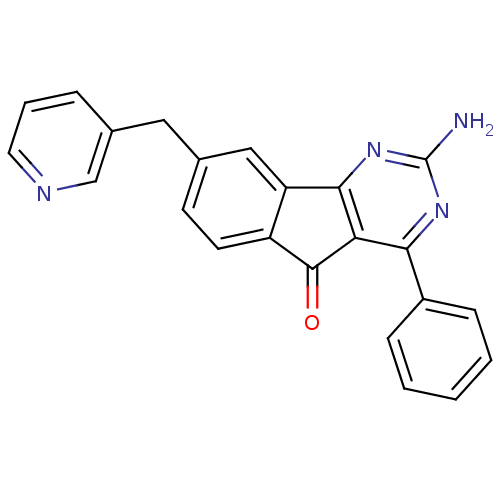 (CHEMBL2165807) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50428131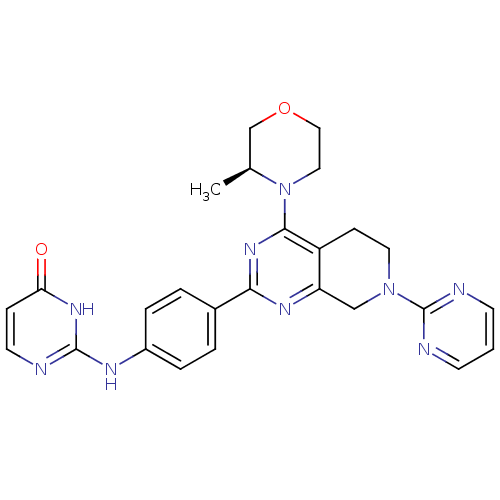 (CHEMBL2331687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay | ACS Med Chem Lett 4: 103-7 (2013) Article DOI: 10.1021/ml3003132 BindingDB Entry DOI: 10.7270/Q28C9XK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50317007 (2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394717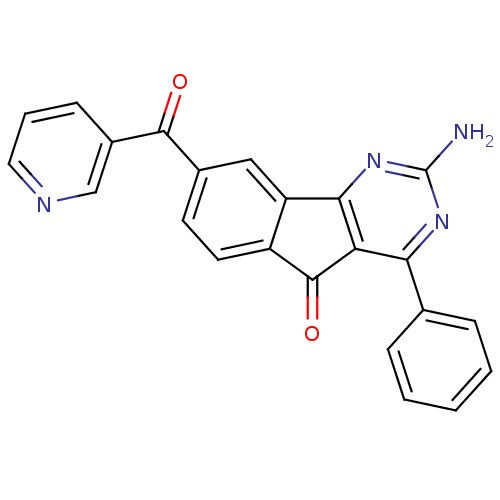 (CHEMBL2165802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50317008 (2-amino-4-(5-chlorofuran-2-yl)-5H-indeno[1,2-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317011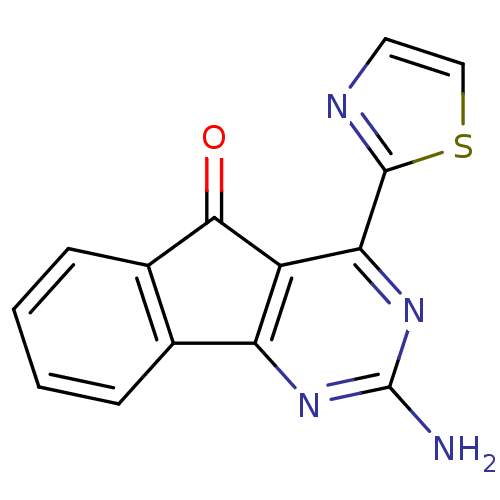 (2-amino-4-(thiazol-2-yl)-5H-indeno[1,2-d]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394718 (CHEMBL2165801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317010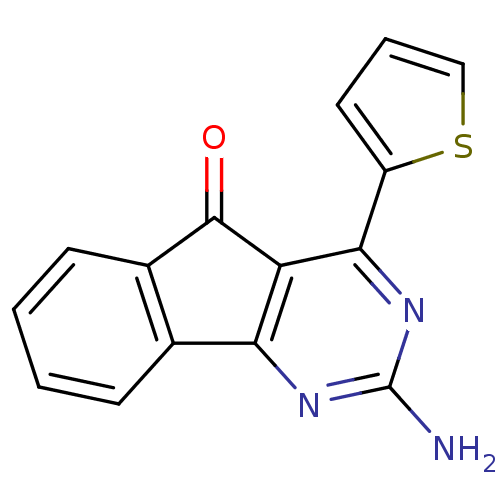 (2-amino-4-(thiophen-2-yl)-5H-indeno[1,2-d]pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19775 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50125017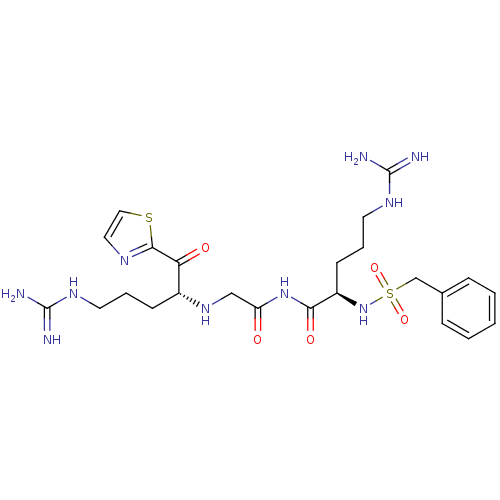 (CHEMBL350976 | N-((R)-5-Guanidino-2-phenylmethanes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against serine protease thrombin | Bioorg Med Chem Lett 13: 729-32 (2003) BindingDB Entry DOI: 10.7270/Q2T72GTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394719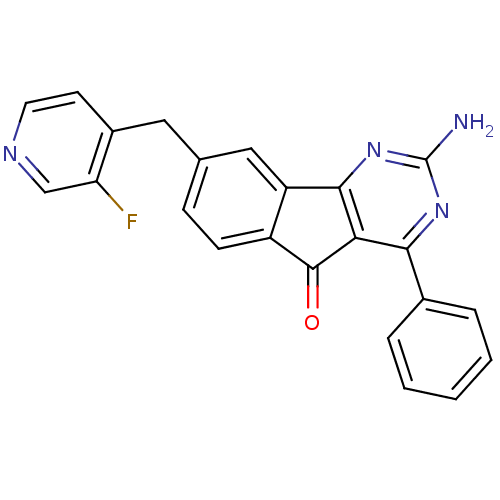 (CHEMBL2165800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316893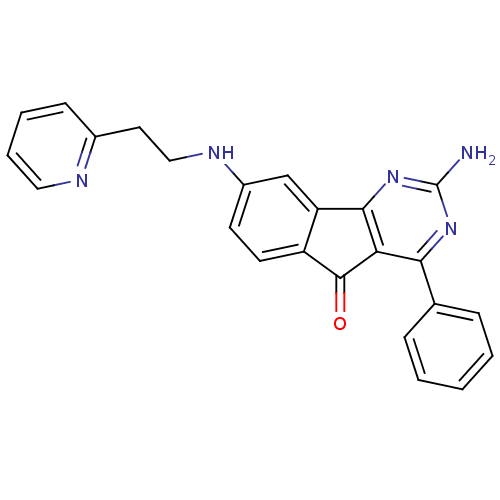 (2-amino-4-phenyl-8-(2-(pyridin-2-yl)ethylamino)-5H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316892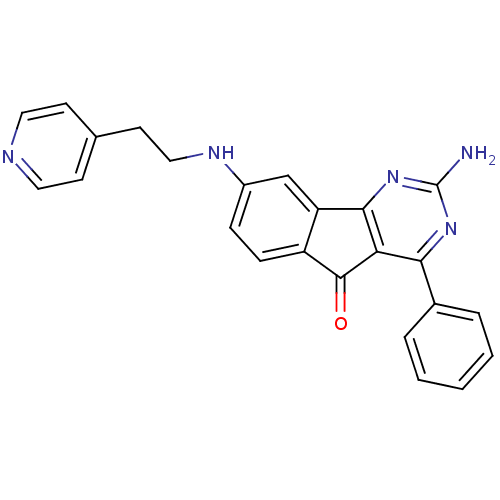 (2-amino-4-phenyl-8-(2-(pyridin-4-yl)ethylamino)-5H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317003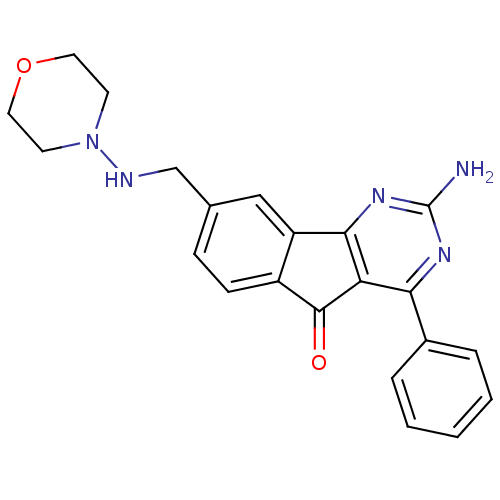 (2-amino-8-((morpholinoamino)methyl)-4-phenyl-5H-in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50161342 (2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394720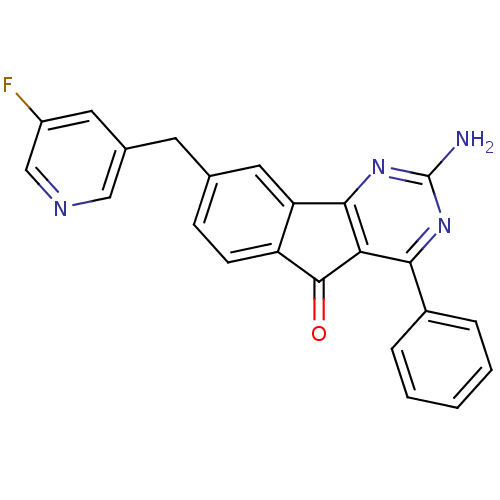 (CHEMBL2165799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50124979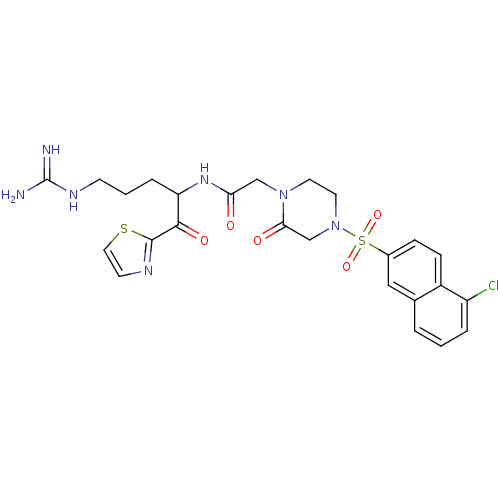 (2-[4-(5-Chloro-naphthalene-2-sulfonyl)-2-oxo-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards factor Xa | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394721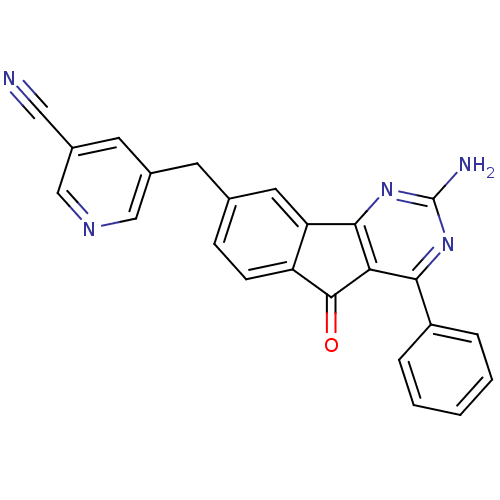 (CHEMBL2165808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50428125 (CHEMBL2331677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay | ACS Med Chem Lett 4: 103-7 (2013) Article DOI: 10.1021/ml3003132 BindingDB Entry DOI: 10.7270/Q28C9XK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50124992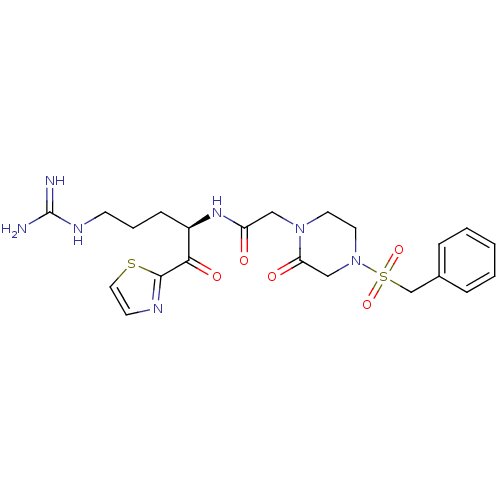 (CHEMBL162461 | N-[(R)-4-Guanidino-1-(thiazole-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro binding affinity towards factor Xa | Bioorg Med Chem Lett 13: 723-8 (2003) BindingDB Entry DOI: 10.7270/Q2Z037JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19780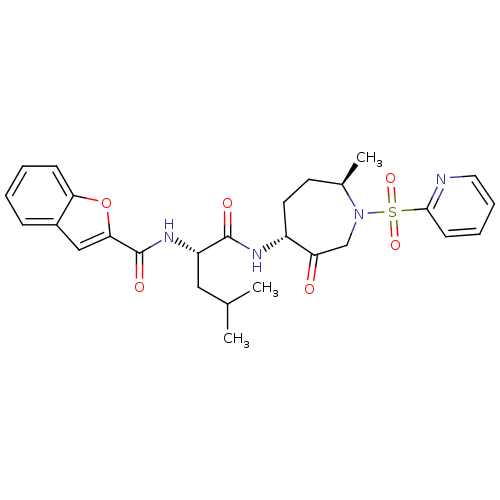 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19771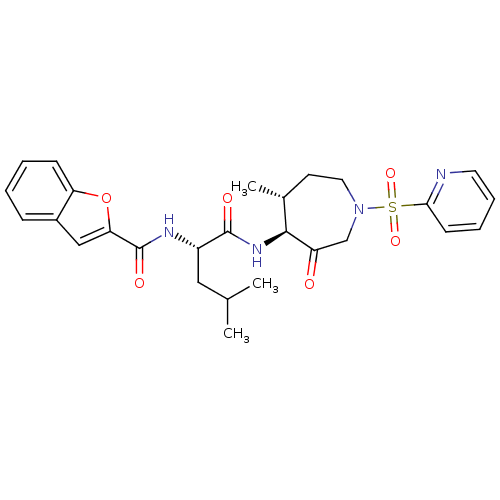 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50400338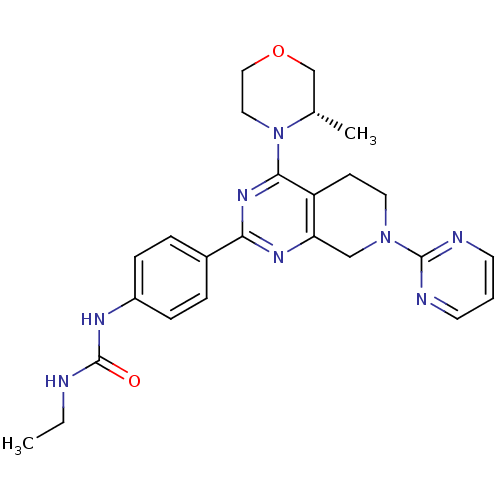 (CHEMBL2181522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay | ACS Med Chem Lett 4: 103-7 (2013) Article DOI: 10.1021/ml3003132 BindingDB Entry DOI: 10.7270/Q28C9XK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394716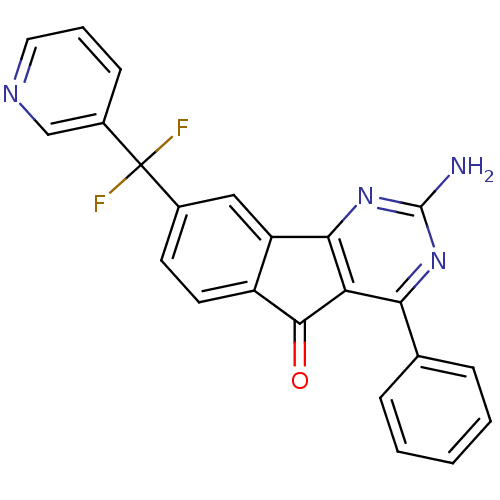 (CHEMBL2165803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50317014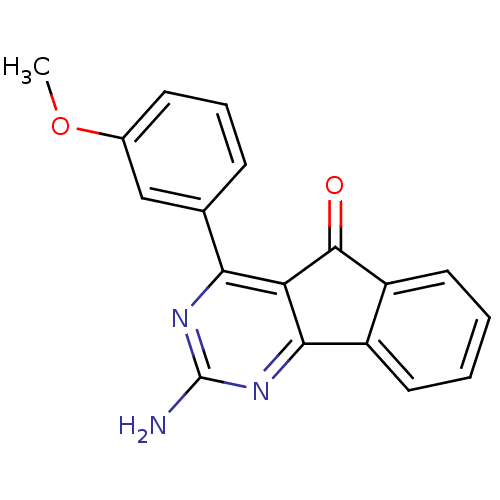 (2-amino-4-(3-methoxyphenyl)-5H-indeno[1,2-d]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394717 (CHEMBL2165802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50317003 (2-amino-8-((morpholinoamino)methyl)-4-phenyl-5H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | Bioorg Med Chem Lett 20: 2864-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.042 BindingDB Entry DOI: 10.7270/Q2GB246H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394715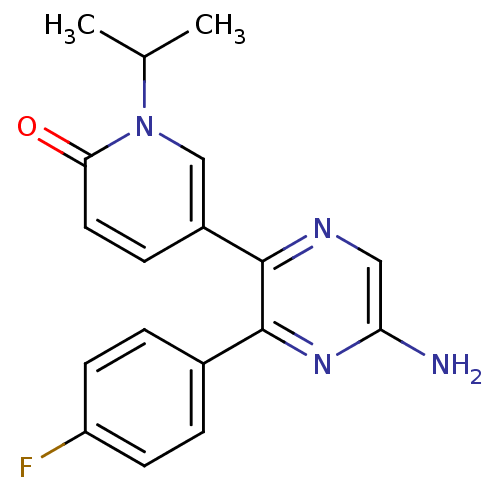 (CHEMBL2165804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1946 total ) | Next | Last >> |