

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (RAT) | BDBM50370083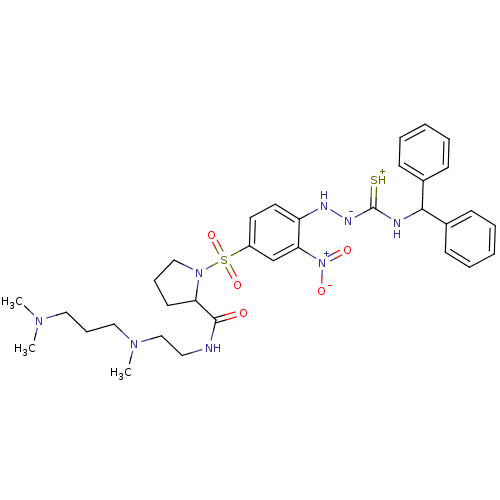 (CHEMBL1907651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427363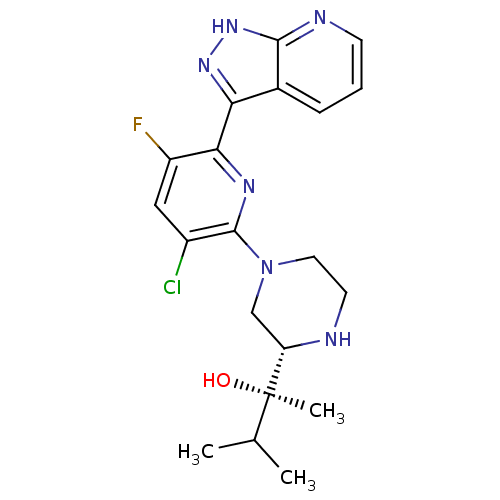 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427365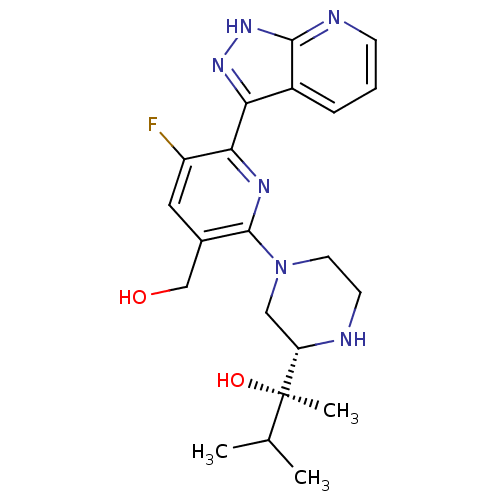 (CHEMBL2326000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427367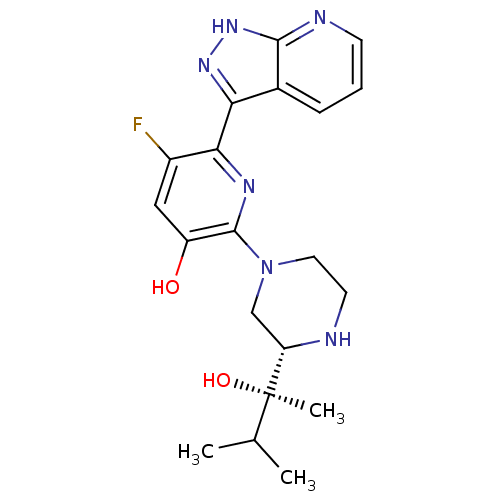 (CHEMBL2325998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427364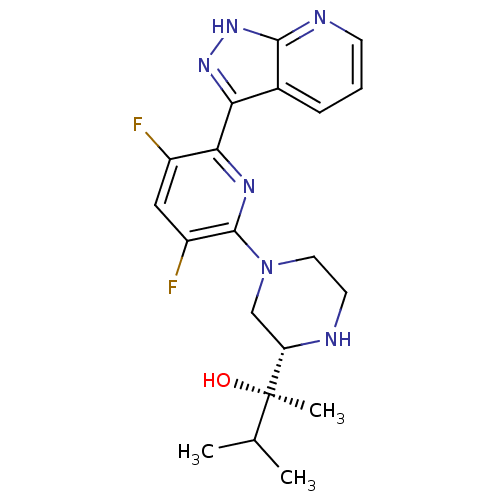 (CHEMBL2326001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427370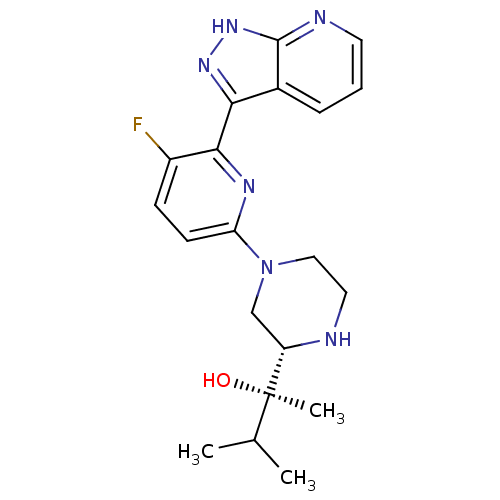 (CHEMBL2326007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525600 (CHEMBL4469412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50181745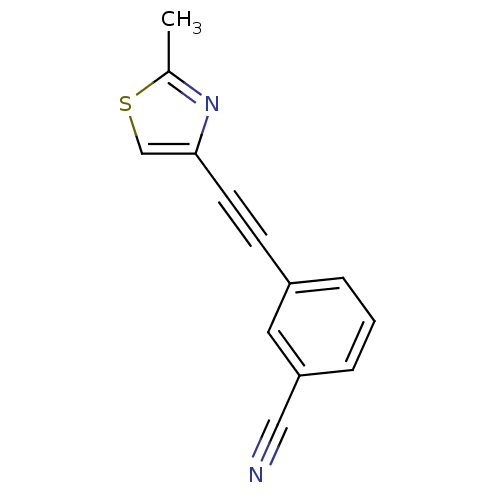 (3-[(2-methyl-4-thiazolyl)ethynyl]benzonitrile | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from mGluR5 in rat brain membranes | J Med Chem 49: 1080-100 (2006) Article DOI: 10.1021/jm050570f BindingDB Entry DOI: 10.7270/Q27S7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012544 (CHEMBL3260768) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525603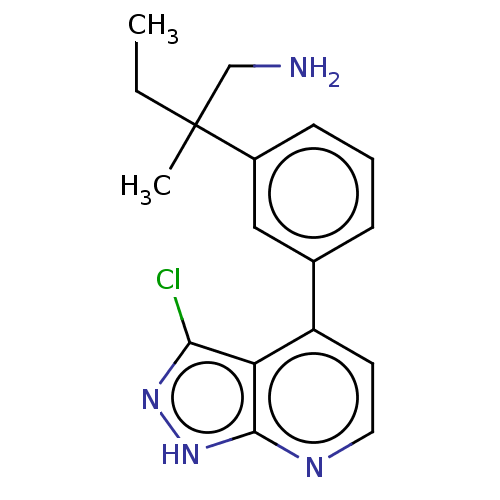 (CHEMBL4528271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370077 (CHEMBL1907652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multifunctional protein ADE2 (Gallus gallus) | BDBM50247807 (((2R,3S,4R,5R)-5-(5-AMINO-4-NITRO-1H-IMIDAZOL-1-YL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of Gallus gallus AIR carboxylase by CAIR decarboxylation assay | Bioorg Med Chem 17: 794-803 (2009) Article DOI: 10.1016/j.bmc.2008.11.057 BindingDB Entry DOI: 10.7270/Q2RR204K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50181745 (3-[(2-methyl-4-thiazolyl)ethynyl]benzonitrile | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from cloned human mGluR5 transfected in HEK293-T cells | J Med Chem 49: 1080-100 (2006) Article DOI: 10.1021/jm050570f BindingDB Entry DOI: 10.7270/Q27S7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525602 (CHEMBL4569479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM97372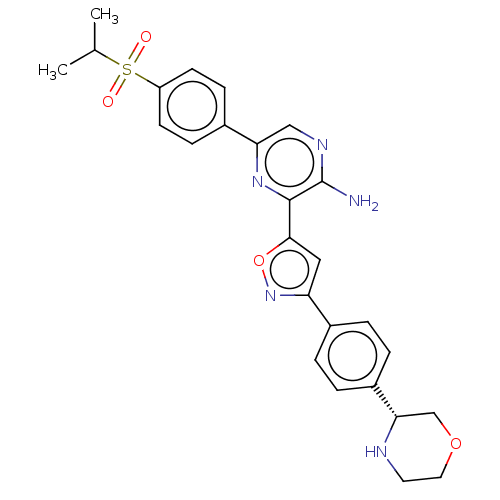 (US20130089624, 1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.503 | -53.1 | 200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Vertex Pharmaceuticals US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. | US Patent US20130089624 (2013) BindingDB Entry DOI: 10.7270/Q2PZ57DN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012543 (CHEMBL3260767) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human Angiotensin receptor 1 | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50181753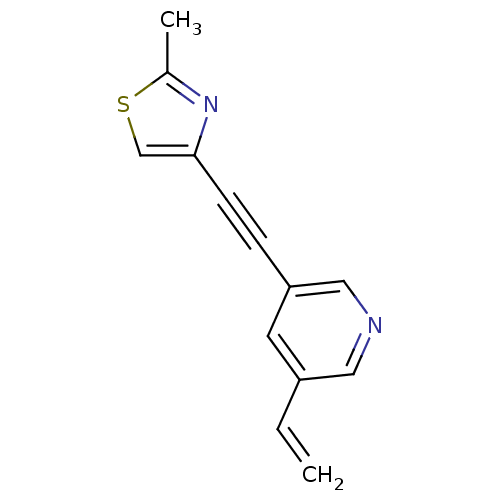 (3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from mGluR5 in rat brain membranes | J Med Chem 49: 1080-100 (2006) Article DOI: 10.1021/jm050570f BindingDB Entry DOI: 10.7270/Q27S7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409527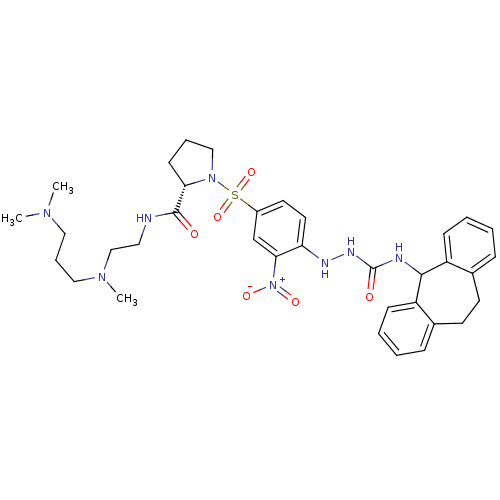 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525604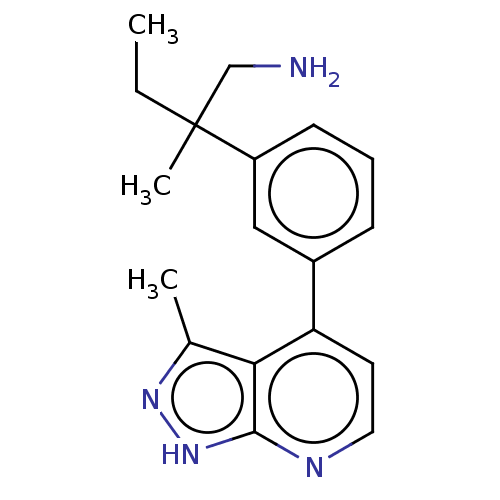 (CHEMBL4532737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427366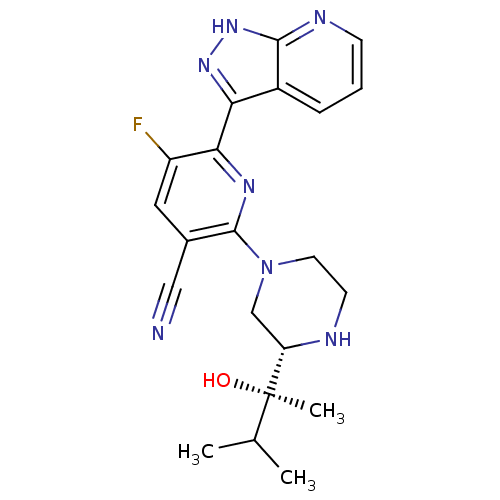 (CHEMBL2325999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50251493 ((2S,4S)-1-(2-(1-(4-cyano-3,5-difluorophenyl)piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma DPP4 | Bioorg Med Chem Lett 18: 4087-91 (2008) Article DOI: 10.1016/j.bmcl.2008.05.101 BindingDB Entry DOI: 10.7270/Q2T153FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50427363 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of PKC epsilon (unknown origin) | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427374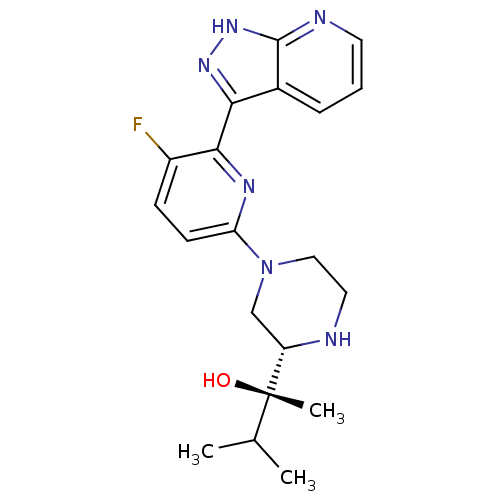 (CHEMBL2326009) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50181753 (3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from cloned human mGluR5 transfected in HEK293-T cells | J Med Chem 49: 1080-100 (2006) Article DOI: 10.1021/jm050570f BindingDB Entry DOI: 10.7270/Q27S7NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012502 (CHEMBL3260457) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012532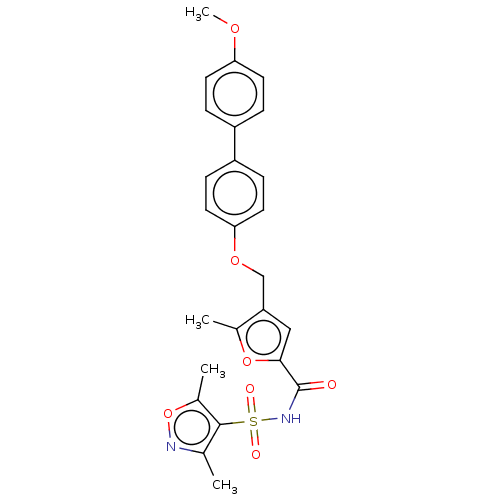 (CHEMBL3260463) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409529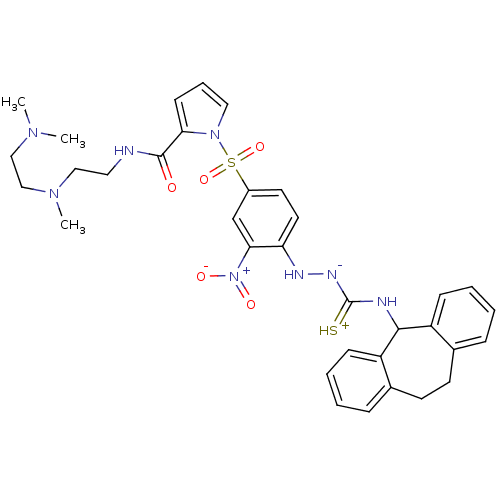 (CHEMBL2112221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085685 (4-benzhydrylamino(thioxo)methylhydrazine-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012542 (CHEMBL3260766) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525587 (CHEMBL4589844) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427382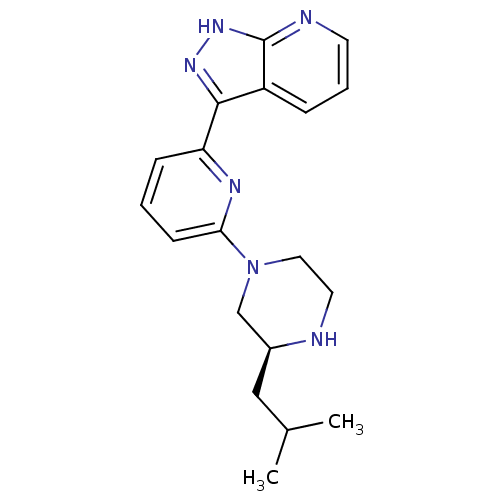 (CHEMBL2326019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409528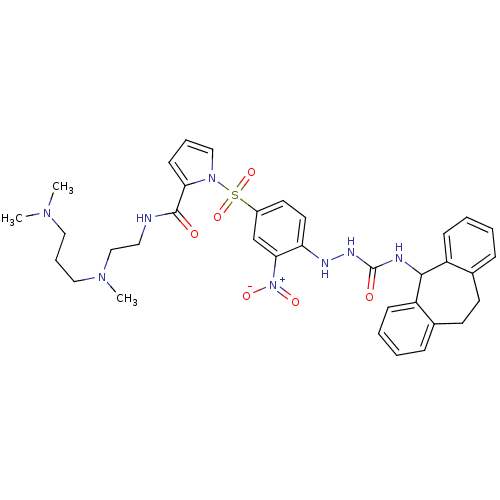 (CHEMBL2112220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012548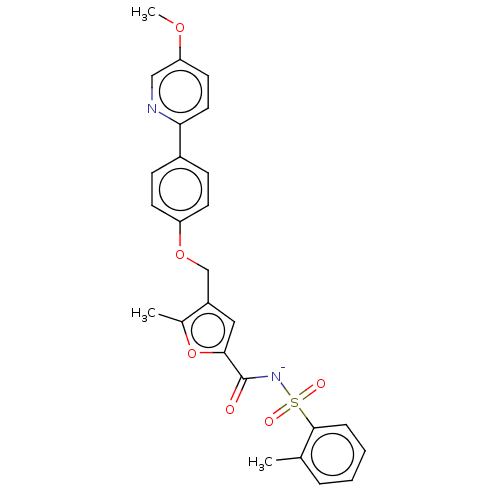 (CHEMBL3260771) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525584 (CHEMBL4453941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012534 (CHEMBL3260758) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012545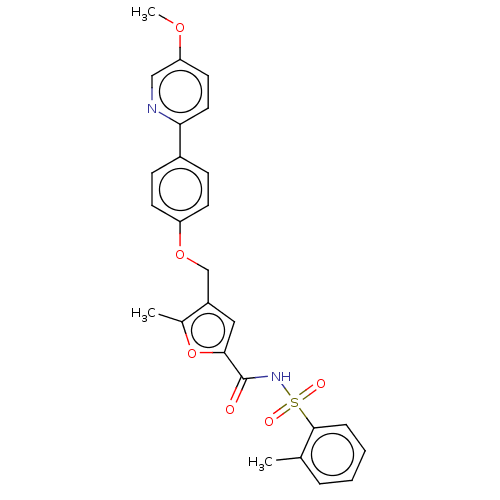 (BGC-201531 | CHEMBL1628698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525596 (CHEMBL4590477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50525584 (CHEMBL4453941) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50525599 (CHEMBL4461370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His tagged PKC theta expressed in baculovirus using ERMRPRKRQGSVRRRV peptide as substrate incubated for 6... | ACS Med Chem Lett 10: 1134-1139 (2019) Article DOI: 10.1021/acsmedchemlett.9b00134 BindingDB Entry DOI: 10.7270/Q2NC64NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50427363 (CHEMBL2326002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of PKC eta (unknown origin) | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50012533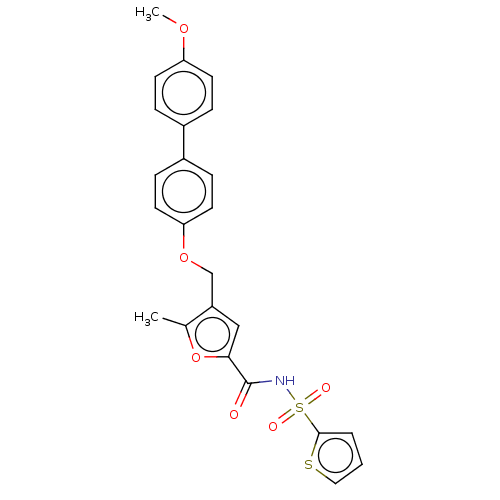 (CHEMBL3260757) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting | Bioorg Med Chem Lett 24: 2212-21 (2014) Article DOI: 10.1016/j.bmcl.2014.02.068 BindingDB Entry DOI: 10.7270/Q2125V6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085684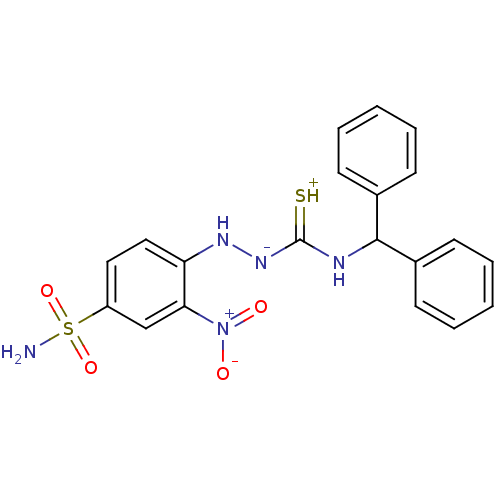 (4-benzhydrylamino(thioxo)methylhydrazine-3-nitro-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C theta type (Homo sapiens (Human)) | BDBM50427379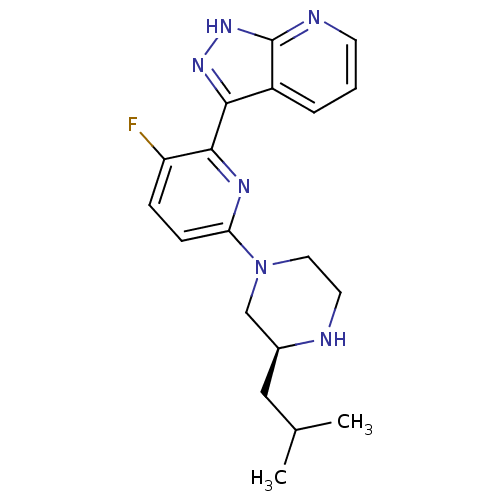 (CHEMBL2326003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant PKC theta (unknown origin) using ERMRPRKRQGSVRRRV as substrate after 60 mins by scintillation counting analysis... | J Med Chem 56: 1799-810 (2013) Article DOI: 10.1021/jm301465a BindingDB Entry DOI: 10.7270/Q2M046R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50370080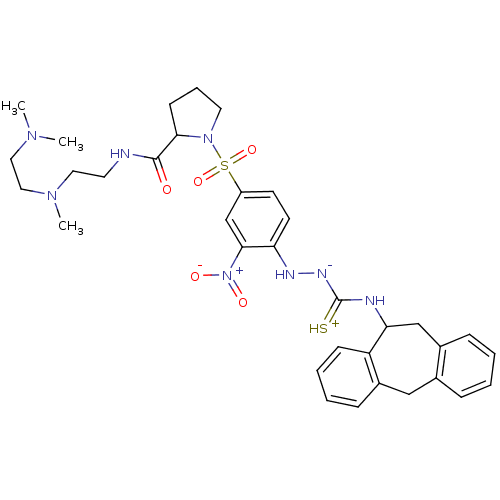 (CHEMBL1907656) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50251494 ((2S,4S)-1-(2-(1-(4-cyano-2-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human plasma DPP4 | Bioorg Med Chem Lett 18: 4087-91 (2008) Article DOI: 10.1016/j.bmcl.2008.05.101 BindingDB Entry DOI: 10.7270/Q2T153FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2699 total ) | Next | Last >> |