 Found 359 hits with Last Name = 'dirico' and Initial = 'kj'
Found 359 hits with Last Name = 'dirico' and Initial = 'kj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122928

(5-{6-[5-Chloro-2-(3-methyl-isoxazol-5-ylmethoxy)-b...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H24ClN7O6/c1-11-5-14(37-30-11)8-35-15-4-3-13(24)6-12(15)7-26-20-16-21(28-9-27-20)31(10-29-16)23-18(33)17(32)19(36-23)22(34)25-2/h3-6,9-10,17-19,23,32-33H,7-8H2,1-2H3,(H,25,34)(H,26,27,28)/t17-,18+,19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182312
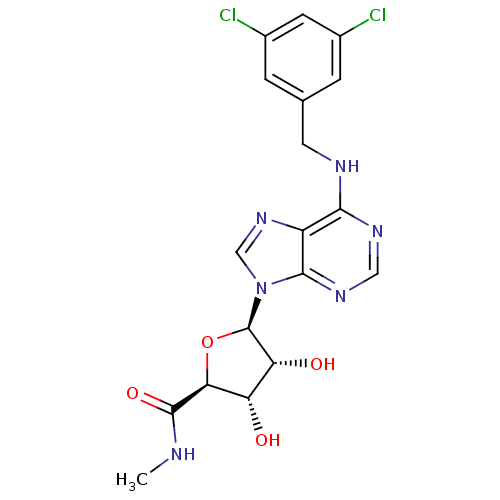
((2S,3S,4R,5R)-5-(6-(3,5-dichlorobenzylamino)-9H-pu...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)cc(Cl)c3)ncnc12 Show InChI InChI=1S/C18H18Cl2N6O4/c1-21-17(29)14-12(27)13(28)18(30-14)26-7-25-11-15(23-6-24-16(11)26)22-5-8-2-9(19)4-10(20)3-8/h2-4,6-7,12-14,18,27-28H,5H2,1H3,(H,21,29)(H,22,23,24)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182322
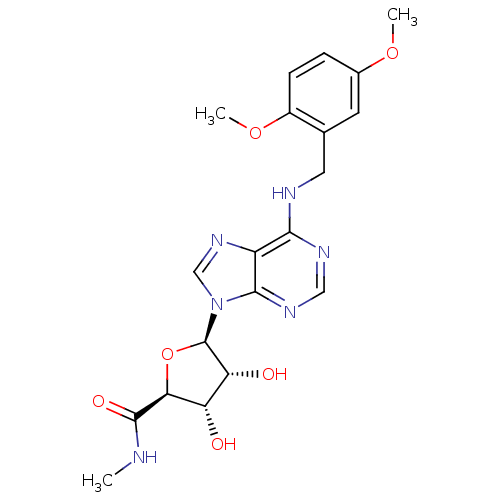
((2S,3S,4R,5R)-5-(6-(2,5-dimethoxybenzylamino)-9H-p...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(OC)ccc3OC)ncnc12 Show InChI InChI=1S/C20H24N6O6/c1-21-19(29)16-14(27)15(28)20(32-16)26-9-25-13-17(23-8-24-18(13)26)22-7-10-6-11(30-2)4-5-12(10)31-3/h4-6,8-9,14-16,20,27-28H,7H2,1-3H3,(H,21,29)(H,22,23,24)/t14-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122927
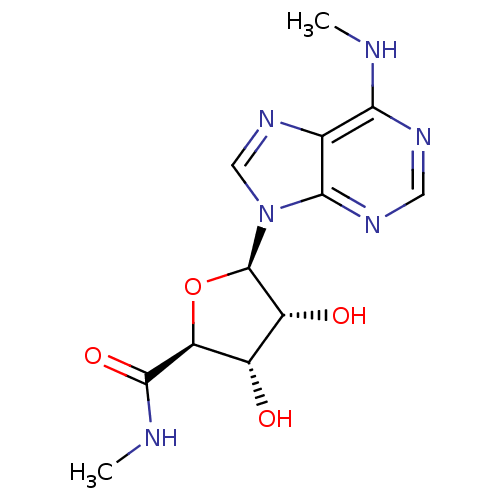
((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122927

((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122926
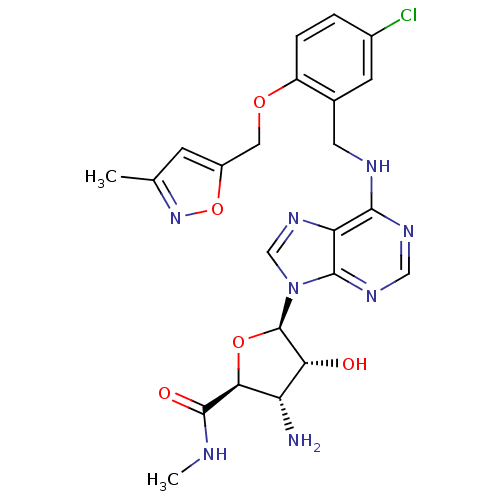
(3-Amino-5-{6-[5-chloro-2-(3-methyl-isoxazol-5-ylme...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H25ClN8O5/c1-11-5-14(37-31-11)8-35-15-4-3-13(24)6-12(15)7-27-20-17-21(29-9-28-20)32(10-30-17)23-18(33)16(25)19(36-23)22(34)26-2/h3-6,9-10,16,18-19,23,33H,7-8,25H2,1-2H3,(H,26,34)(H,27,28,29)/t16-,18+,19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182326

((2S,3S,4R,5R)-5-(6-(2-(2-amino-2-oxoethoxy)-5-chlo...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(N)=O)ncnc12 Show InChI InChI=1S/C20H23ClN8O5/c1-24-19(32)16-13(23)15(31)20(34-16)29-8-28-14-17(26-7-27-18(14)29)25-5-9-4-10(21)2-3-11(9)33-6-12(22)30/h2-4,7-8,13,15-16,20,31H,5-6,23H2,1H3,(H2,22,30)(H,24,32)(H,25,26,27)/t13-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182313

((2S,3S,4R,5R)-5-(6-(5-chloro-2-(2-(4-(dimethylamin...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(=O)N3CCC(CC3)N(C)C)ncnc12 Show InChI InChI=1S/C27H36ClN9O5/c1-30-26(40)23-20(29)22(39)27(42-23)37-14-34-21-24(32-13-33-25(21)37)31-11-15-10-16(28)4-5-18(15)41-12-19(38)36-8-6-17(7-9-36)35(2)3/h4-5,10,13-14,17,20,22-23,27,39H,6-9,11-12,29H2,1-3H3,(H,30,40)(H,31,32,33)/t20-,22+,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182324
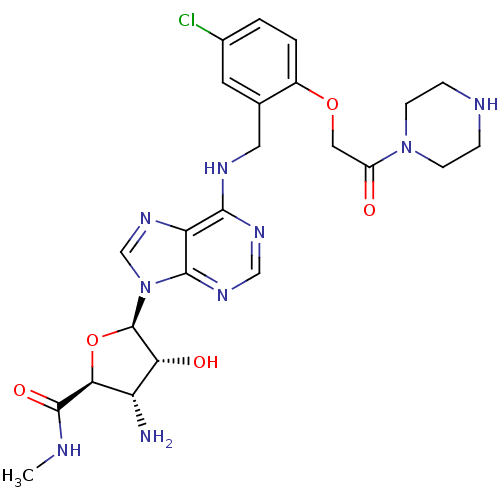
((2S,3S,4R,5R)-5-(6-(5-chloro-2-(2-oxo-2-(piperazin...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(=O)N3CCNCC3)ncnc12 Show InChI InChI=1S/C24H30ClN9O5/c1-27-23(37)20-17(26)19(36)24(39-20)34-12-32-18-21(30-11-31-22(18)34)29-9-13-8-14(25)2-3-15(13)38-10-16(35)33-6-4-28-5-7-33/h2-3,8,11-12,17,19-20,24,28,36H,4-7,9-10,26H2,1H3,(H,27,37)(H,29,30,31)/t17-,19+,20-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182323

((2S,3S,4R,5R)-5-(6-(5-chloro-2-methoxybenzylamino)...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OC)ncnc12 Show InChI InChI=1S/C19H22ClN7O4/c1-22-18(29)15-12(21)14(28)19(31-15)27-8-26-13-16(24-7-25-17(13)27)23-6-9-5-10(20)3-4-11(9)30-2/h3-5,7-8,12,14-15,19,28H,6,21H2,1-2H3,(H,22,29)(H,23,24,25)/t12-,14+,15-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182321

((2S,3S,4R,5R)-5-(6-(2-(benzyloxy)-5-chlorobenzylam...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCc3ccccc3)ncnc12 Show InChI InChI=1S/C25H26ClN7O4/c1-28-24(35)21-18(27)20(34)25(37-21)33-13-32-19-22(30-12-31-23(19)33)29-10-15-9-16(26)7-8-17(15)36-11-14-5-3-2-4-6-14/h2-9,12-13,18,20-21,25,34H,10-11,27H2,1H3,(H,28,35)(H,29,30,31)/t18-,20+,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182311
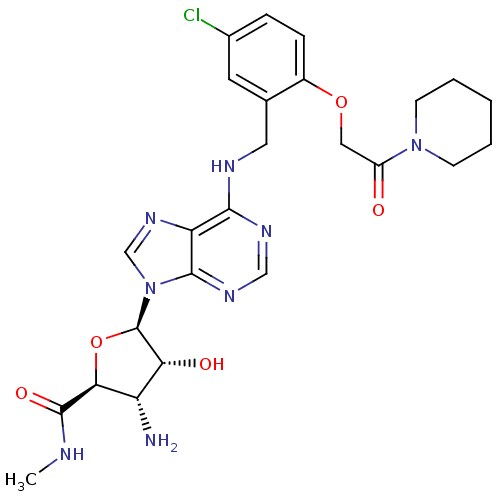
((2S,3S,4R,5R)-5-(6-(5-chloro-2-(2-oxo-2-(piperidin...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(=O)N3CCCCC3)ncnc12 Show InChI InChI=1S/C25H31ClN8O5/c1-28-24(37)21-18(27)20(36)25(39-21)34-13-32-19-22(30-12-31-23(19)34)29-10-14-9-15(26)5-6-16(14)38-11-17(35)33-7-3-2-4-8-33/h5-6,9,12-13,18,20-21,25,36H,2-4,7-8,10-11,27H2,1H3,(H,28,37)(H,29,30,31)/t18-,20+,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182318
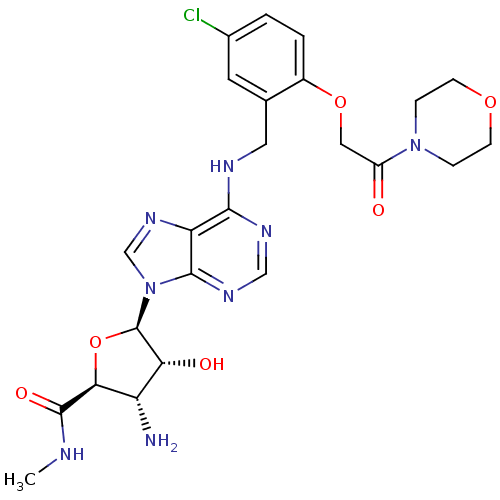
((2S,3S,4R,5R)-5-(6-(5-chloro-2-(2-morpholino-2-oxo...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(=O)N3CCOCC3)ncnc12 Show InChI InChI=1S/C24H29ClN8O6/c1-27-23(36)20-17(26)19(35)24(39-20)33-12-31-18-21(29-11-30-22(18)33)28-9-13-8-14(25)2-3-15(13)38-10-16(34)32-4-6-37-7-5-32/h2-3,8,11-12,17,19-20,24,35H,4-7,9-10,26H2,1H3,(H,27,36)(H,28,29,30)/t17-,19+,20-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182325
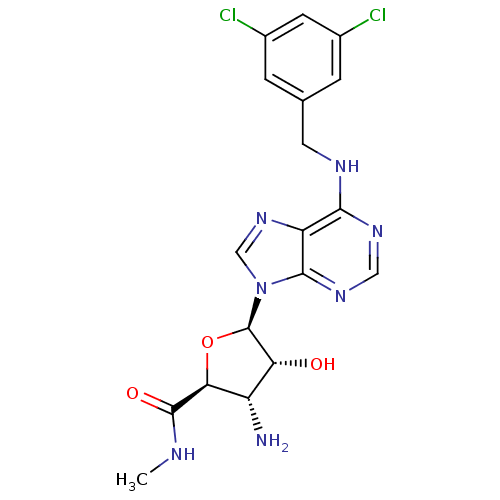
((2S,3S,4R,5R)-5-(6-(3,5-dichlorobenzylamino)-9H-pu...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)cc(Cl)c3)ncnc12 Show InChI InChI=1S/C18H19Cl2N7O3/c1-22-17(29)14-11(21)13(28)18(30-14)27-7-26-12-15(24-6-25-16(12)27)23-5-8-2-9(19)4-10(20)3-8/h2-4,6-7,11,13-14,18,28H,5,21H2,1H3,(H,22,29)(H,23,24,25)/t11-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182320
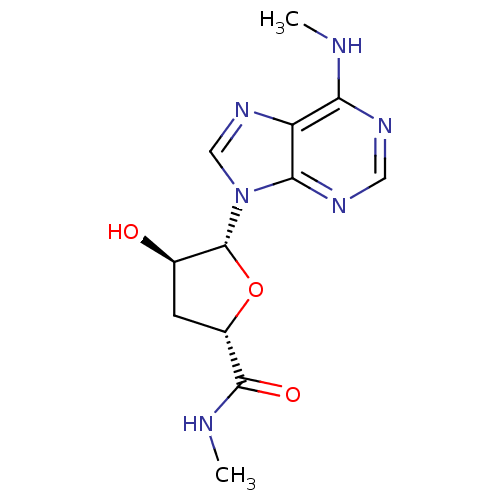
((2S,4R,5R)-4-hydroxy-N-methyl-5-(6-(methylamino)-9...)Show SMILES CNC(=O)[C@@H]1C[C@@H](O)[C@@H](O1)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O3/c1-13-9-8-10(16-4-15-9)18(5-17-8)12-6(19)3-7(21-12)11(20)14-2/h4-7,12,19H,3H2,1-2H3,(H,14,20)(H,13,15,16)/t6-,7+,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50122927

((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50122928

(5-{6-[5-Chloro-2-(3-methyl-isoxazol-5-ylmethoxy)-b...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H24ClN7O6/c1-11-5-14(37-30-11)8-35-15-4-3-13(24)6-12(15)7-26-20-16-21(28-9-27-20)31(10-29-16)23-18(33)17(32)19(36-23)22(34)25-2/h3-6,9-10,17-19,23,32-33H,7-8H2,1-2H3,(H,25,34)(H,26,27,28)/t17-,18+,19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122929

(3-Amino-4-hydroxy-5-[6-(3-iodo-benzylamino)-purin-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H20IN7O3/c1-21-17(28)14-11(20)13(27)18(29-14)26-8-25-12-15(23-7-24-16(12)26)22-6-9-3-2-4-10(19)5-9/h2-5,7-8,11,13-14,18,27H,6,20H2,1H3,(H,21,28)(H,22,23,24)/t11-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182317
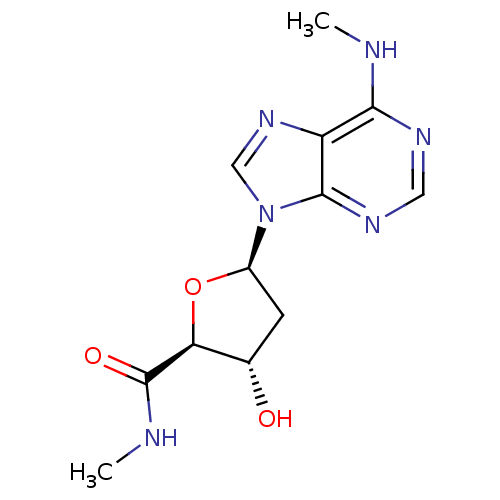
((2S,3S,5R)-3-hydroxy-N-methyl-5-(6-(methylamino)-9...)Show SMILES CNC(=O)[C@H]1O[C@H](C[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O3/c1-13-10-8-11(16-4-15-10)18(5-17-8)7-3-6(19)9(21-7)12(20)14-2/h4-7,9,19H,3H2,1-2H3,(H,14,20)(H,13,15,16)/t6-,7+,9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122930

((2S,3S,4R,5R)-3-amino-4-hydroxy-N-methyl-5-(6-(met...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H17N7O3/c1-14-9-6-10(17-3-16-9)19(4-18-6)12-7(20)5(13)8(22-12)11(21)15-2/h3-5,7-8,12,20H,13H2,1-2H3,(H,15,21)(H,14,16,17)/t5-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122930

((2S,3S,4R,5R)-3-amino-4-hydroxy-N-methyl-5-(6-(met...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H17N7O3/c1-14-9-6-10(17-3-16-9)19(4-18-6)12-7(20)5(13)8(22-12)11(21)15-2/h3-5,7-8,12,20H,13H2,1-2H3,(H,15,21)(H,14,16,17)/t5-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182319

(2-(2-((9-((2R,3R,4S,5S)-4-amino-3-hydroxy-5-(methy...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(O)=O)ncnc12 Show InChI InChI=1S/C20H22ClN7O6/c1-23-19(32)16-13(22)15(31)20(34-16)28-8-27-14-17(25-7-26-18(14)28)24-5-9-4-10(21)2-3-11(9)33-6-12(29)30/h2-4,7-8,13,15-16,20,31H,5-6,22H2,1H3,(H,23,32)(H,29,30)(H,24,25,26)/t13-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182327
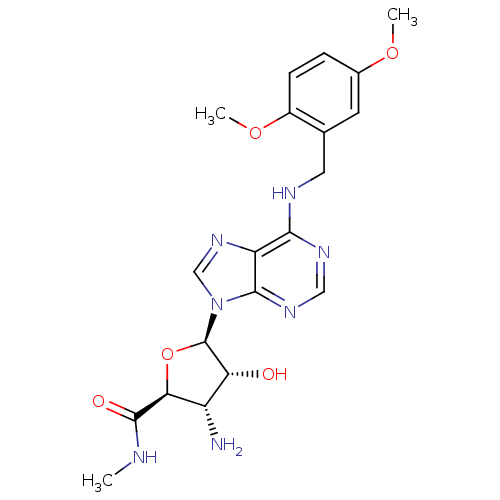
((2S,3S,4R,5R)-5-(6-(2,5-dimethoxybenzylamino)-9H-p...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(OC)ccc3OC)ncnc12 Show InChI InChI=1S/C20H25N7O5/c1-22-19(29)16-13(21)15(28)20(32-16)27-9-26-14-17(24-8-25-18(14)27)23-7-10-6-11(30-2)4-5-12(10)31-3/h4-6,8-9,13,15-16,20,28H,7,21H2,1-3H3,(H,22,29)(H,23,24,25)/t13-,15+,16-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182316
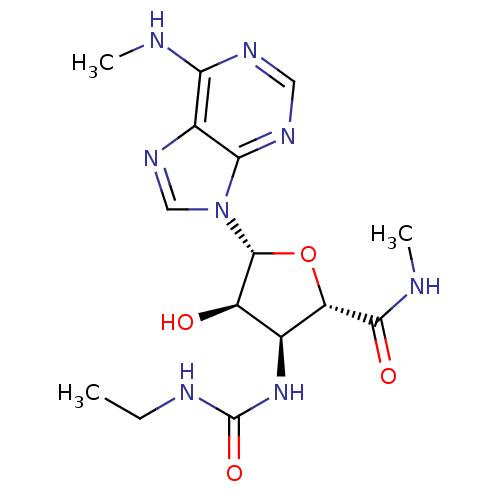
(1-ethyl-3-((2S,3S,4R,5R)-4-hydroxy-5-(6-(methylami...)Show SMILES CCNC(=O)N[C@H]1[C@@H](O)[C@@H](O[C@@H]1C(=O)NC)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C15H22N8O4/c1-4-18-15(26)22-7-9(24)14(27-10(7)13(25)17-3)23-6-21-8-11(16-2)19-5-20-12(8)23/h5-7,9-10,14,24H,4H2,1-3H3,(H,17,25)(H,16,19,20)(H2,18,22,26)/t7-,9+,10-,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182315

((2S,3S,4R,5R)-4-hydroxy-N-methyl-5-(6-(methylamino...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1NS(C)(=O)=O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C13H19N7O5S/c1-14-10-7-11(17-4-16-10)20(5-18-7)13-8(21)6(19-26(3,23)24)9(25-13)12(22)15-2/h4-6,8-9,13,19,21H,1-3H3,(H,15,22)(H,14,16,17)/t6-,8+,9-,13+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182314
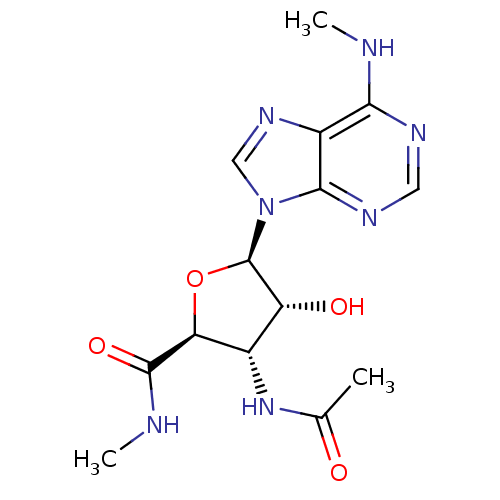
((2S,3S,4R,5R)-3-acetamido-4-hydroxy-N-methyl-5-(6-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1NC(C)=O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C14H19N7O4/c1-6(22)20-7-9(23)14(25-10(7)13(24)16-3)21-5-19-8-11(15-2)17-4-18-12(8)21/h4-5,7,9-10,14,23H,1-3H3,(H,16,24)(H,20,22)(H,15,17,18)/t7-,9+,10-,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50122929

(3-Amino-4-hydroxy-5-[6-(3-iodo-benzylamino)-purin-...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C18H20IN7O3/c1-21-17(28)14-11(20)13(27)18(29-14)26-8-25-12-15(23-7-24-16(12)26)22-6-9-3-2-4-10(19)5-9/h2-5,7-8,11,13-14,18,27H,6,20H2,1H3,(H,21,28)(H,22,23,24)/t11-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50122926

(3-Amino-5-{6-[5-chloro-2-(3-methyl-isoxazol-5-ylme...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H25ClN8O5/c1-11-5-14(37-31-11)8-35-15-4-3-13(24)6-12(15)7-27-20-17-21(29-9-28-20)32(10-30-17)23-18(33)16(25)19(36-23)22(34)26-2/h3-6,9-10,16,18-19,23,33H,7-8,25H2,1-2H3,(H,26,34)(H,27,28,29)/t16-,18+,19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50122930

((2S,3S,4R,5R)-3-amino-4-hydroxy-N-methyl-5-(6-(met...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H17N7O3/c1-14-9-6-10(17-3-16-9)19(4-18-6)12-7(20)5(13)8(22-12)11(21)15-2/h3-5,7-8,12,20H,13H2,1-2H3,(H,15,21)(H,14,16,17)/t5-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A1 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50485444

(CHEMBL2059813)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(CC12CC(C1)(C2)c1ncon1)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H20ClF3N4O4S/c20-12-1-3-13(4-2-12)32(29,30)27(14(15(24)28)5-6-19(21,22)23)10-17-7-18(8-17,9-17)16-25-11-31-26-16/h1-4,11,14H,5-10H2,(H2,24,28)/t14-,17?,18?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50314930
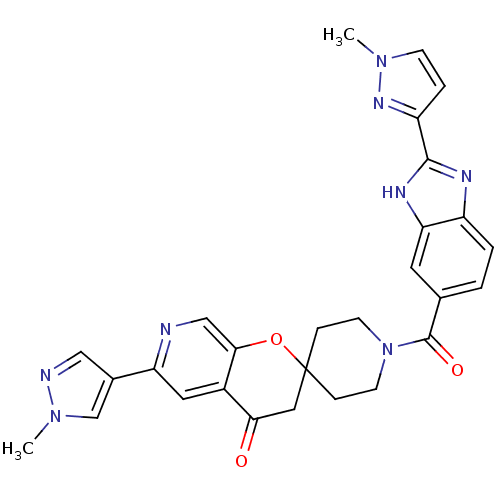
(1-(2-(1-methyl-1H-pyrazol-3-yl)-1H-benzo[d]imidazo...)Show SMILES Cn1ccc(n1)-c1nc2ccc(cc2[nH]1)C(=O)N1CCC2(CC1)CC(=O)c1cc(ncc1O2)-c1cnn(C)c1 Show InChI InChI=1S/C28H26N8O3/c1-34-8-5-21(33-34)26-31-20-4-3-17(11-23(20)32-26)27(38)36-9-6-28(7-10-36)13-24(37)19-12-22(29-15-25(19)39-28)18-14-30-35(2)16-18/h3-5,8,11-12,14-16H,6-7,9-10,13H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader |
Bioorg Med Chem Lett 20: 2383-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.04.091
BindingDB Entry DOI: 10.7270/Q22N52DW |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50458169

(Avagacestat | BMS 708163 | BMS-708163 | BMS-708163...)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(Cc1ccc(cc1F)-c1ncon1)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H17ClF4N4O4S/c21-14-3-5-15(6-4-14)34(31,32)29(17(18(26)30)7-8-20(23,24)25)10-13-2-1-12(9-16(13)22)19-27-11-33-28-19/h1-6,9,11,17H,7-8,10H2,(H2,26,30)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50314931
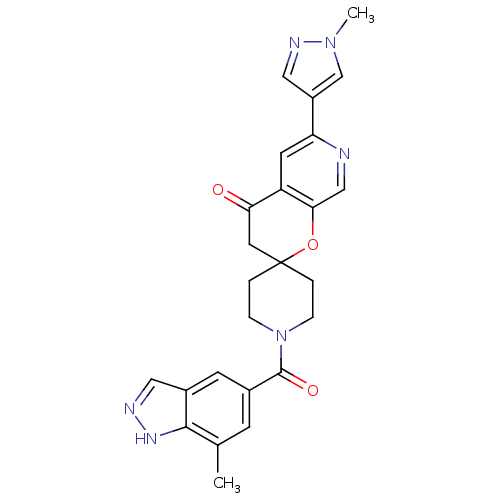
(1-(7-methyl-1H-indazole-5-carbonyl)-6'-(1-methyl-1...)Show SMILES Cc1cc(cc2cn[nH]c12)C(=O)N1CCC2(CC1)CC(=O)c1cc(ncc1O2)-c1cnn(C)c1 Show InChI InChI=1S/C25H24N6O3/c1-15-7-16(8-17-11-27-29-23(15)17)24(33)31-5-3-25(4-6-31)10-21(32)19-9-20(26-13-22(19)34-25)18-12-28-30(2)14-18/h7-9,11-14H,3-6,10H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader |
Bioorg Med Chem Lett 20: 2383-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.04.091
BindingDB Entry DOI: 10.7270/Q22N52DW |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50314932
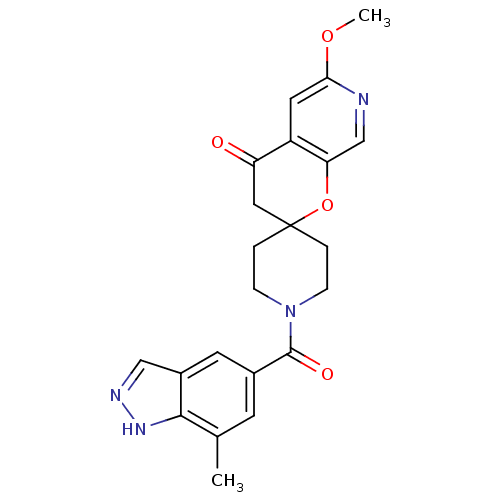
(6'-methoxy-1-(7-methyl-1H-indazole-5-carbonyl)spir...)Show SMILES COc1cc2C(=O)CC3(CCN(CC3)C(=O)c3cc(C)c4[nH]ncc4c3)Oc2cn1 Show InChI InChI=1S/C22H22N4O4/c1-13-7-14(8-15-11-24-25-20(13)15)21(28)26-5-3-22(4-6-26)10-17(27)16-9-19(29-2)23-12-18(16)30-22/h7-9,11-12H,3-6,10H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader |
Bioorg Med Chem Lett 20: 2383-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.04.091
BindingDB Entry DOI: 10.7270/Q22N52DW |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50390333

(CHEMBL2070733)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4nccs4)CCO3)nnc12)C1CC1 |r| Show InChI InChI=1S/C16H20N8OS/c17-14-13-16(20-15(19-14)10-1-2-10)24(22-21-13)8-11-7-23(4-5-25-11)9-12-18-3-6-26-12/h3,6,10-11H,1-2,4-5,7-9H2,(H2,17,19,20)/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B expressed in Sf9 insect cells |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50485438
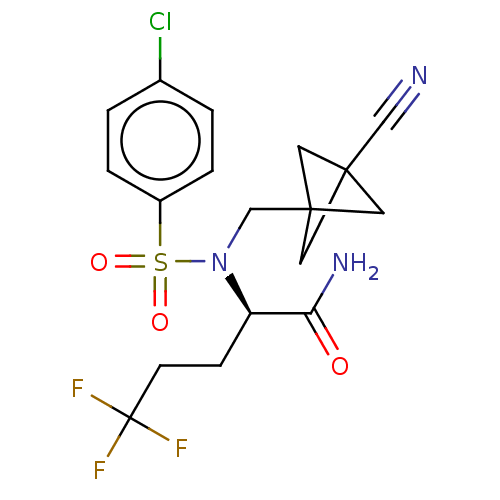
(CHEMBL2059021)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(CC12CC(C1)(C2)C#N)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H19ClF3N3O3S/c19-12-1-3-13(4-2-12)29(27,28)25(11-17-7-16(8-17,9-17)10-23)14(15(24)26)5-6-18(20,21)22/h1-4,14H,5-9,11H2,(H2,24,26)/t14-,16?,17?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50485443

(CHEMBL2059814)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(Cc1ccc(cc1)C#N)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H17ClF3N3O3S/c20-15-5-7-16(8-6-15)30(28,29)26(12-14-3-1-13(11-24)2-4-14)17(18(25)27)9-10-19(21,22)23/h1-8,17H,9-10,12H2,(H2,25,27)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50390334

(CHEMBL2070735)Show SMILES Cc1nc(N)c2nnn(C[C@H]3CN(Cc4nccs4)CCO3)c2n1 |r| Show InChI InChI=1S/C14H18N8OS/c1-9-17-13(15)12-14(18-9)22(20-19-12)7-10-6-21(3-4-23-10)8-11-16-2-5-24-11/h2,5,10H,3-4,6-8H2,1H3,(H2,15,17,18)/t10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B expressed in Sf9 insect cells |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B
(Homo sapiens (Human)) | BDBM50390332
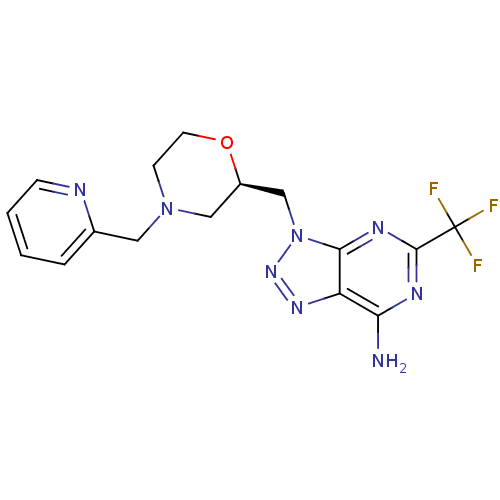
(CHEMBL2070732)Show SMILES Nc1nc(nc2n(C[C@H]3CN(Cc4ccccn4)CCO3)nnc12)C(F)(F)F |r| Show InChI InChI=1S/C16H17F3N8O/c17-16(18,19)15-22-13(20)12-14(23-15)27(25-24-12)9-11-8-26(5-6-28-11)7-10-3-1-2-4-21-10/h1-4,11H,5-9H2,(H2,20,22,23)/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8B expressed in Sf9 insect cells |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50485431

(CHEMBL2059815)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(Cc1ccc(cc1F)C#N)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H16ClF4N3O3S/c20-14-3-5-15(6-4-14)31(29,30)27(17(18(26)28)7-8-19(22,23)24)11-13-2-1-12(10-25)9-16(13)21/h1-6,9,17H,7-8,11H2,(H2,26,28)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A
(Homo sapiens (Human)) | BDBM50390334

(CHEMBL2070735)Show SMILES Cc1nc(N)c2nnn(C[C@H]3CN(Cc4nccs4)CCO3)c2n1 |r| Show InChI InChI=1S/C14H18N8OS/c1-9-17-13(15)12-14(18-9)22(20-19-12)7-10-6-21(3-4-23-10)8-11-16-2-5-24-11/h2,5,10H,3-4,6-8H2,1H3,(H2,15,17,18)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE8A |
Bioorg Med Chem Lett 22: 5721-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.079
BindingDB Entry DOI: 10.7270/Q2J67J0X |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50277775
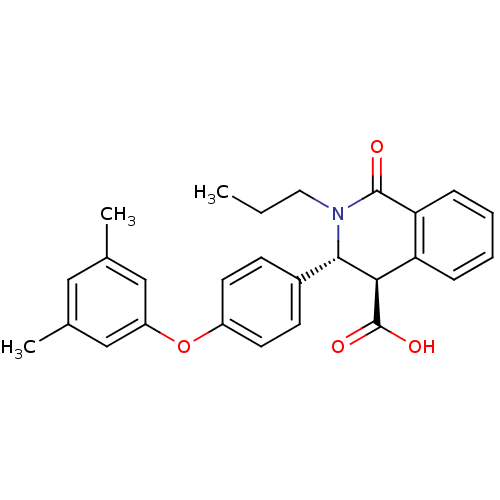
((3R,4R)-3-(4-(3,5-dimethylphenoxy)phenyl)-1-oxo-2-...)Show SMILES CCCN1[C@H]([C@H](C(O)=O)c2ccccc2C1=O)c1ccc(Oc2cc(C)cc(C)c2)cc1 |r| Show InChI InChI=1S/C27H27NO4/c1-4-13-28-25(24(27(30)31)22-7-5-6-8-23(22)26(28)29)19-9-11-20(12-10-19)32-21-15-17(2)14-18(3)16-21/h5-12,14-16,24-25H,4,13H2,1-3H3,(H,30,31)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay |
Bioorg Med Chem Lett 19: 2400-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.082
BindingDB Entry DOI: 10.7270/Q23N238P |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50485434
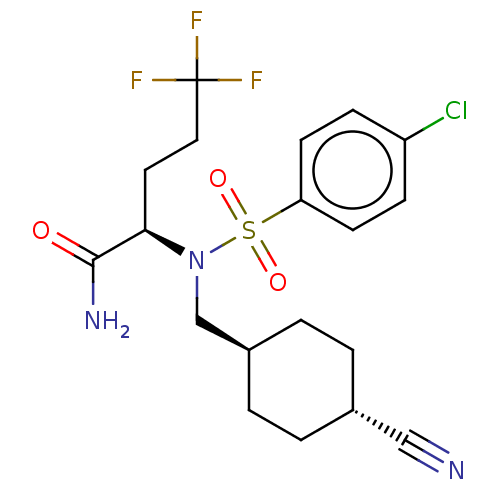
(CHEMBL2059016)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(C[C@H]1CC[C@@H](CC1)C#N)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:15.18,wD:3.3,12.11,(.6,-42.56,;-.73,-41.8,;-2.06,-42.57,;-.74,-40.26,;.59,-39.49,;1.93,-40.26,;3.25,-39.49,;4.59,-40.26,;3.26,-37.95,;4.59,-38.71,;-2.1,-39.49,;-3.43,-40.27,;-4.77,-39.51,;-4.79,-37.97,;-6.12,-37.21,;-7.46,-37.99,;-7.44,-39.53,;-6.1,-40.3,;-8.79,-37.23,;-10.14,-36.47,;-2.1,-37.94,;-2.87,-36.61,;-3.64,-37.94,;-.77,-37.18,;-.74,-35.65,;.6,-34.9,;1.92,-35.69,;3.26,-34.94,;1.9,-37.22,;.56,-37.98,)| Show InChI InChI=1S/C19H23ClF3N3O3S/c20-15-5-7-16(8-6-15)30(28,29)26(12-14-3-1-13(11-24)2-4-14)17(18(25)27)9-10-19(21,22)23/h5-8,13-14,17H,1-4,9-10,12H2,(H2,25,27)/t13-,14-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50485434

(CHEMBL2059016)Show SMILES NC(=O)[C@@H](CCC(F)(F)F)N(C[C@H]1CC[C@@H](CC1)C#N)S(=O)(=O)c1ccc(Cl)cc1 |r,wU:15.18,wD:3.3,12.11,(.6,-42.56,;-.73,-41.8,;-2.06,-42.57,;-.74,-40.26,;.59,-39.49,;1.93,-40.26,;3.25,-39.49,;4.59,-40.26,;3.26,-37.95,;4.59,-38.71,;-2.1,-39.49,;-3.43,-40.27,;-4.77,-39.51,;-4.79,-37.97,;-6.12,-37.21,;-7.46,-37.99,;-7.44,-39.53,;-6.1,-40.3,;-8.79,-37.23,;-10.14,-36.47,;-2.1,-37.94,;-2.87,-36.61,;-3.64,-37.94,;-.77,-37.18,;-.74,-35.65,;.6,-34.9,;1.92,-35.69,;3.26,-34.94,;1.9,-37.22,;.56,-37.98,)| Show InChI InChI=1S/C19H23ClF3N3O3S/c20-15-5-7-16(8-6-15)30(28,29)26(12-14-3-1-13(11-24)2-4-14)17(18(25)27)9-10-19(21,22)23/h5-8,13-14,17H,1-4,9-10,12H2,(H2,25,27)/t13-,14-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in human H4 cells expressing human APP swedish mutant |
J Med Chem 55: 3414-24 (2012)
Article DOI: 10.1021/jm300094u
BindingDB Entry DOI: 10.7270/Q23F4SHS |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50277776
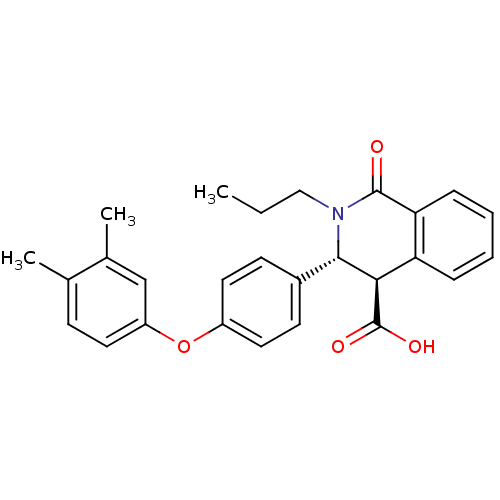
((3R,4R)-3-(4-(3,4-dimethylphenoxy)phenyl)-1-oxo-2-...)Show SMILES CCCN1[C@H]([C@H](C(O)=O)c2ccccc2C1=O)c1ccc(Oc2ccc(C)c(C)c2)cc1 |r| Show InChI InChI=1S/C27H27NO4/c1-4-15-28-25(24(27(30)31)22-7-5-6-8-23(22)26(28)29)19-10-13-20(14-11-19)32-21-12-9-17(2)18(3)16-21/h5-14,16,24-25H,4,15H2,1-3H3,(H,30,31)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR40 expressed HEK293 cells assessed as effect on intracellular calcium concentration by FLIPR assay |
Bioorg Med Chem Lett 19: 2400-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.082
BindingDB Entry DOI: 10.7270/Q23N238P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data