

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648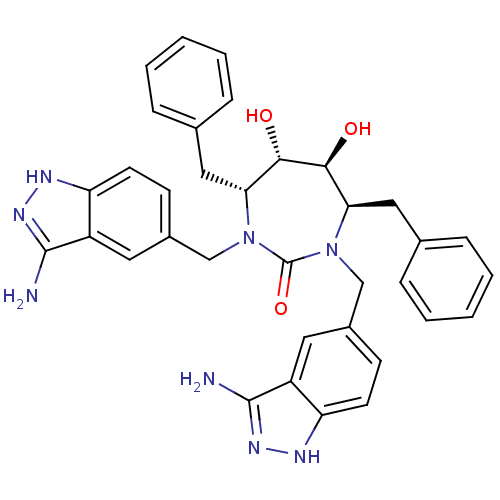 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647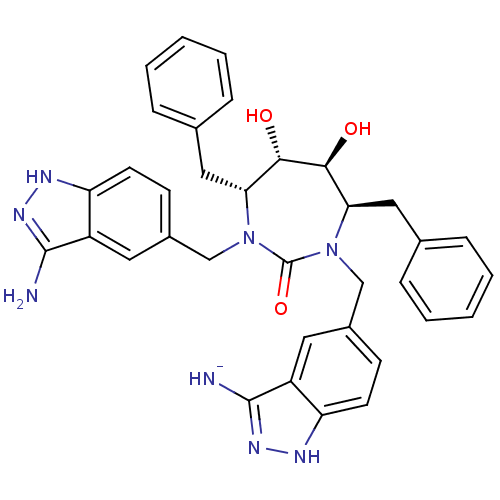 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7084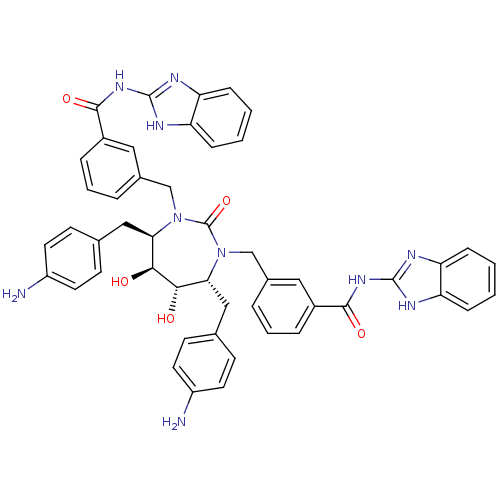 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-4,7-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | -64.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM161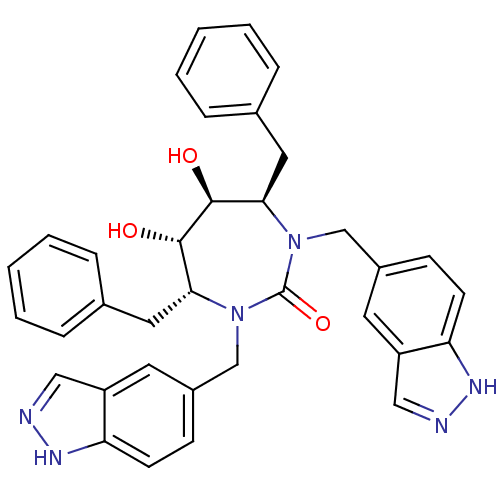 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36649 (3-alkylaminoindazole cyclic urea, (Me)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36655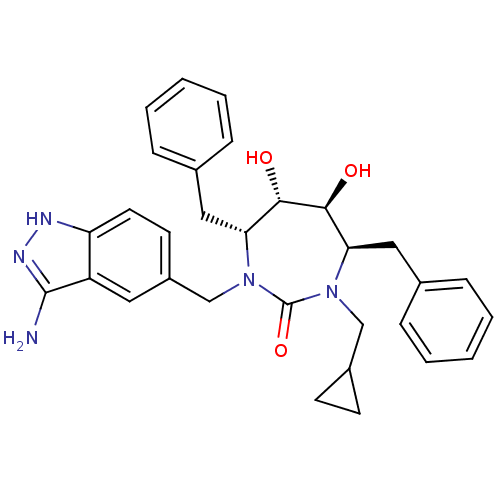 (Cyclopropylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50124714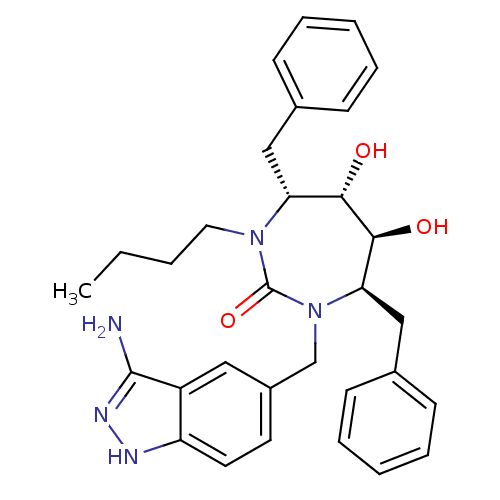 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36657 (2-Naphthylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155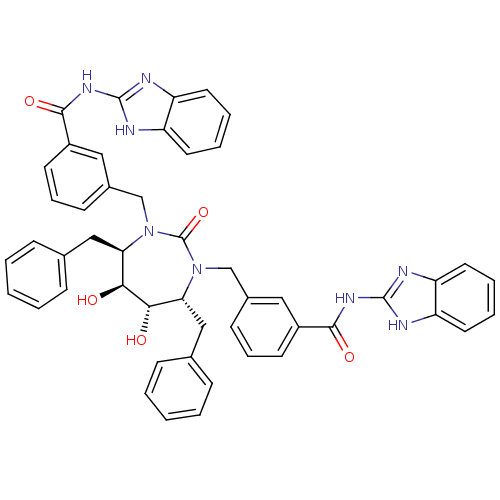 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0240 | -63.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7088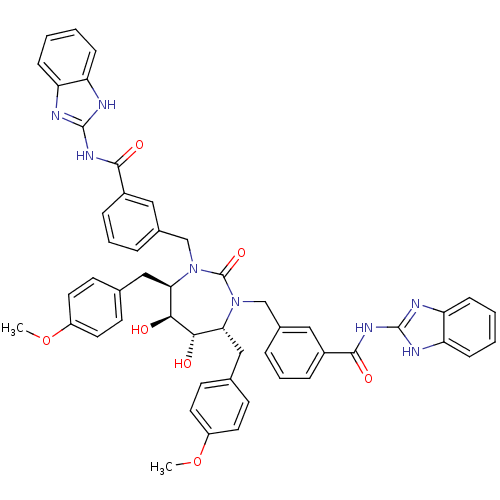 (SD146 Analog 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | -62.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7026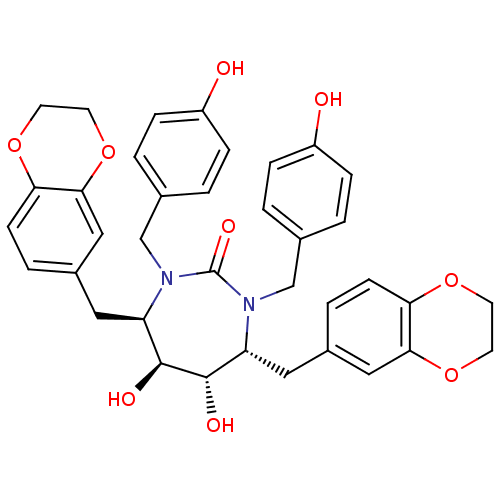 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36646 (DMP850) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7086 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | -62.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7090 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | -61.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7085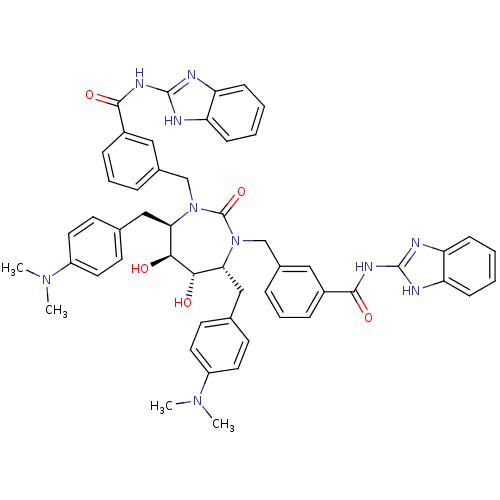 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | -61.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50063687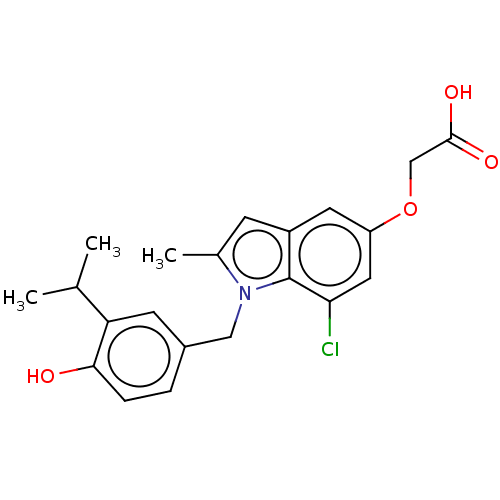 (CHEMBL3397339) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... | Bioorg Med Chem Lett 25: 1377-80 (2015) Article DOI: 10.1016/j.bmcl.2015.02.062 BindingDB Entry DOI: 10.7270/Q2PV6N2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7024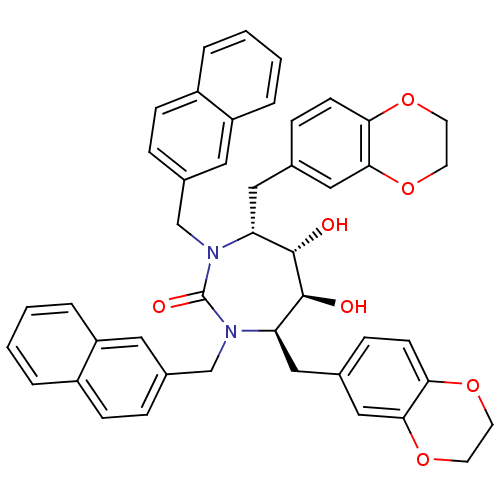 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7020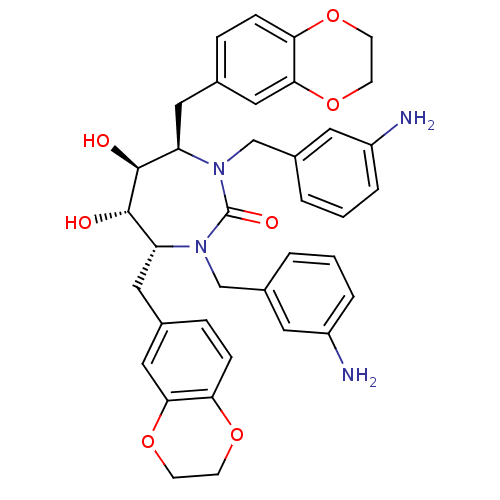 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7019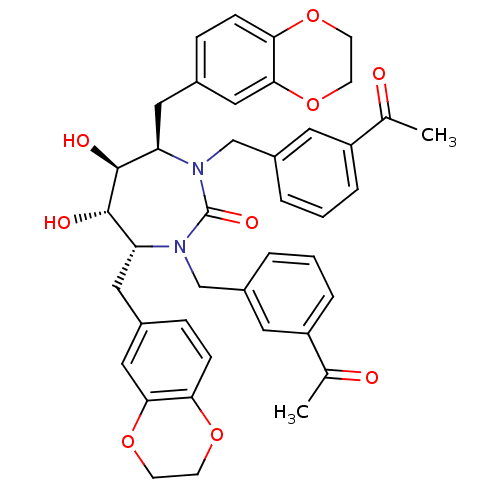 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36650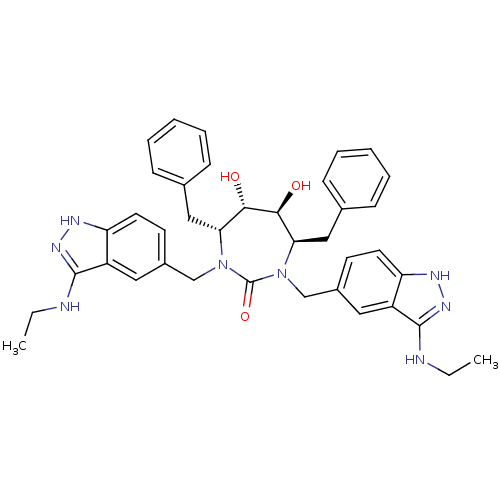 (3-alkylaminoindazole cyclic urea, (Et)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36658 (n-Pentyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7091 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | -61.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7028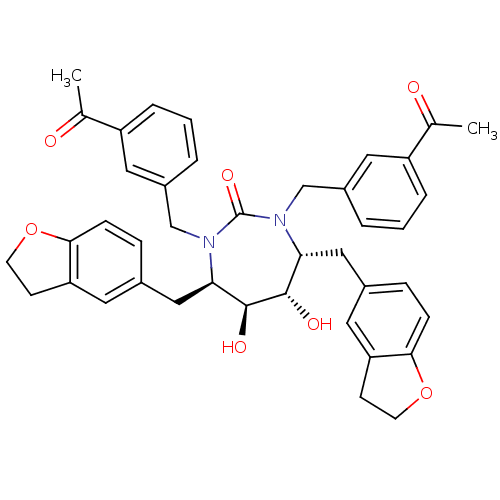 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1-benzofuran-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | -61.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7025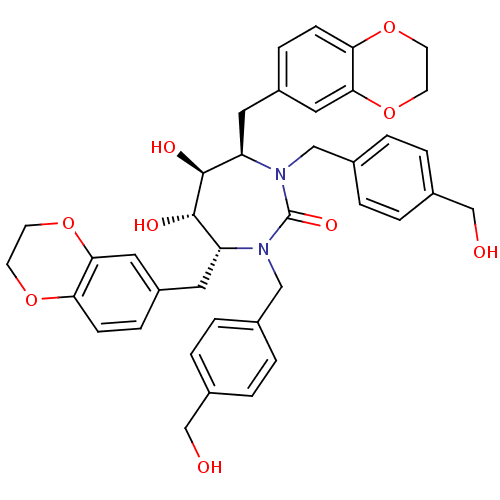 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -61.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192240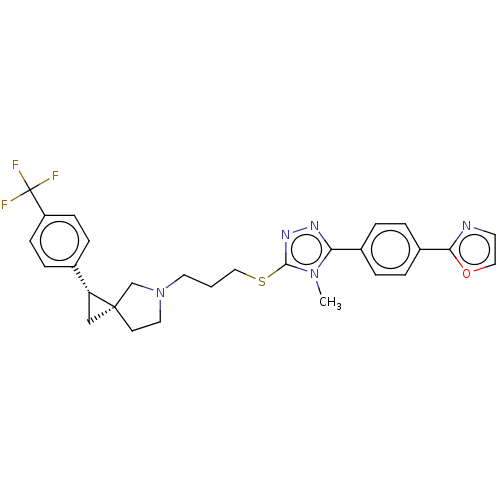 (CHEMBL3941818 | US10239870, Example 278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7089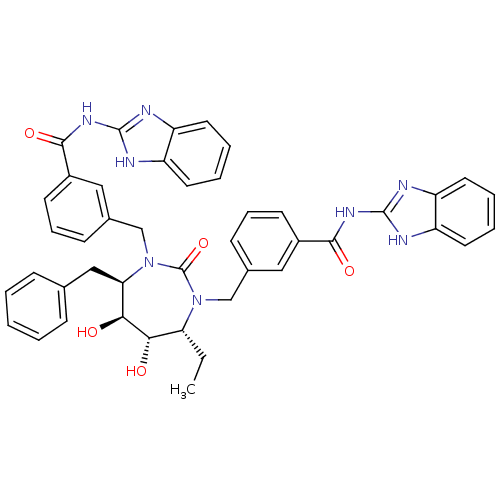 (N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,5S,6S,7R)-3-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0690 | -60.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50063689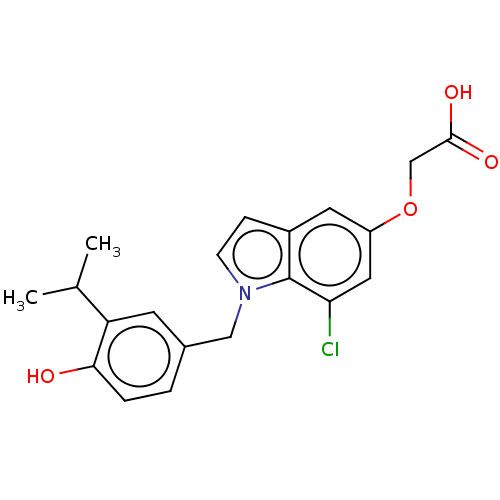 (CHEMBL3397337) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... | Bioorg Med Chem Lett 25: 1377-80 (2015) Article DOI: 10.1016/j.bmcl.2015.02.062 BindingDB Entry DOI: 10.7270/Q2PV6N2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192240 (CHEMBL3941818 | US10239870, Example 278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192240 (CHEMBL3941818 | US10239870, Example 278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36651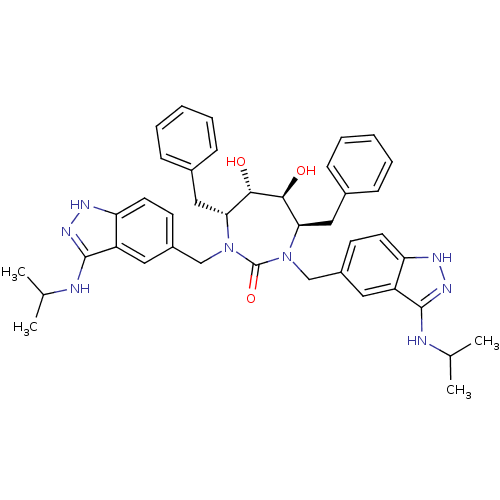 (3-alkylaminoindazole cyclic urea, (i-Pr)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50063755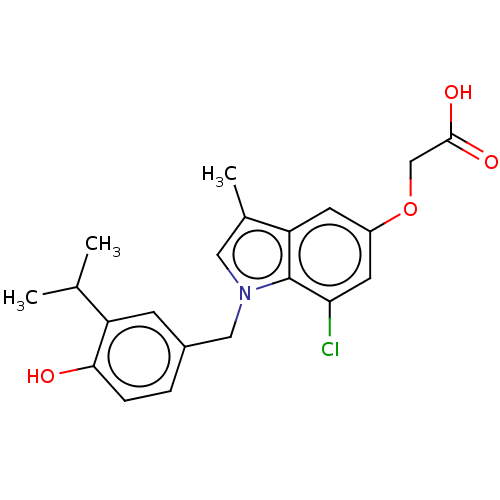 (CHEMBL3397341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... | Bioorg Med Chem Lett 25: 1377-80 (2015) Article DOI: 10.1016/j.bmcl.2015.02.062 BindingDB Entry DOI: 10.7270/Q2PV6N2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7075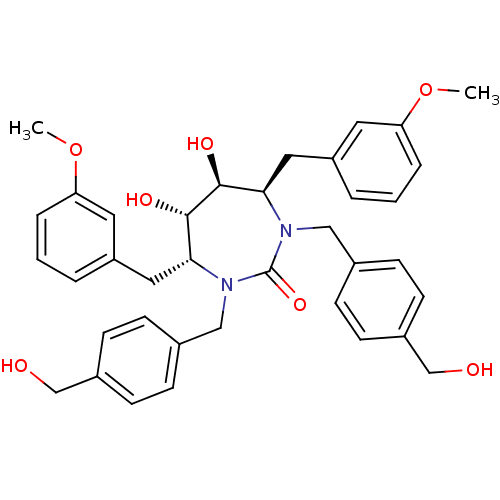 ((4R,5S,6S,7R)-5,6-dihydroxy-1,3-bis({[4-(hydroxyme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -59.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | Bioorg Med Chem Lett 8: 1077-82 (1998) Article DOI: 10.1016/s0960-894x(98)00175-9 BindingDB Entry DOI: 10.7270/Q21G0JGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7029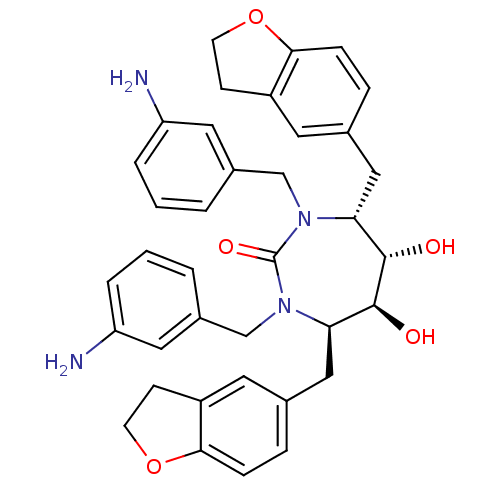 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -59.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7021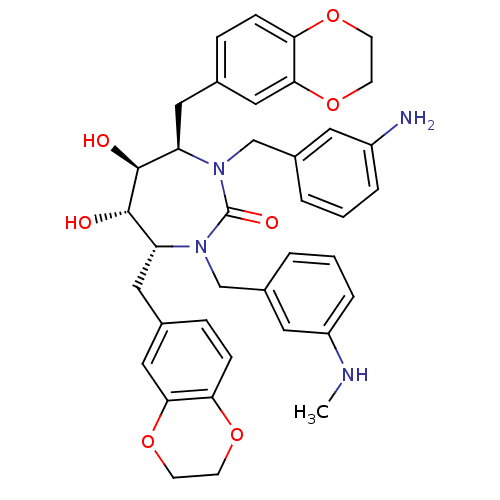 ((4R,5S,6S,7R)-1-[(3-aminophenyl)methyl]-4,7-bis(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -59.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50154039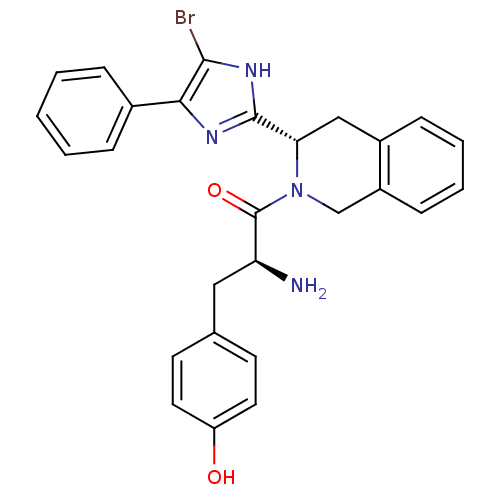 (2-Amino-1-[3-(5-bromo-4-phenyl-1H-imidazol-2-yl)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptor of rat brain | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36659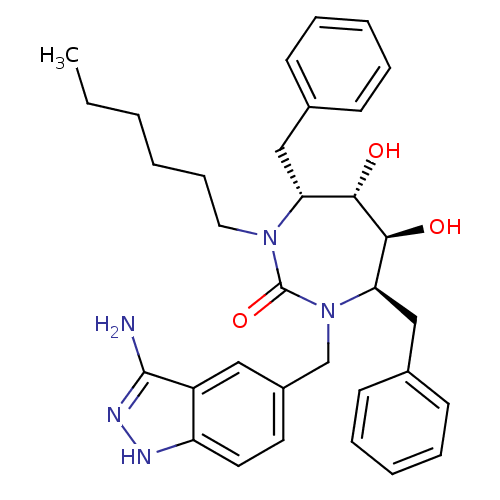 (n-Hexyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7022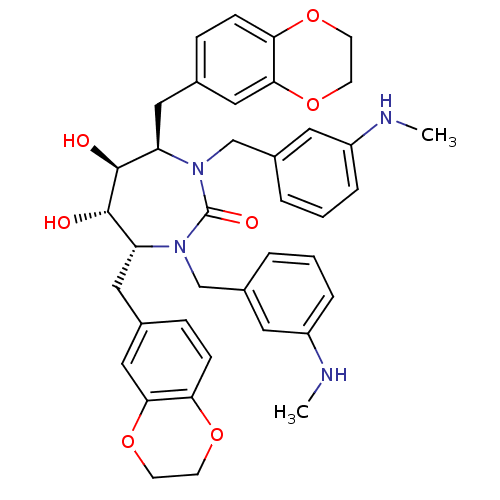 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | -58.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50063688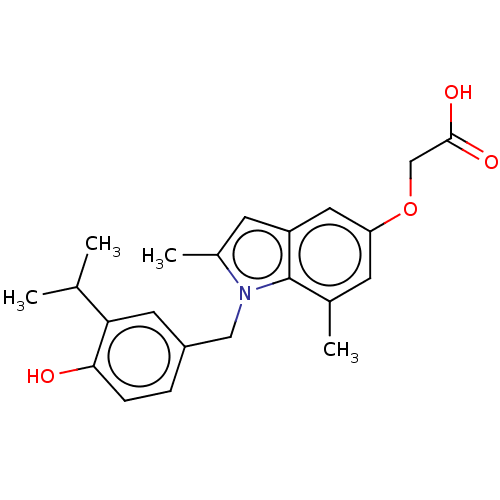 (CHEMBL3397338) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... | Bioorg Med Chem Lett 25: 1377-80 (2015) Article DOI: 10.1016/j.bmcl.2015.02.062 BindingDB Entry DOI: 10.7270/Q2PV6N2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM82247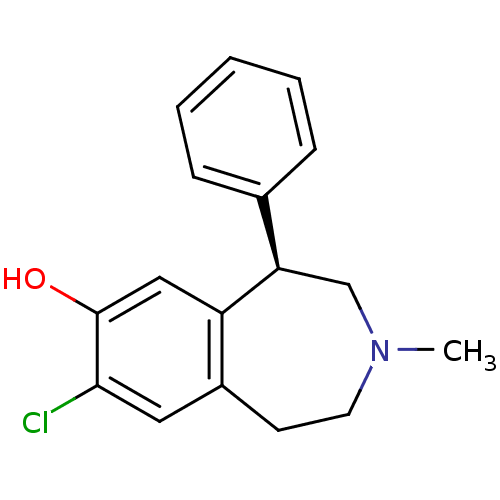 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 | J Med Chem 30: 1433-54 (1987) BindingDB Entry DOI: 10.7270/Q2D50NJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192317 (CHEMBL3899125 | US10239870, Example 281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50028288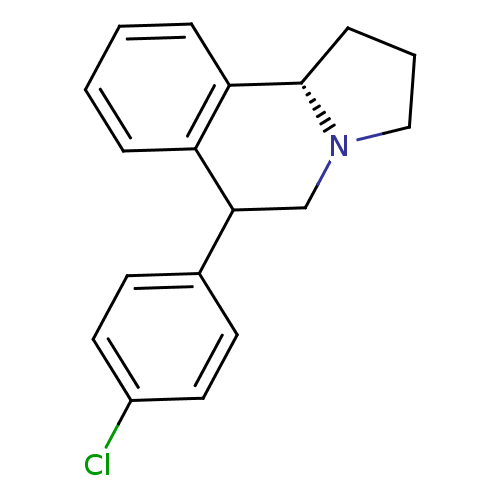 (6-(4-Chloro-phenyl)-1,2,3,5,6,10b-hexahydro-pyrrol...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of norepinephrine (NE) into rat brain synaptosomes | J Med Chem 27: 943-6 (1984) BindingDB Entry DOI: 10.7270/Q2VT1SPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50017698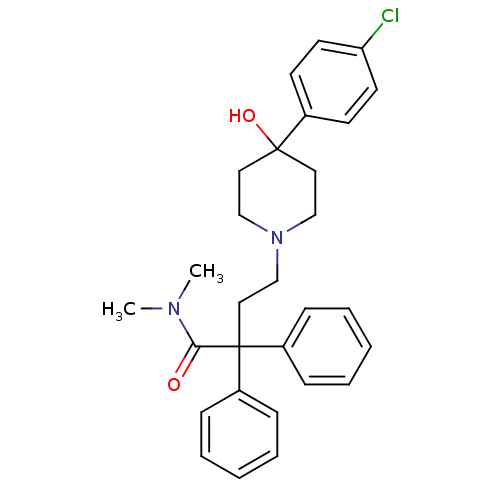 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity for Mu opioid receptor of rat brain | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50367554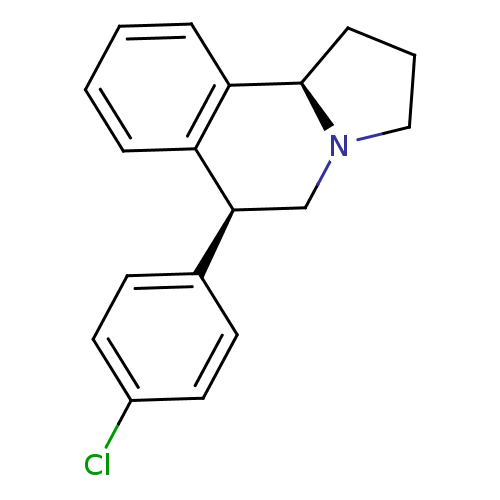 (CHEMBL1743782) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomes | J Med Chem 30: 1433-54 (1987) BindingDB Entry DOI: 10.7270/Q2D50NJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50063686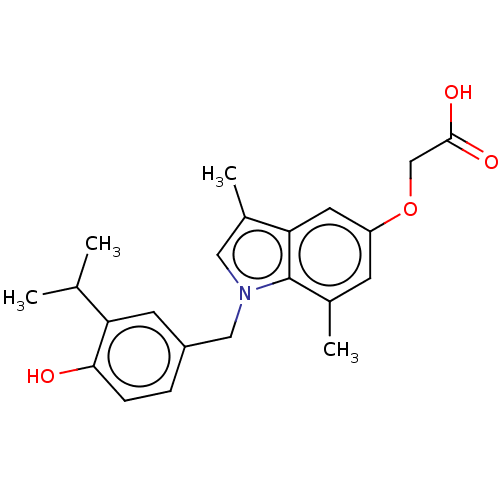 (CHEMBL3397340) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-L-3,5,3'-triiodothyronine from human TRbeta expressed in human Hela cell lysate measured after overnight incubation by competi... | Bioorg Med Chem Lett 25: 1377-80 (2015) Article DOI: 10.1016/j.bmcl.2015.02.062 BindingDB Entry DOI: 10.7270/Q2PV6N2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192319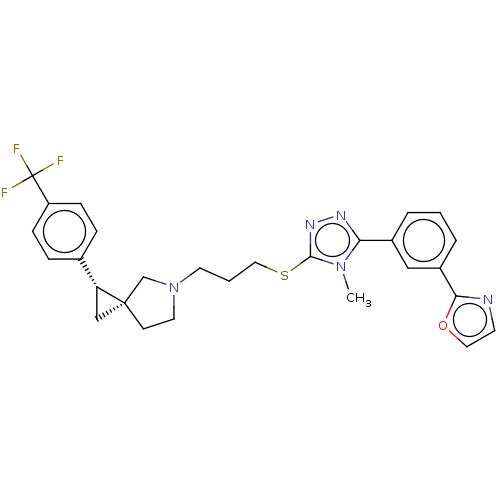 (CHEMBL3920167 | US10239870, Example 279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50154890 (CHEMBL3774425 | US10273244, Example 113 | US105841...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counter metho... | Bioorg Med Chem 24: 1619-36 (2016) Article DOI: 10.1016/j.bmc.2016.02.031 BindingDB Entry DOI: 10.7270/Q29P33JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50452190 (CHEMBL2093944) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of norepinephrine (NE) into rat brain synaptosomes | J Med Chem 27: 943-6 (1984) BindingDB Entry DOI: 10.7270/Q2VT1SPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5532 total ) | Next | Last >> |