

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450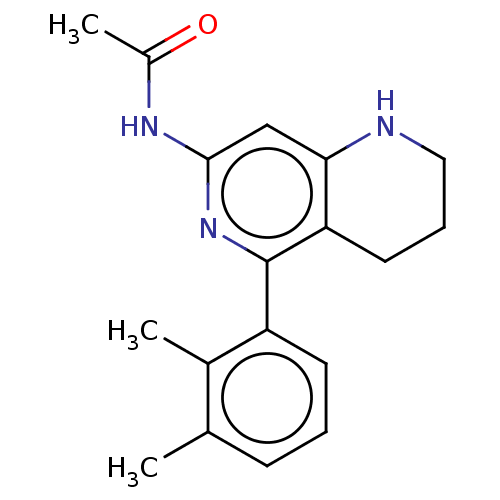 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335985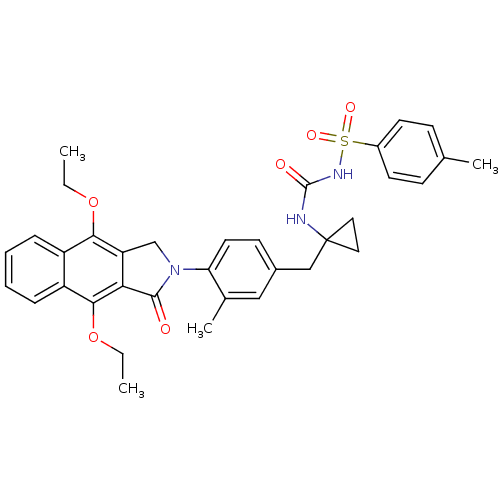 (CHEMBL1669013 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372064 (CHEMBL256873) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335986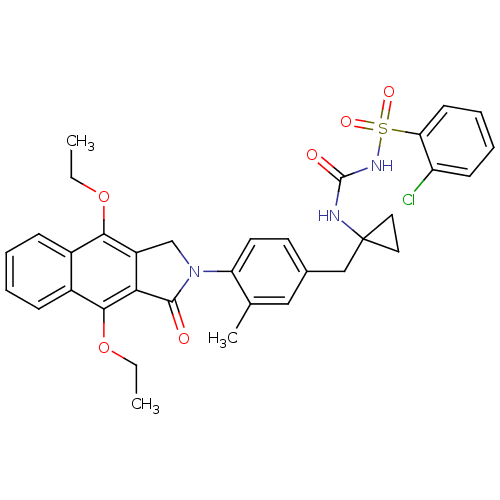 (2-chloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335980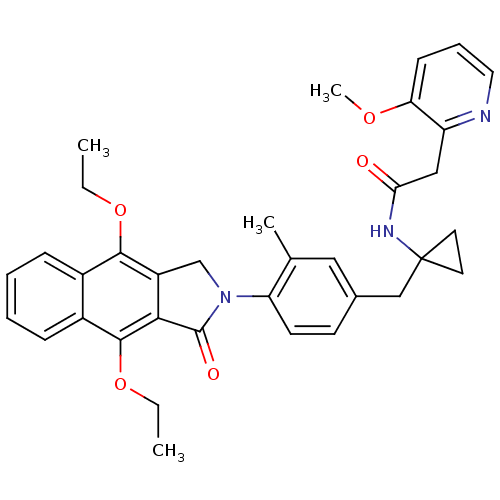 (CHEMBL1669017 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335989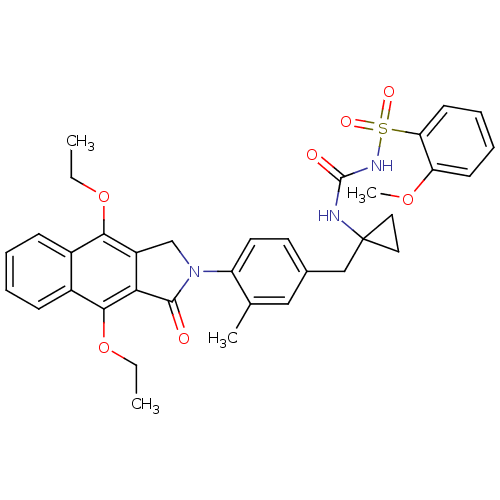 (CHEMBL1669009 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335981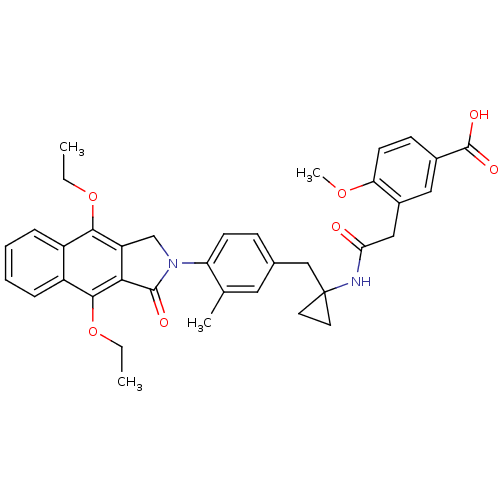 (3-(2-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]isoindol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335984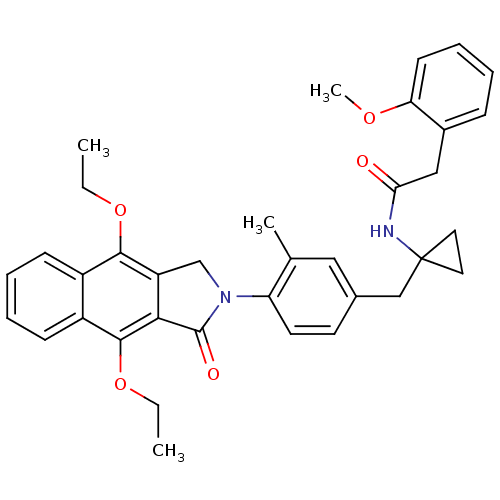 (CHEMBL1669018 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372051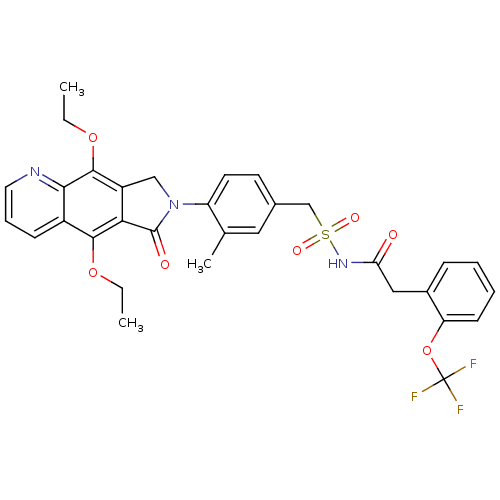 (CHEMBL269987) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335988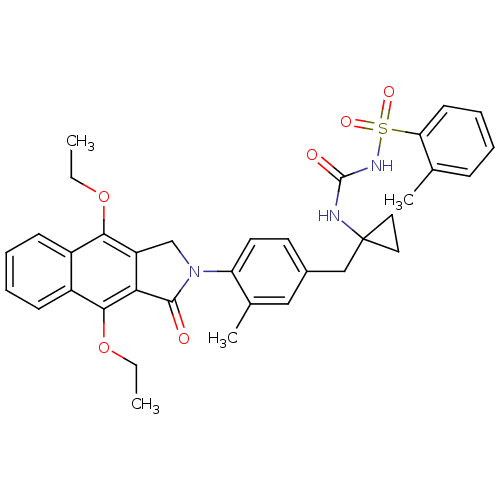 (CHEMBL1669010 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372054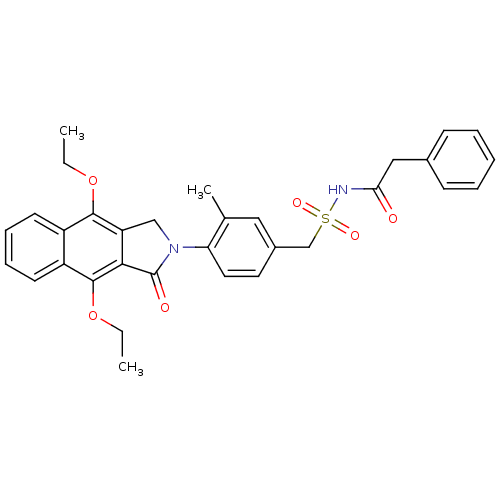 (CHEMBL255527) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372052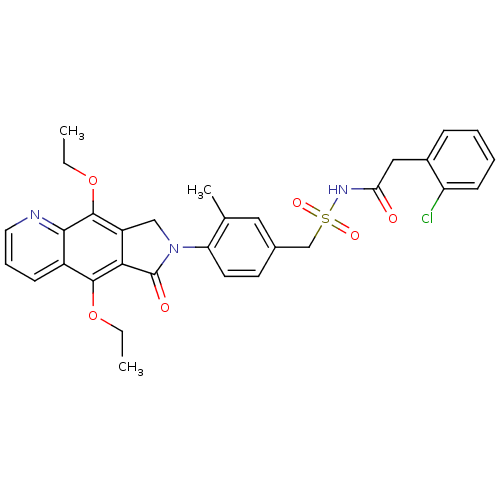 (CHEMBL218699) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372050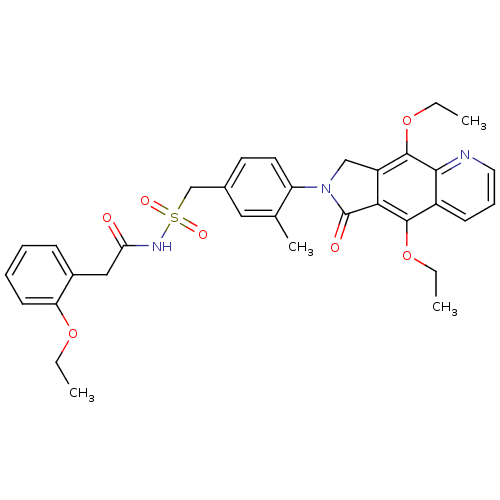 (CHEMBL255906) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372065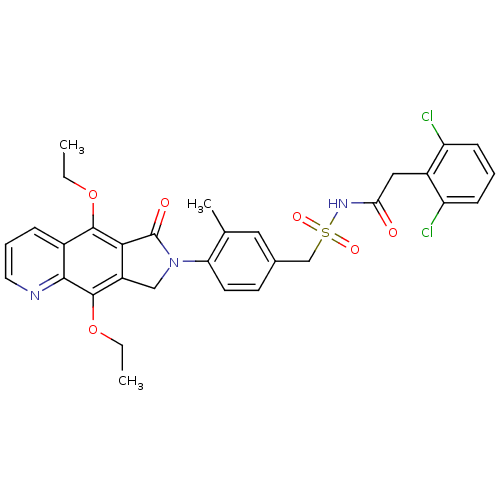 (CHEMBL272363) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335990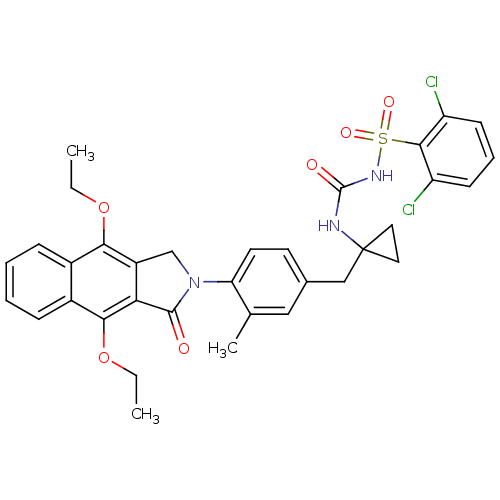 (2,6-dichloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372056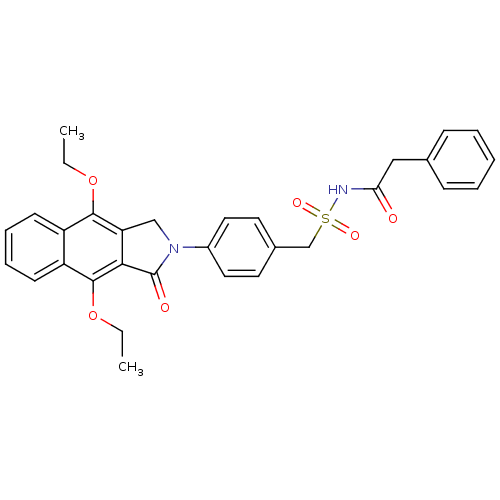 (CHEMBL255422) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335982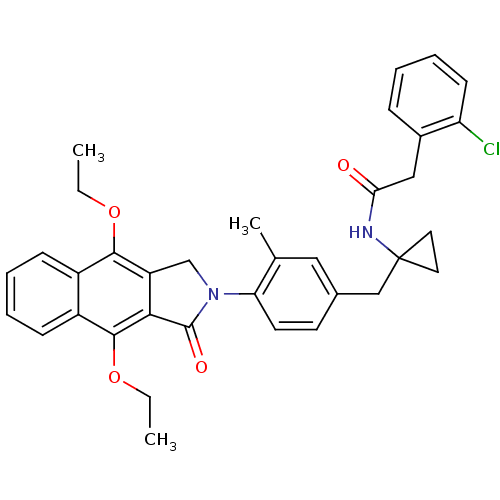 (2-(2-chlorophenyl)-N-(1-(4-(4,9-diethoxy-1-oxo-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50336003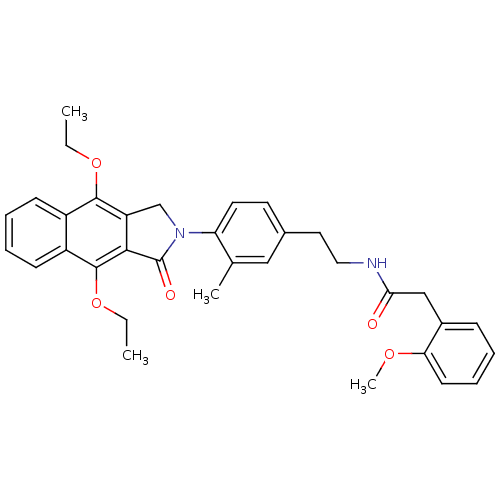 (CHEMBL1669023 | N-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372049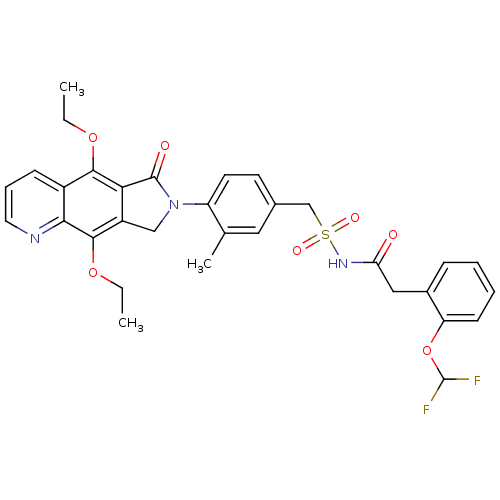 (CHEMBL257255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335983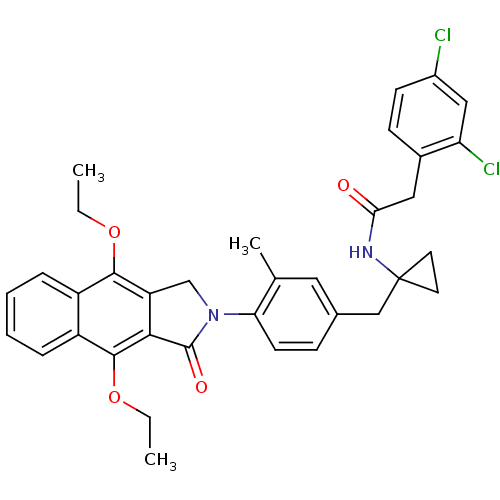 (2-(2,4-dichlorophenyl)-N-(1-(4-(4,9-diethoxy-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335993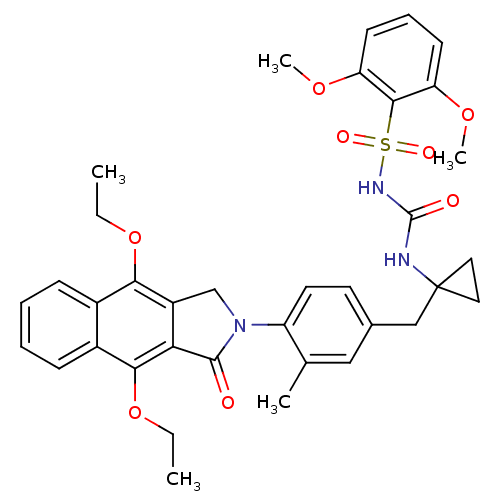 (CHEMBL1669005 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372062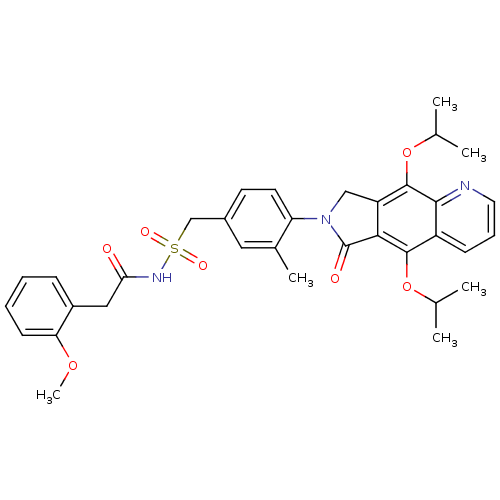 (CHEMBL272498) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372073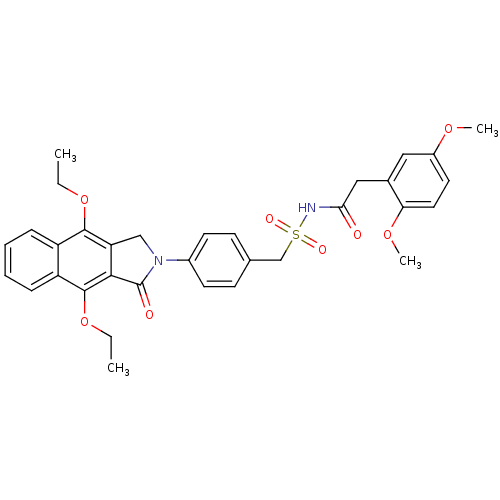 (CHEMBL256220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372063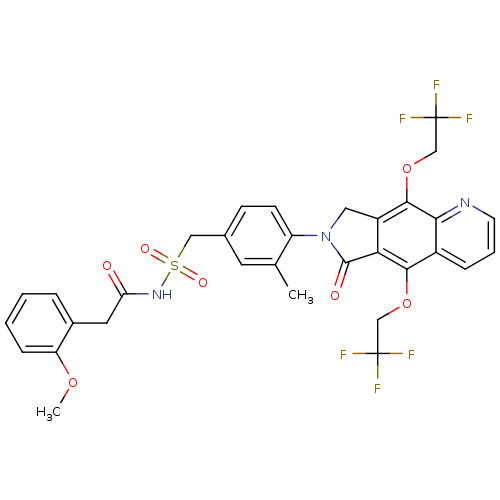 (CHEMBL271095) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372075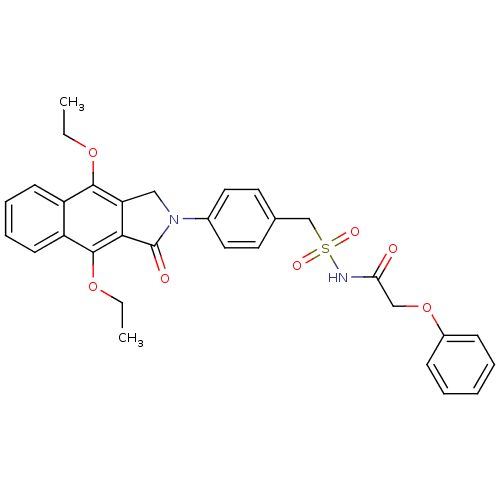 (CHEMBL256183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335992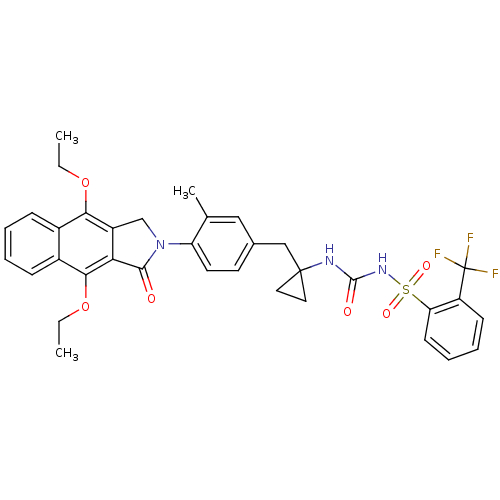 (CHEMBL1669006 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335998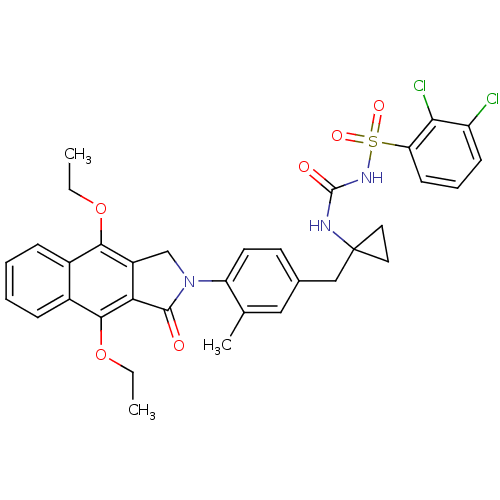 (2,3-dichloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335999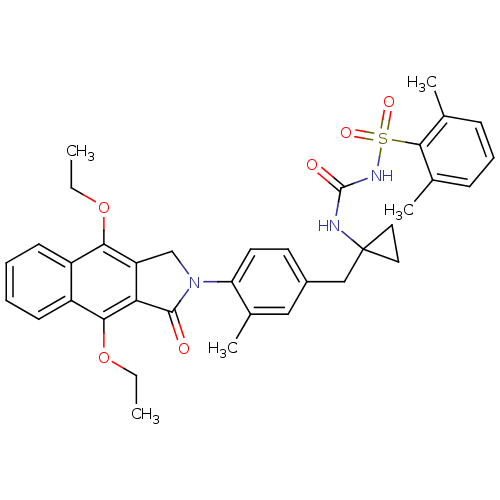 (2,6-dimethyl-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372077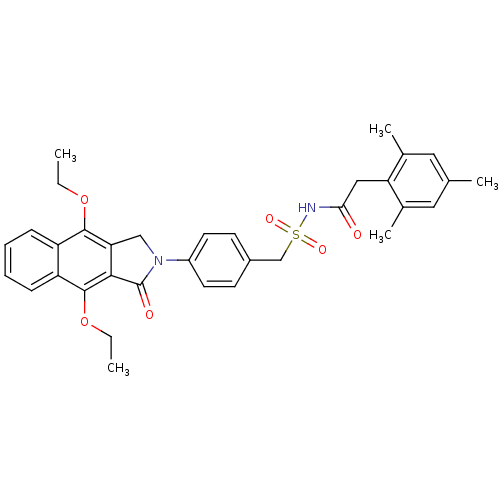 (CHEMBL271070) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372067 (CHEMBL270397) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372071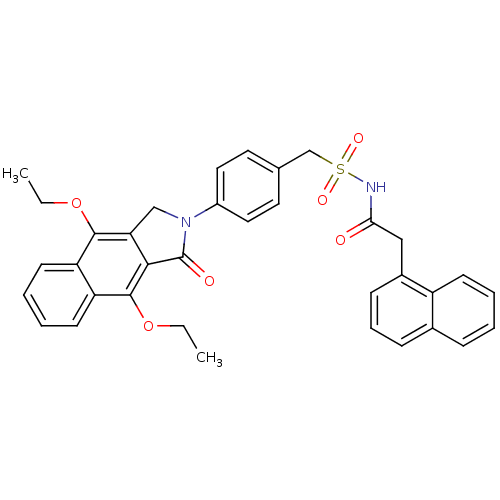 (CHEMBL255529) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335997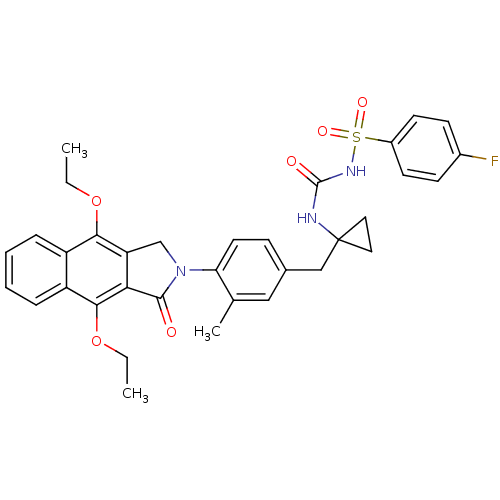 (CHEMBL1669024 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372053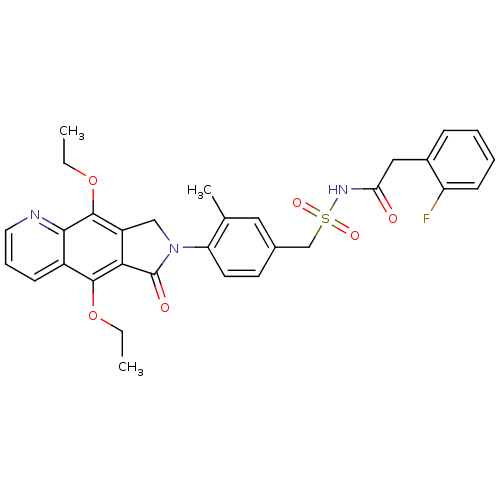 (CHEMBL402960) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335987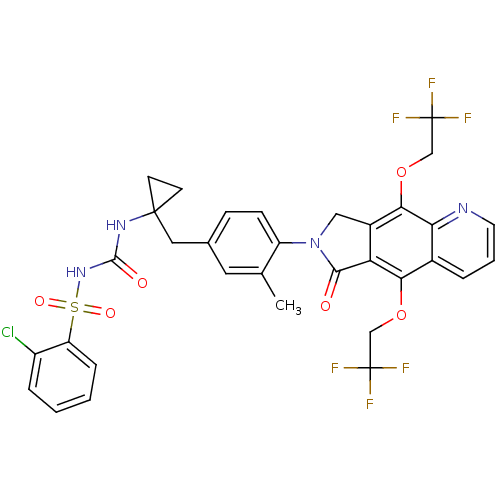 (2-chloro-N-(1-(3-methyl-4-(6-oxo-5,9-bis(2,2,2-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335994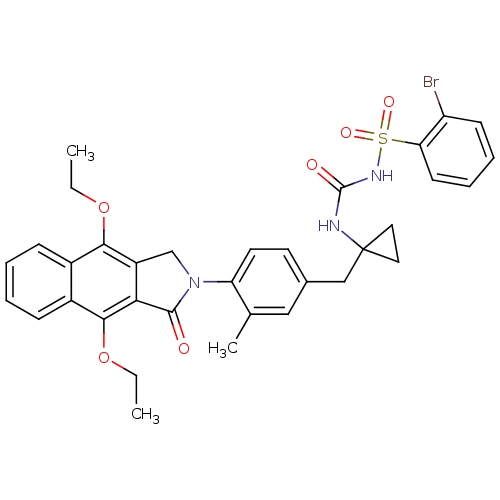 (2-bromo-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335991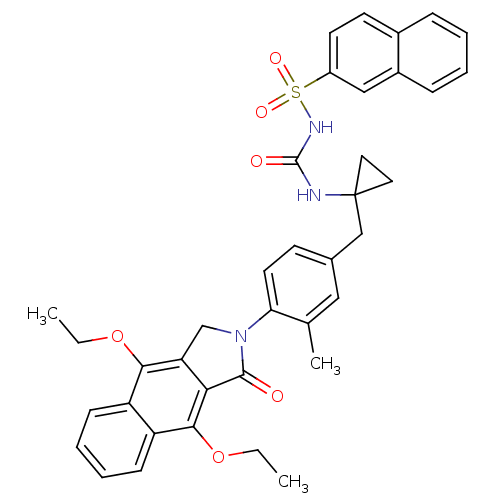 (CHEMBL1669007 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335983 (2-(2,4-dichlorophenyl)-N-(1-(4-(4,9-diethoxy-1-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay in pr... | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372059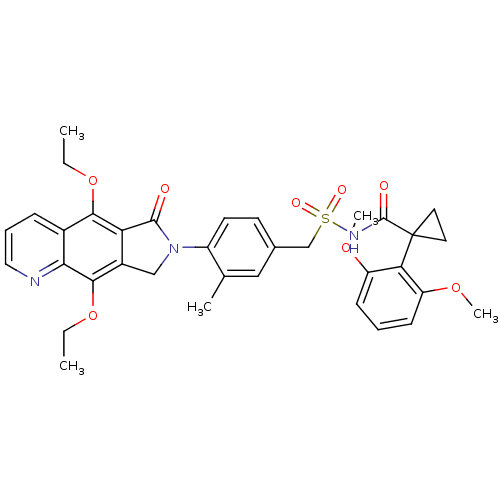 (CHEMBL405446) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372061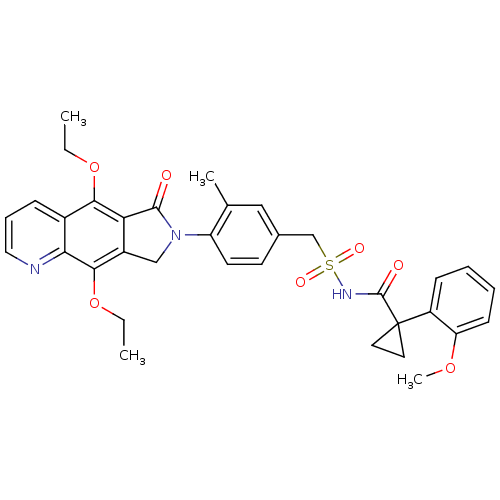 (CHEMBL256822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308131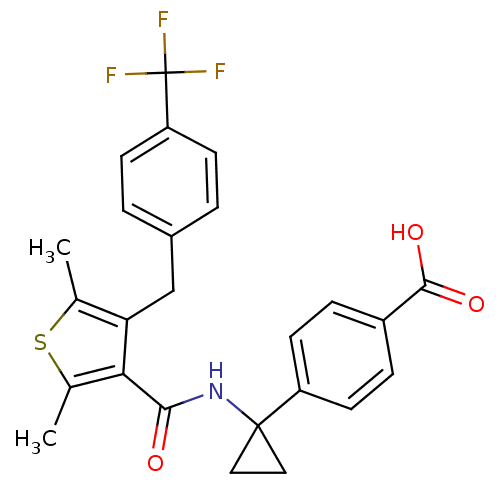 (4-{1-[({2,5-Dimethyl-4-[4-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308132 (2,5-Dimethyl-N-{1-[4-(2H-tetrazol-5-yl)phenyl]cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335996 (CHEMBL1669002 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50336002 (CHEMBL1669019 | N-(1-(4-(5,9-diethoxy-6-oxo-6H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335984 (CHEMBL1669018 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay in pr... | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50308131 (4-{1-[({2,5-Dimethyl-4-[4-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from rat EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50336004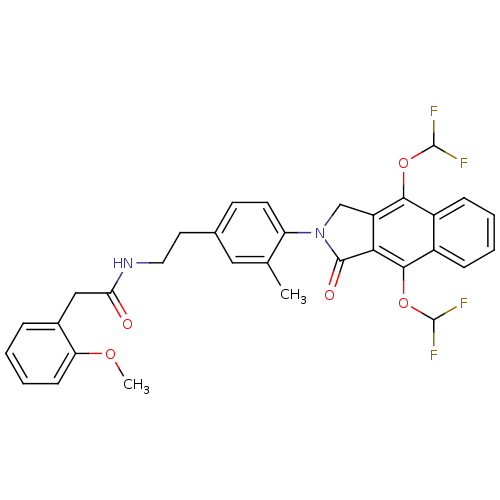 (CHEMBL1669022 | N-(4-(4,9-bis(difluoromethoxy)-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308134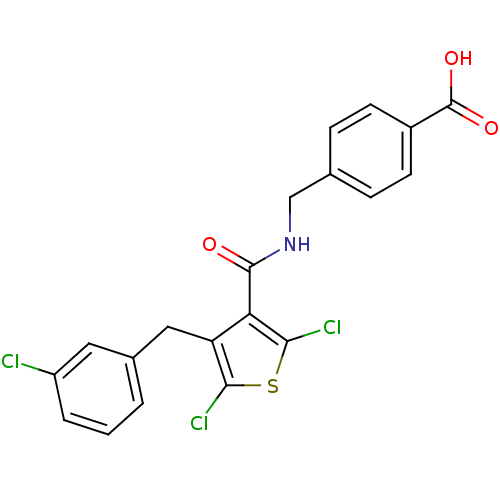 (4-[({[2,5-Dichloro-4-(3-chlorobenzyl)-3-thienyl]ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting in presence of 10% human serum | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372068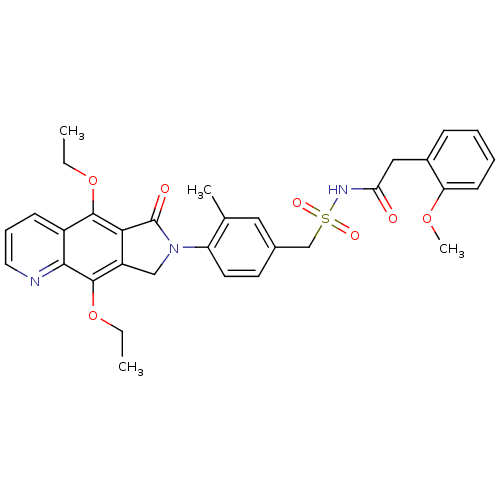 (CHEMBL402162) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308130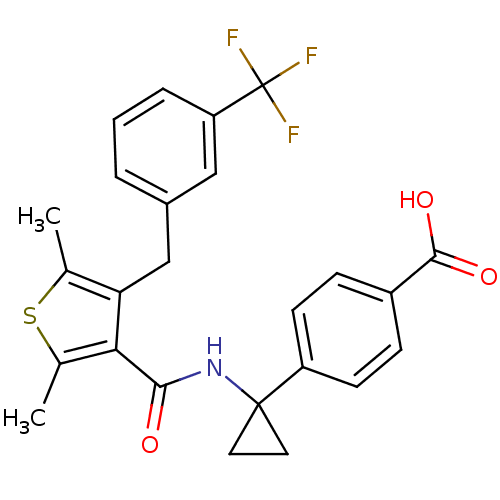 (4-{1-[({2,5-Dimethyl-4-[3-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting in presence of 10% human serum | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 403 total ) | Next | Last >> |