

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451823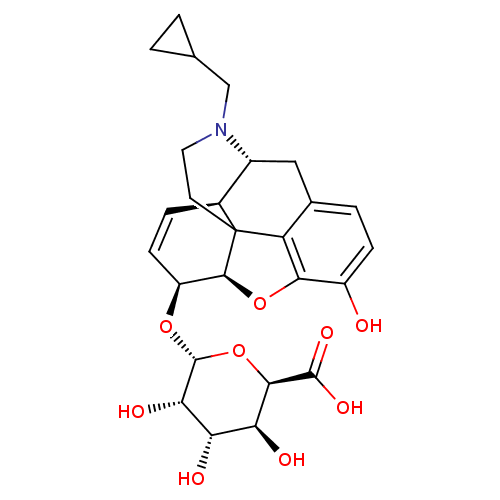 (CHEMBL2112840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125686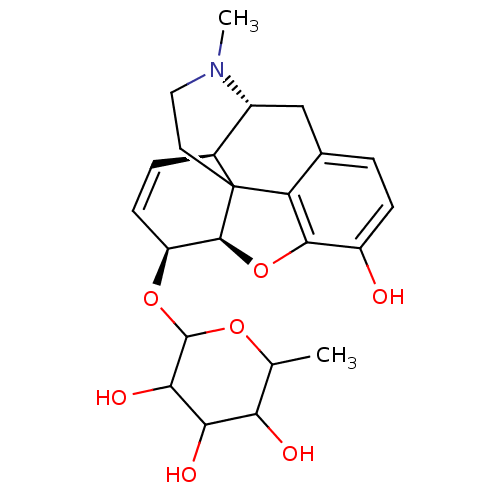 (2-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-12-oxa-4-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451820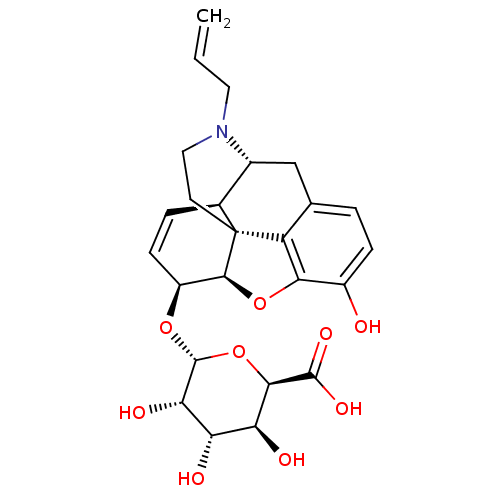 (CHEMBL3085267) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451826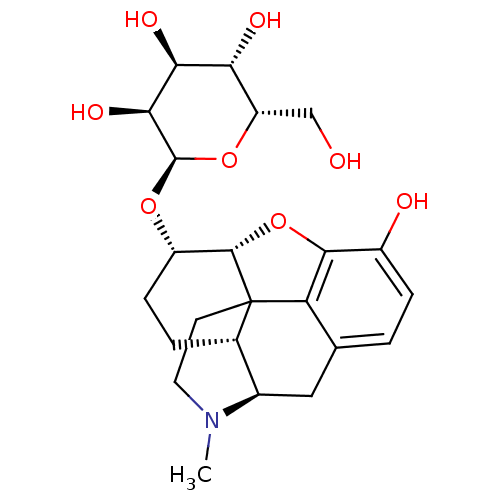 (CHEMBL2112797) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451822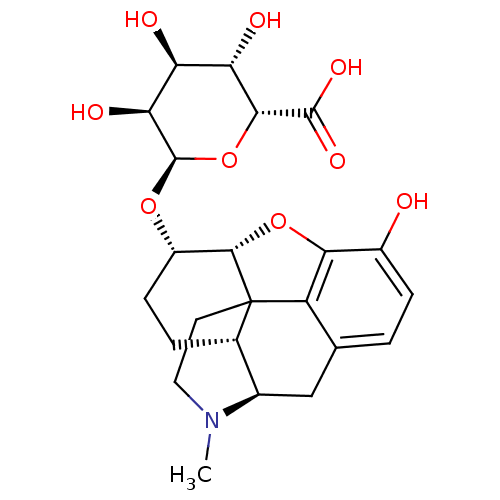 (CHEMBL2112839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451819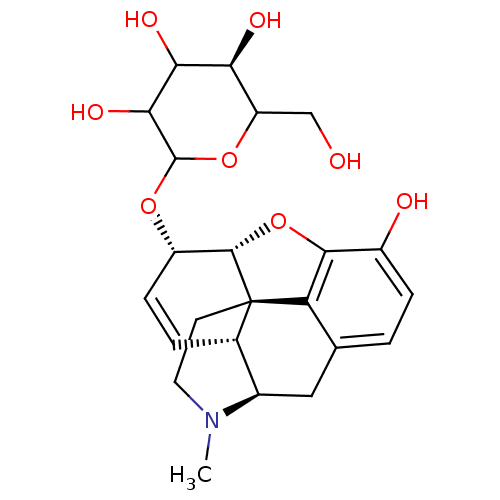 (CHEMBL2079659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125687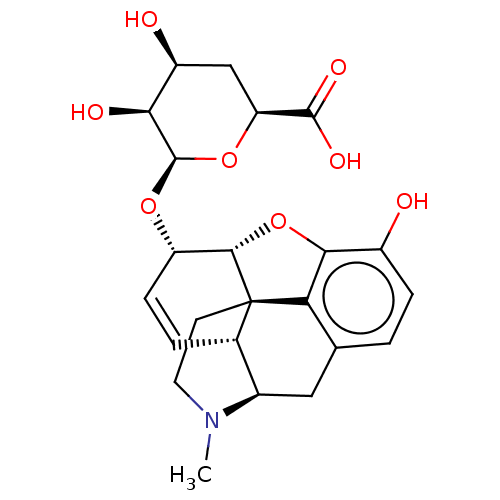 (2-[14-(6-carboxy-3,4,5-trihydroxytetrahydro-2H-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125689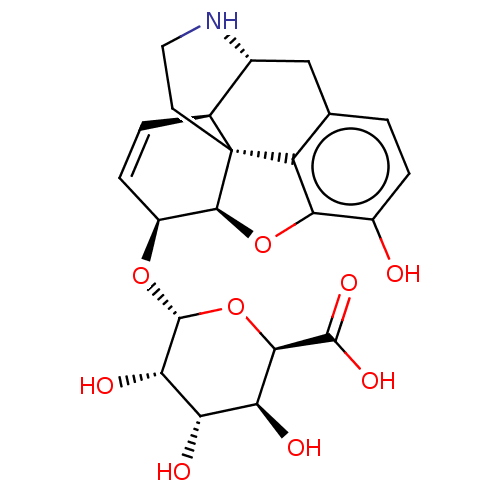 (6-[4-allyl-10-hydroxy-(5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451824 (CHEMBL2092968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125680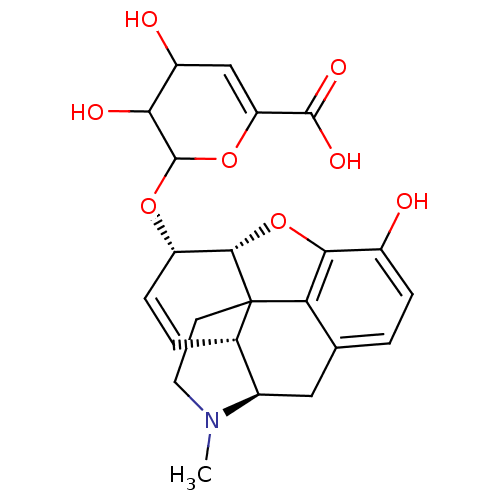 (3,4-dihydroxy-2-[10-hydroxy-4-methyl-(5R,13R,14S,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50370478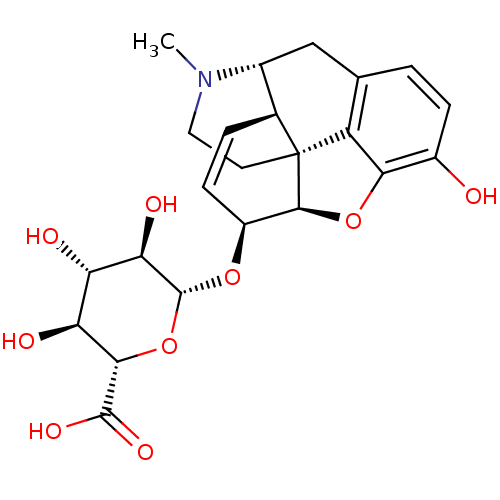 (MORPHINE-6-GLUCURONIDE | Morphine 6-Glucuronide(Mi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451825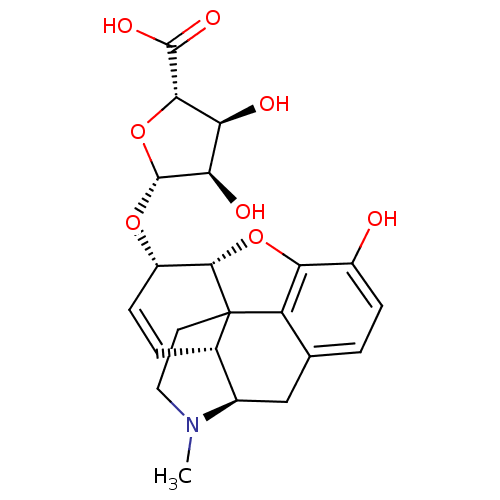 (CHEMBL2113393) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451826 (CHEMBL2112797) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125688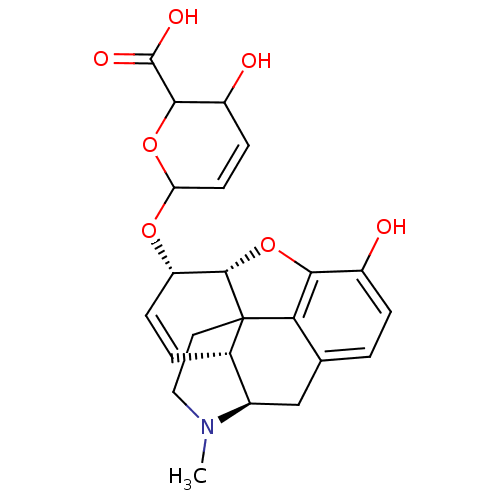 (3-hydroxy-6-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451819 (CHEMBL2079659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451823 (CHEMBL2112840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451824 (CHEMBL2092968) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451822 (CHEMBL2112839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125686 (2-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-12-oxa-4-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451820 (CHEMBL3085267) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50370478 (MORPHINE-6-GLUCURONIDE | Morphine 6-Glucuronide(Mi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451821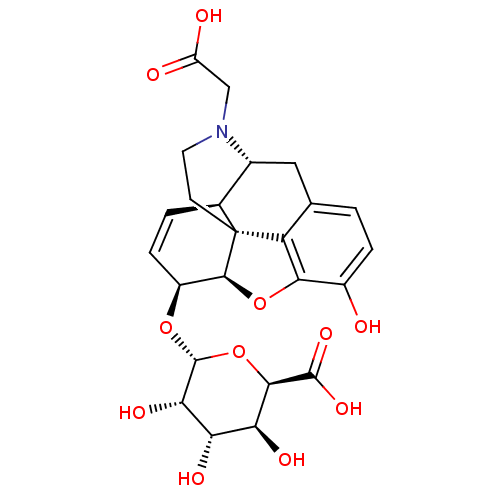 (CHEMBL3085275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125689 (6-[4-allyl-10-hydroxy-(5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451825 (CHEMBL2113393) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125680 (3,4-dihydroxy-2-[10-hydroxy-4-methyl-(5R,13R,14S,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125692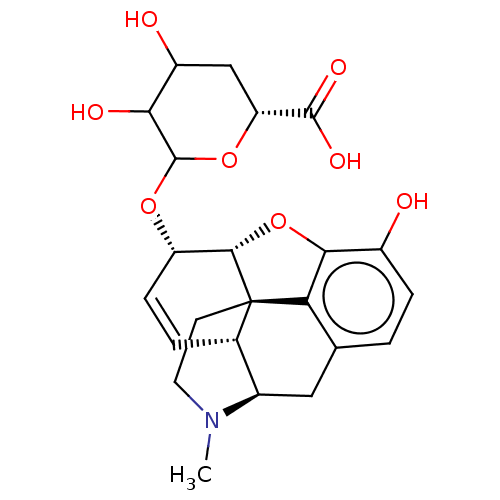 (3,4,5-trihydroxy-6-[10-hydroxy-(5R,13R,14S,17R)-12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125692 (3,4,5-trihydroxy-6-[10-hydroxy-(5R,13R,14S,17R)-12...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125684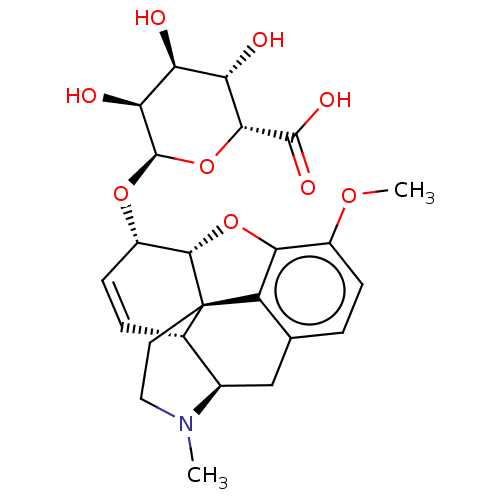 (3,4,5-trihydroxy-6-[10-methoxy-4-methyl-(5R,13R,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125690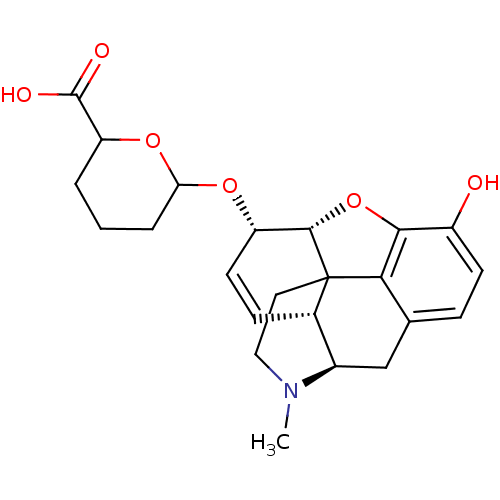 (6-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-12-oxa-4-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125690 (6-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-12-oxa-4-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125687 (2-[14-(6-carboxy-3,4,5-trihydroxytetrahydro-2H-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125688 (3-hydroxy-6-[10-hydroxy-4-methyl-(5R,13R,14S,17R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50451821 (CHEMBL3085275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125684 (3,4,5-trihydroxy-6-[10-methoxy-4-methyl-(5R,13R,14...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd Curated by ChEMBL | Assay Description mu-2 receptor binding affinity in rat brain by 3H [d-Ala2, (N-Me)Phe4, Gly5-ol] enkephalin displacement. | Bioorg Med Chem Lett 13: 1207-14 (2003) BindingDB Entry DOI: 10.7270/Q2251JQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50293788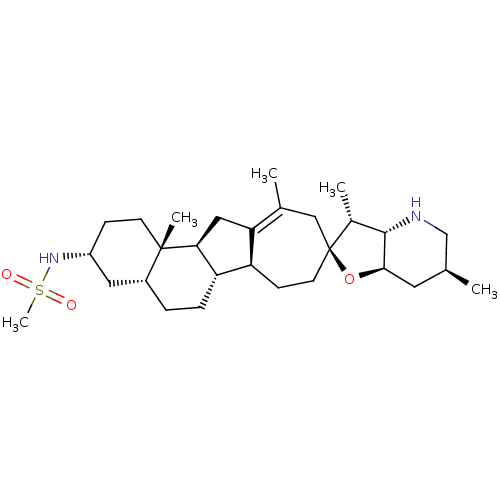 (CHEMBL538867 | N-((2S,3R,3aS,3'R,4a'R,6S,6a'R,6b'S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant SMO expressed in mouse C3H10T1/2 cells assessed as inhibition of association of BODIPY-cyclopamine | J Med Chem 52: 4400-18 (2009) Article DOI: 10.1021/jm900305z BindingDB Entry DOI: 10.7270/Q2CC10QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557895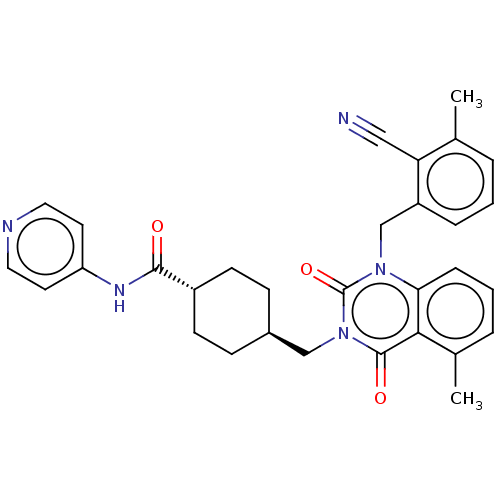 (CHEMBL4747523) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557896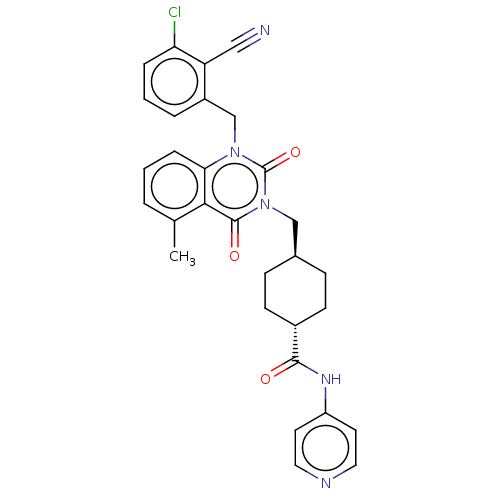 (CHEMBL4786379) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557880 (CHEMBL4794878) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557892 (CHEMBL4757303) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557894 (CHEMBL4759993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557885 (CHEMBL4788947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557884 (CHEMBL4759701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557882 (CHEMBL4754447) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557888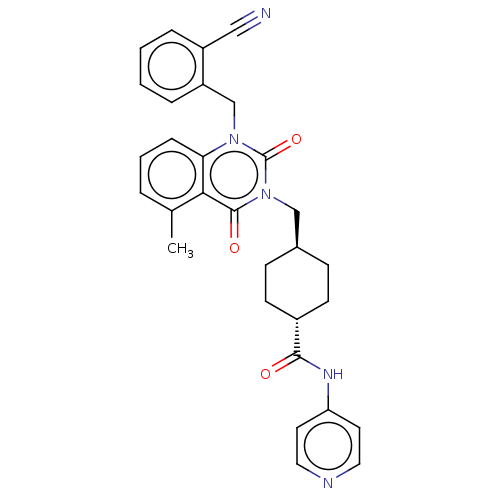 (CHEMBL4758600) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557881 (CHEMBL4799048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557897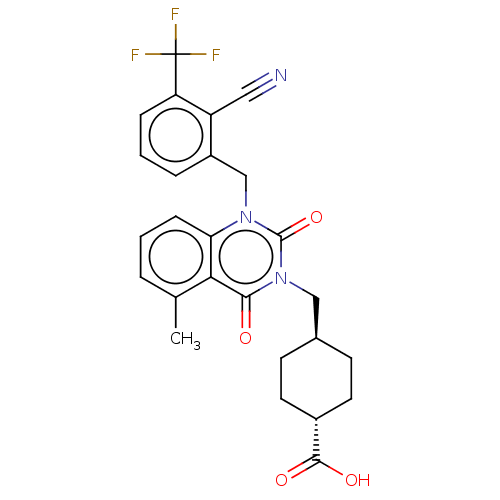 (CHEMBL4761298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557879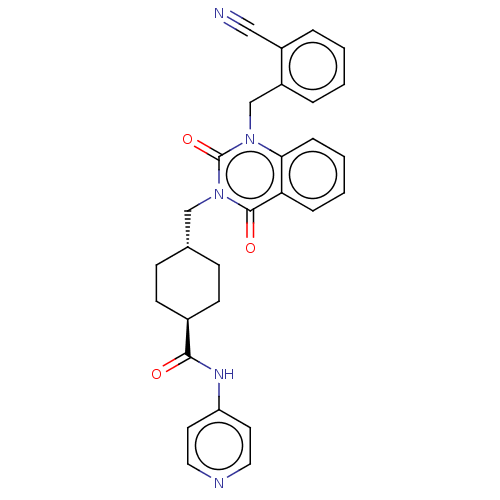 (CHEMBL4791639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50557883 (CHEMBL4798387) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNKS1 incubated for 1 to 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00160 BindingDB Entry DOI: 10.7270/Q2W099KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 202 total ) | Next | Last >> |