

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50495103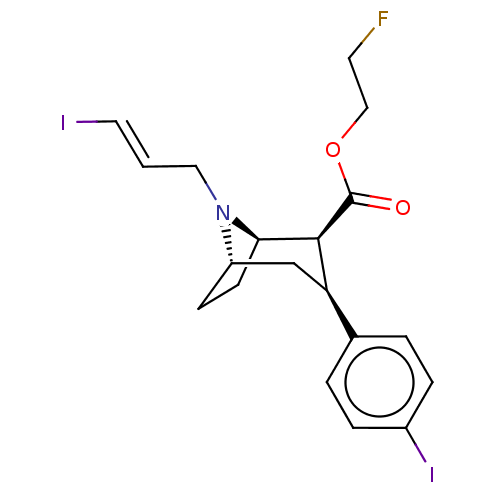 (CHEMBL3105243) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50495101 (CHEMBL1945251) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human SERT after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50495101 (CHEMBL1945251) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50495103 (CHEMBL3105243) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human SERT after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Binding affinity to DAT (unknown origin) | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50296782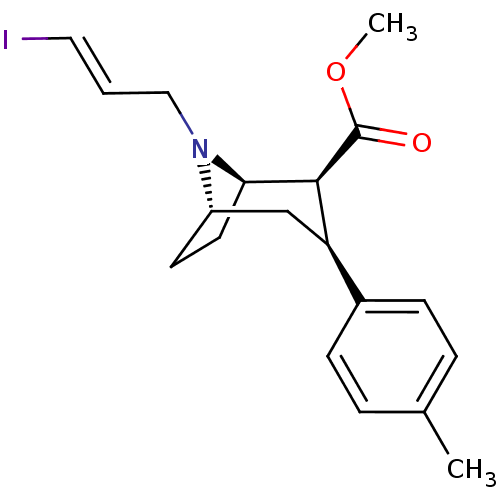 ((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Binding affinity to DAT (unknown origin) | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50495101 (CHEMBL1945251) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]Imipramin from human NET after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50283607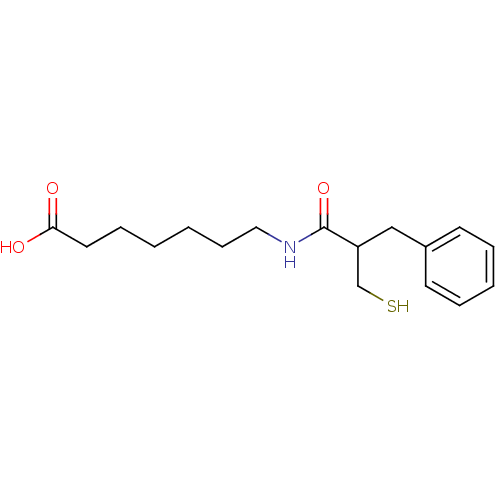 (7-(2-Mercaptomethyl-3-phenyl-propionylamino)-hepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Binding affinity to SERT (unknown origin) | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM22166 (3-(4-iodophenyl)tropane-2-carboxylic acid methyl e...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Binding affinity to NET (unknown origin) | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50495102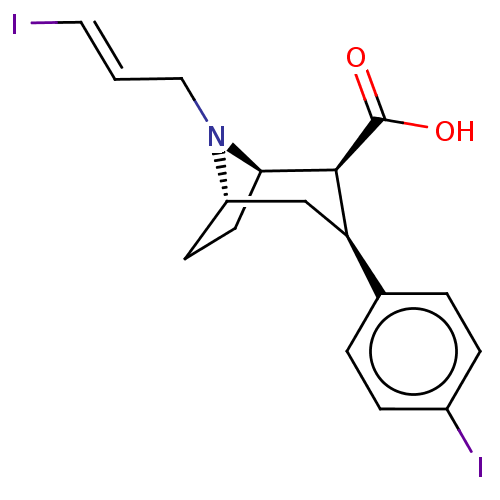 (CHEMBL3105242) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human DAT after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50495102 (CHEMBL3105242) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human SERT after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50495103 (CHEMBL3105243) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]Imipramin from human NET after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50296782 ((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Binding affinity to SERT (unknown origin) | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50495102 (CHEMBL3105242) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Displacement of [3H]Imipramin from human NET after 2 hrs by liquid scintillation counting analysis | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50296782 ((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna Curated by ChEMBL | Assay Description Binding affinity to NET (unknown origin) | Bioorg Med Chem 21: 7562-9 (2013) Article DOI: 10.1016/j.bmc.2013.10.046 BindingDB Entry DOI: 10.7270/Q27P92BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107813 ((S)-methyl 5-(6-methyl-4'-(trifluoromethyl)bipheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107808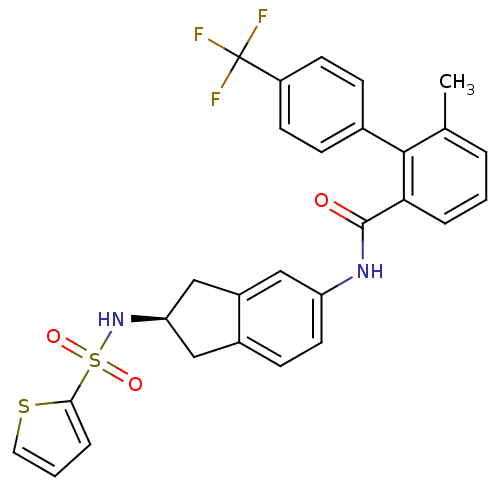 (6-Methyl-4'-trifluoromethyl-biphenyl-2-carboxylic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM105283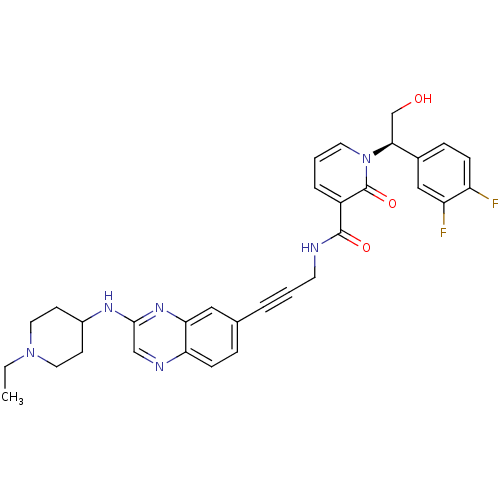 (US8575203, I-50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The activity of the compounds according to the invention on the kinase PDK1 which inhibits the signal transduction pathway is determined in an in vit... | US Patent US8575203 (2013) BindingDB Entry DOI: 10.7270/Q2HD7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50581660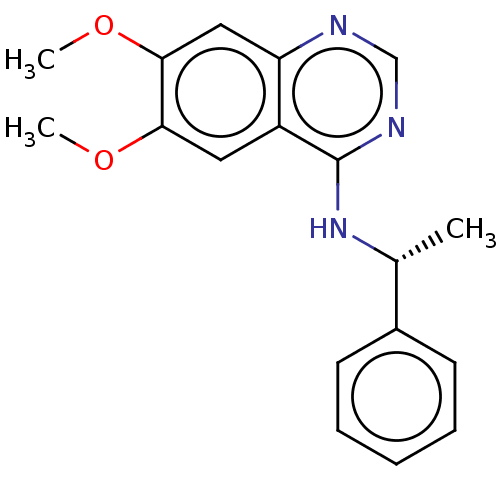 (CHEMBL1714813) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01949 BindingDB Entry DOI: 10.7270/Q2154MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM105308 (US8575203, I-75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The activity of the compounds according to the invention on the kinase PDK1 which inhibits the signal transduction pathway is determined in an in vit... | US Patent US8575203 (2013) BindingDB Entry DOI: 10.7270/Q2HD7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139785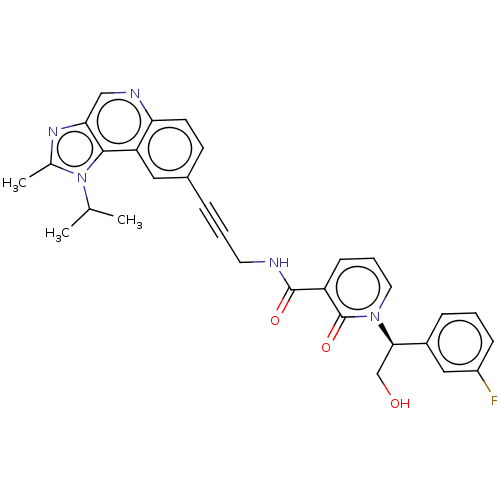 (US8895581, II-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139796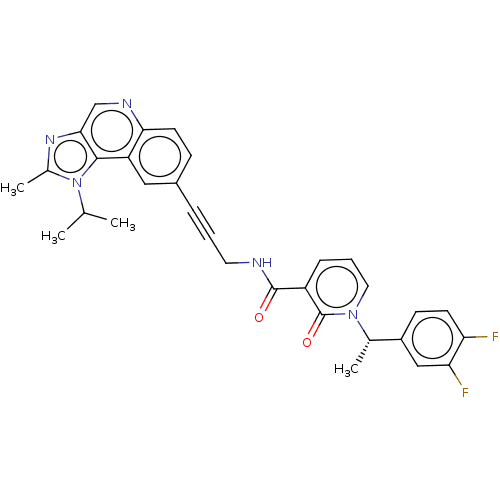 (US8895581, II-17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139798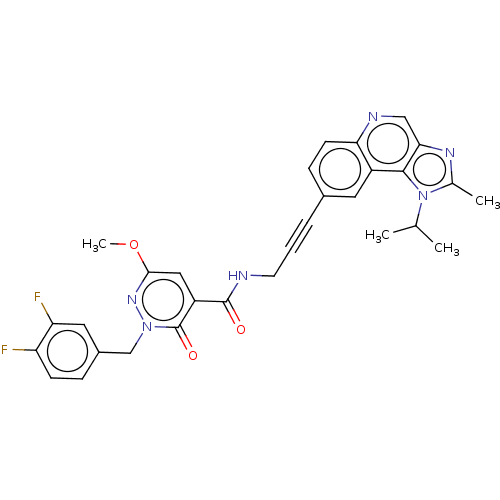 (US8895581, II-19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139819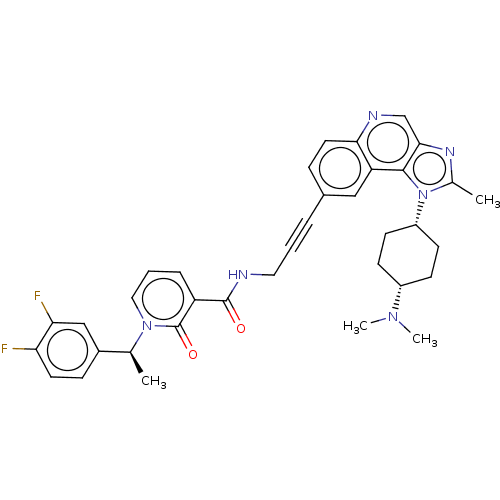 (US8895581, III-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139828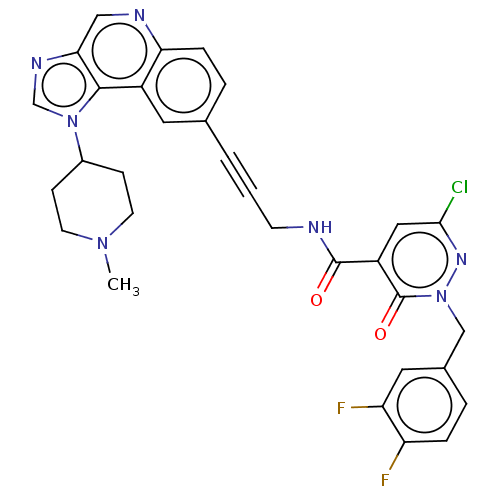 (US8895581, III-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139837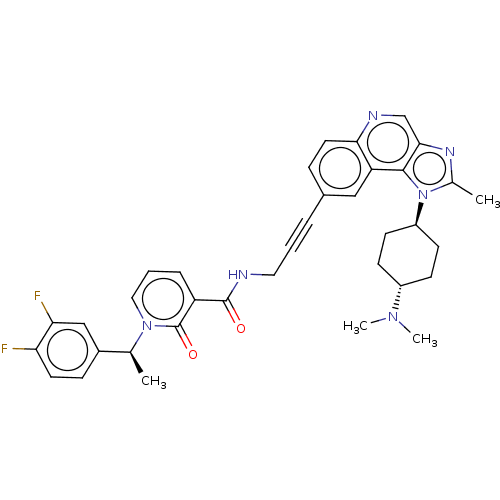 (US8895581, III-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139840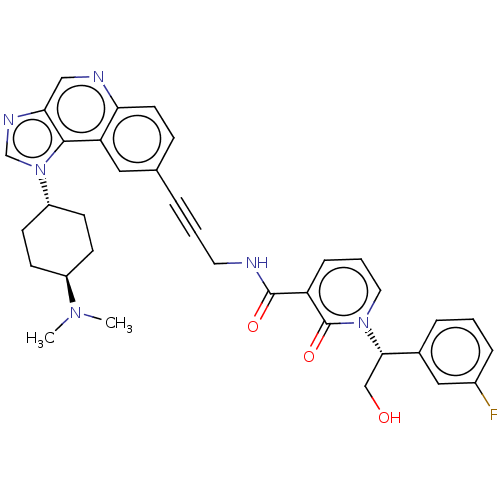 (US8895581, III-26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139845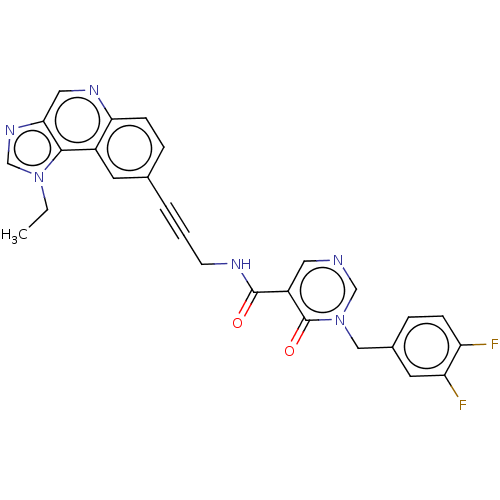 (US8895581, III-31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM139765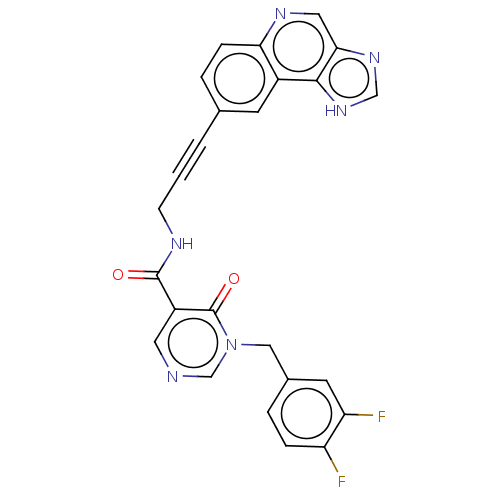 (US8895581, I-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Recombinant human PDK1 enzyme (aa 52-556) linked at its N-terminal end to His6 is isolated from baculovirus-infected insect cells. Purified enzyme ma... | US Patent US8895581 (2014) BindingDB Entry DOI: 10.7270/Q2HM5742 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM105303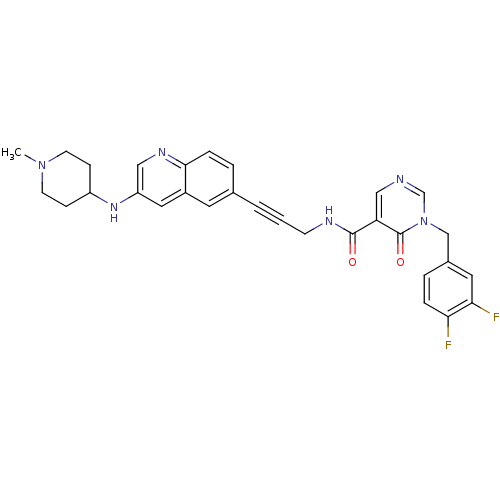 (US8575203, I-70) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The activity of the compounds according to the invention on the kinase PDK1 which inhibits the signal transduction pathway is determined in an in vit... | US Patent US8575203 (2013) BindingDB Entry DOI: 10.7270/Q2HD7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12D]/Son of sevenless homolog 1 [564-1049] () | BDBM472647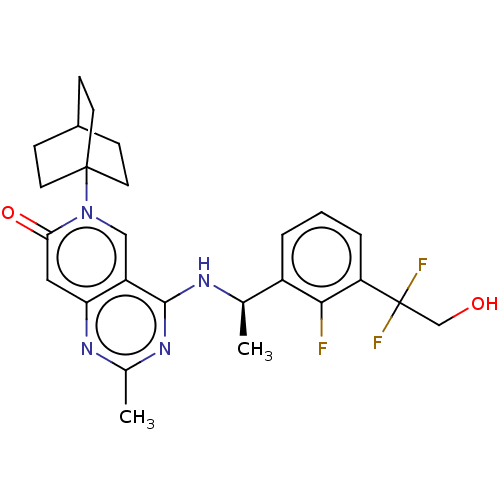 (US10829487, Example I-130) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds are diluted to a final start concentration of 100 μM and are tested in duplicate. Assay-ready plates (ARPs) are generated using an Acc... | US Patent US10829487 (2020) BindingDB Entry DOI: 10.7270/Q2D50R22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [1-169,G12D]/Son of sevenless homolog 1 [564-1049] () | BDBM472582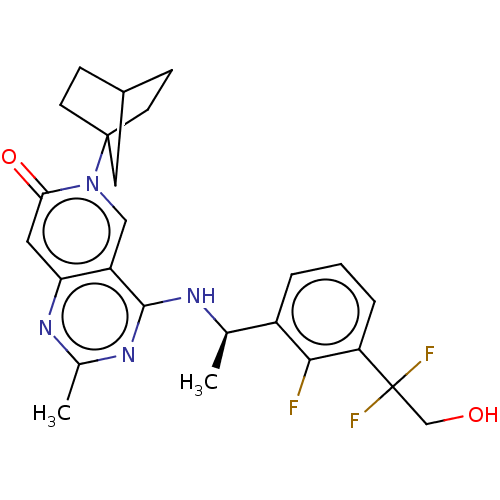 (US10829487, Example I-61) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds are diluted to a final start concentration of 100 μM and are tested in duplicate. Assay-ready plates (ARPs) are generated using an Acc... | US Patent US10829487 (2020) BindingDB Entry DOI: 10.7270/Q2D50R22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107774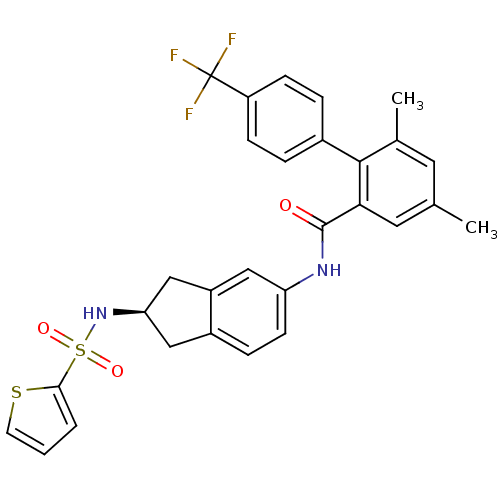 (4,6-Dimethyl-4'-trifluoromethyl-biphenyl-2-carboxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107809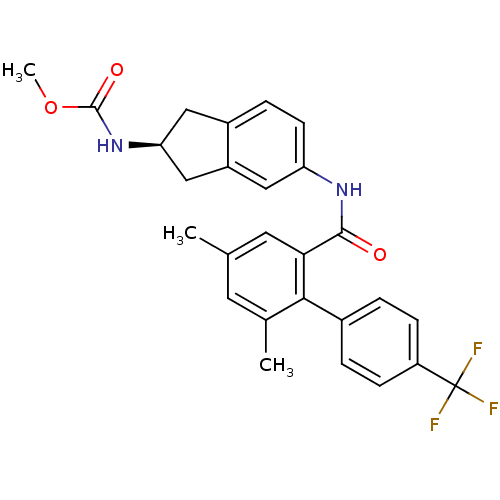 (CHEMBL143284 | {5-[(4,6-Dimethyl-4'-trifluoromethy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048318 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107797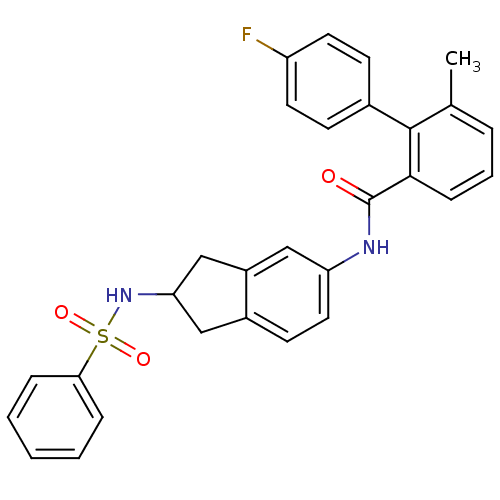 (4'-Fluoro-6-methyl-biphenyl-2-carboxylic acid (2-b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107790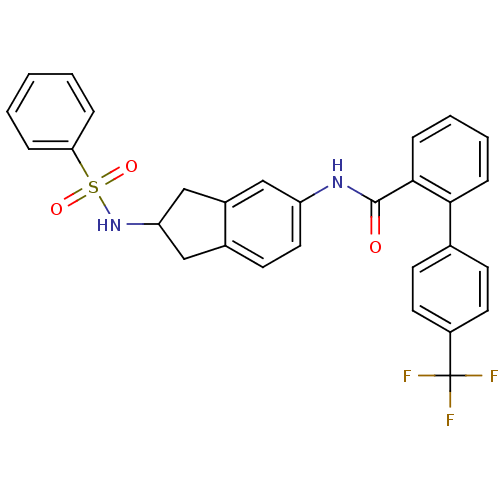 (4'-Trifluoromethyl-biphenyl-2-carboxylic acid (2-b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107772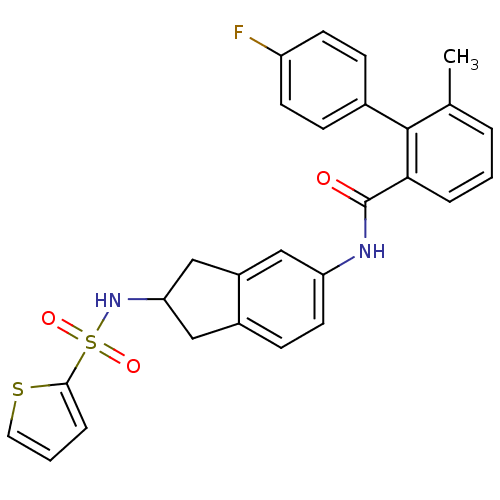 (4'-Fluoro-6-methyl-biphenyl-2-carboxylic acid [2-(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048320 ((S)-2-{[1-((S)-2-Mercapto-3-methyl-butyrylamino)-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048312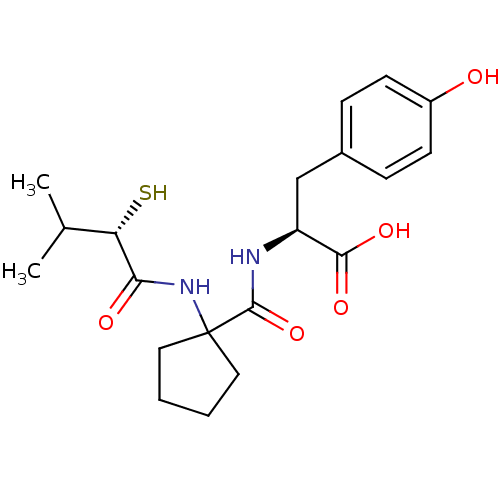 ((S)-3-(4-Hydroxy-phenyl)-2-{[1-((S)-2-mercapto-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107803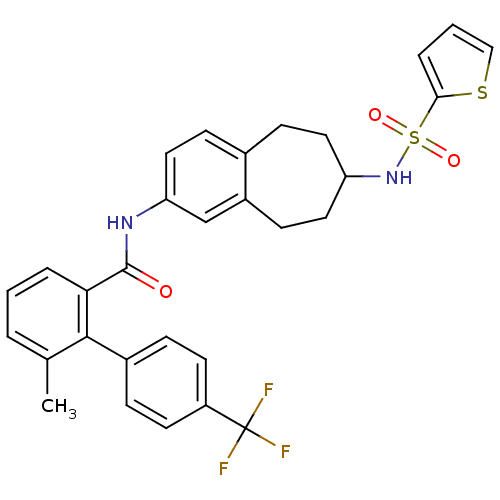 (6-Methyl-4'-trifluoromethyl-biphenyl-2-carboxylic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107804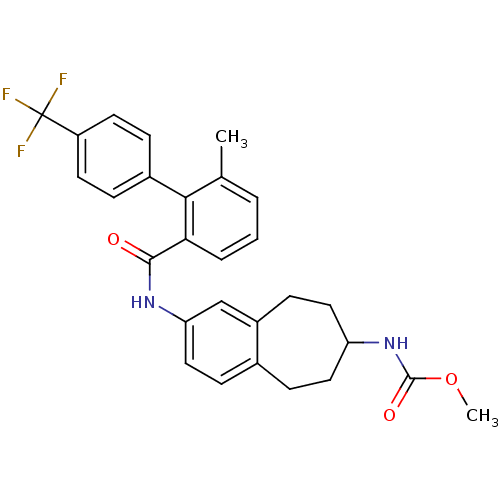 (CHEMBL142975 | {2-[(6-Methyl-4'-trifluoromethyl-bi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107787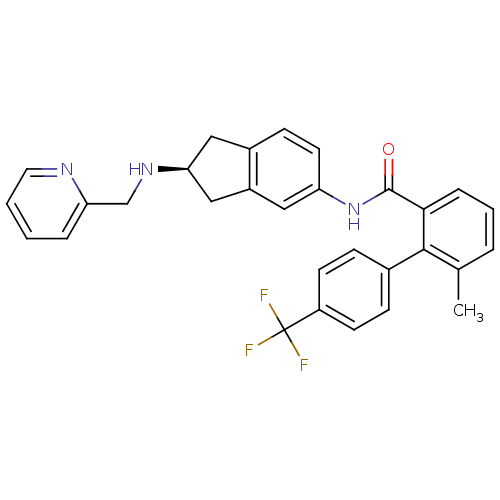 ((S)-6-methyl-N-(2-(pyridin-2-ylmethylamino)-2,3-di...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107775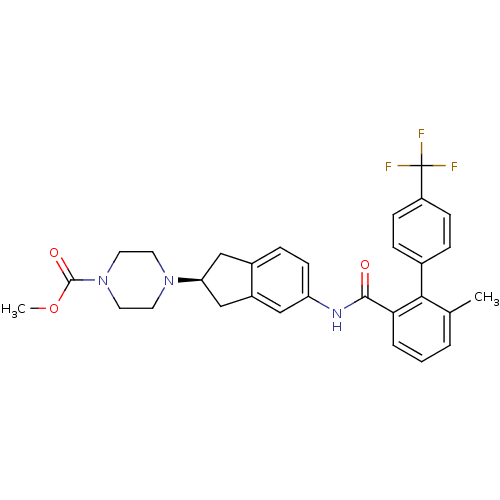 (4-{5-[(6-Methyl-4'-trifluoromethyl-biphenyl-2-carb...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048304 ((S)-3-(4-Fluoro-phenyl)-2-{[1-((S)-2-mercapto-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Evaluation of in vitro inhibitory activity against Neutral endopeptidase | J Med Chem 38: 5023-30 (1996) BindingDB Entry DOI: 10.7270/Q26D5TMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107812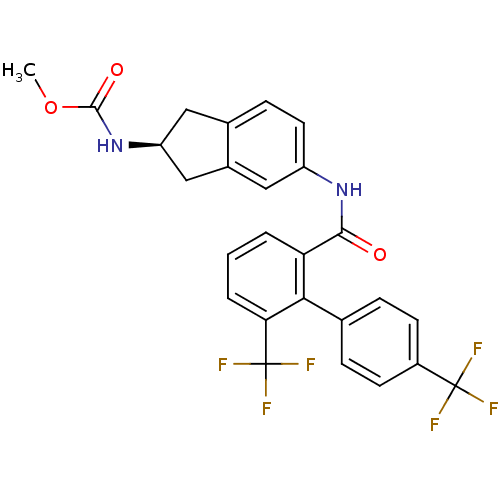 (CHEMBL142928 | {5-[(6,4'-Bis-trifluoromethyl-biphe...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107810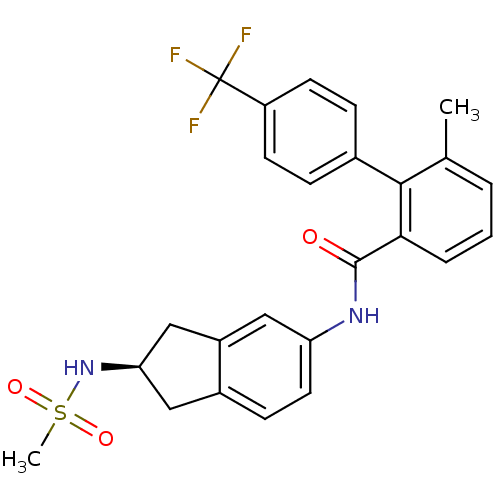 (6-Methyl-4'-trifluoromethyl-biphenyl-2-carboxylic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107815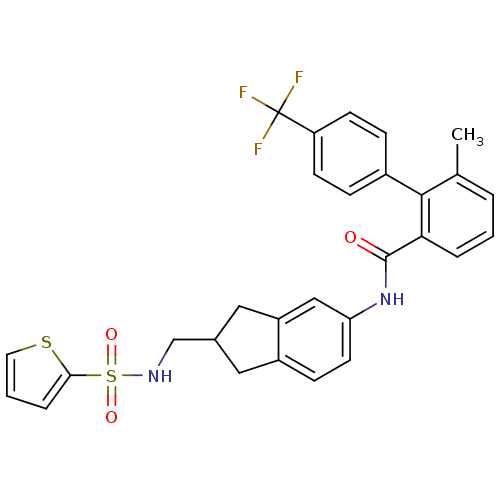 (6-Methyl-4'-trifluoromethyl-biphenyl-2-carboxylic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1355 total ) | Next | Last >> |