 Found 153 hits with Last Name = 'foreman' and Initial = 'mm'
Found 153 hits with Last Name = 'foreman' and Initial = 'mm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247
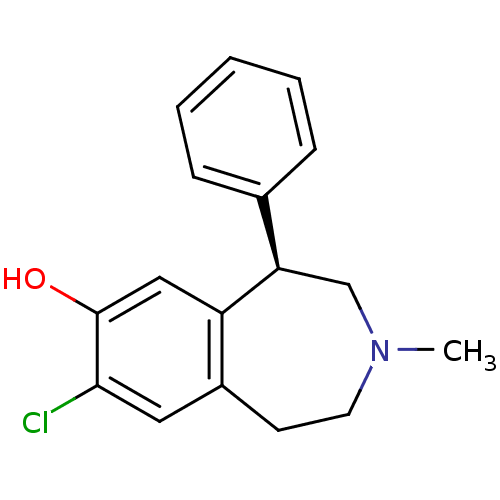
(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM82479
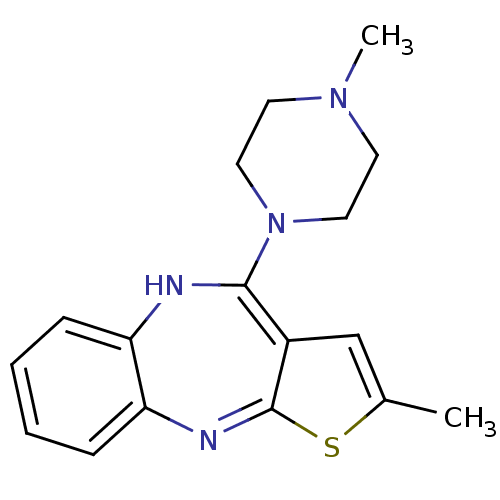
(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50020712
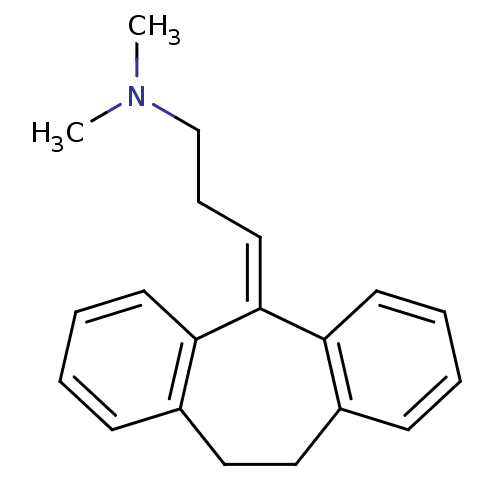
(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50020712

(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50020712

(10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]\[#6]=[#6]-1/c2ccccc2-[#6]-[#6]-c2ccccc-12 Show InChI InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50094670
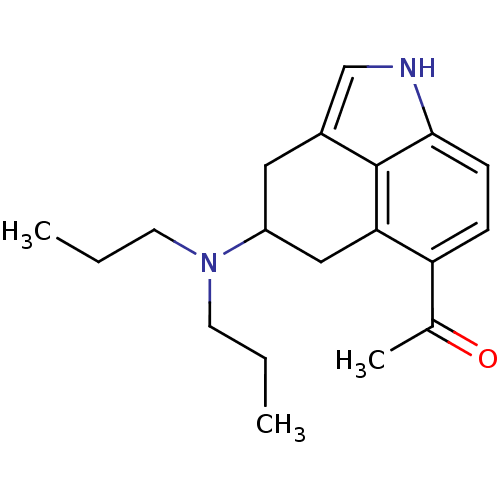
(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 66.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Schizophr Res 37: 107-22 (1999)
Article DOI: 10.1016/s0920-9964(98)00146-7
BindingDB Entry DOI: 10.7270/Q23F4N5N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049319

(CHEMBL42407 | Lanepitant | N-[(R)-1-{[Acetyl-(2-me...)Show SMILES COc1ccccc1CN(C[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CN1CCC(CC1)N1CCCCC1)C(C)=O Show InChI InChI=1S/C33H45N5O3/c1-25(39)38(22-26-10-4-7-13-32(26)41-2)23-28(20-27-21-34-31-12-6-5-11-30(27)31)35-33(40)24-36-18-14-29(15-19-36)37-16-8-3-9-17-37/h4-7,10-13,21,28-29,34H,3,8-9,14-20,22-24H2,1-2H3,(H,35,40)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049315
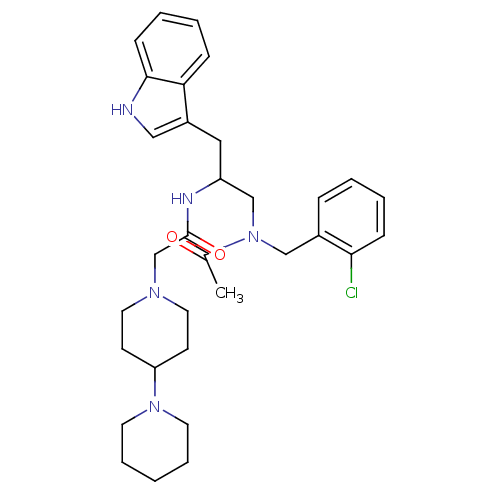
(CHEMBL423623 | N-[1-{[Acetyl-(2-chloro-benzyl)-ami...)Show SMILES CC(=O)N(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCC(CC1)N1CCCCC1)Cc1ccccc1Cl Show InChI InChI=1S/C32H42ClN5O2/c1-24(39)38(21-25-9-3-5-11-30(25)33)22-27(19-26-20-34-31-12-6-4-10-29(26)31)35-32(40)23-36-17-13-28(14-18-36)37-15-7-2-8-16-37/h3-6,9-12,20,27-28,34H,2,7-8,13-19,21-23H2,1H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049309
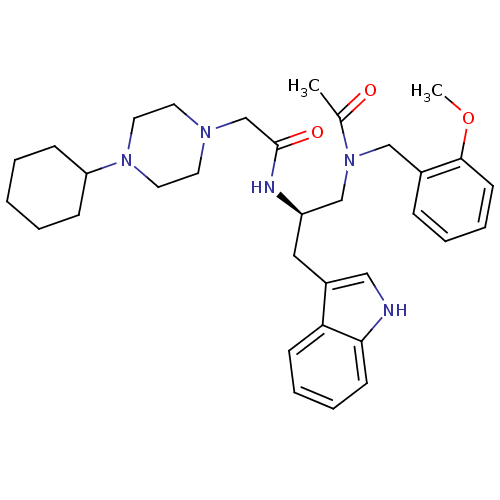
(CHEMBL348976 | N-[(R)-1-{[Acetyl-(2-methoxy-benzyl...)Show SMILES COc1ccccc1CN(C[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(CC1)C1CCCCC1)C(C)=O Show InChI InChI=1S/C33H45N5O3/c1-25(39)38(22-26-10-6-9-15-32(26)41-2)23-28(20-27-21-34-31-14-8-7-13-30(27)31)35-33(40)24-36-16-18-37(19-17-36)29-11-4-3-5-12-29/h6-10,13-15,21,28-29,34H,3-5,11-12,16-20,22-24H2,1-2H3,(H,35,40)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049328
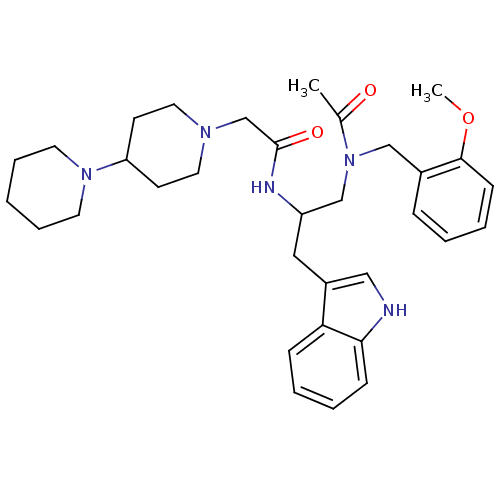
(CHEMBL134500 | LY-303870 | N-[1-{[Acetyl-(2-methox...)Show SMILES COc1ccccc1CN(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCC(CC1)N1CCCCC1)C(C)=O Show InChI InChI=1S/C33H45N5O3/c1-25(39)38(22-26-10-4-7-13-32(26)41-2)23-28(20-27-21-34-31-12-6-5-11-30(27)31)35-33(40)24-36-18-14-29(15-19-36)37-16-8-3-9-17-37/h4-7,10-13,21,28-29,34H,3,8-9,14-20,22-24H2,1-2H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049320
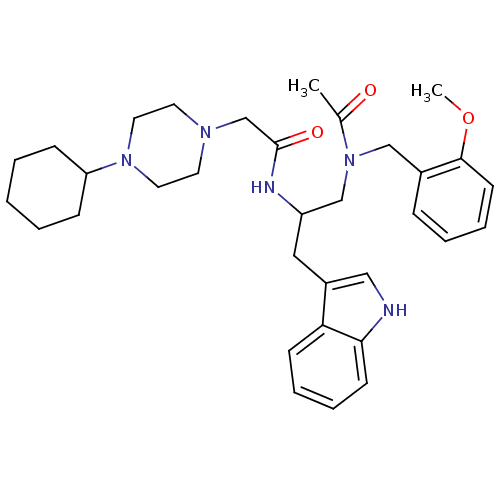
(CHEMBL162337 | N-[1-{[Acetyl-(2-methoxy-benzyl)-am...)Show SMILES COc1ccccc1CN(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(CC1)C1CCCCC1)C(C)=O Show InChI InChI=1S/C33H45N5O3/c1-25(39)38(22-26-10-6-9-15-32(26)41-2)23-28(20-27-21-34-31-14-8-7-13-30(27)31)35-33(40)24-36-16-18-37(19-17-36)29-11-4-3-5-12-29/h6-10,13-15,21,28-29,34H,3-5,11-12,16-20,22-24H2,1-2H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50000040
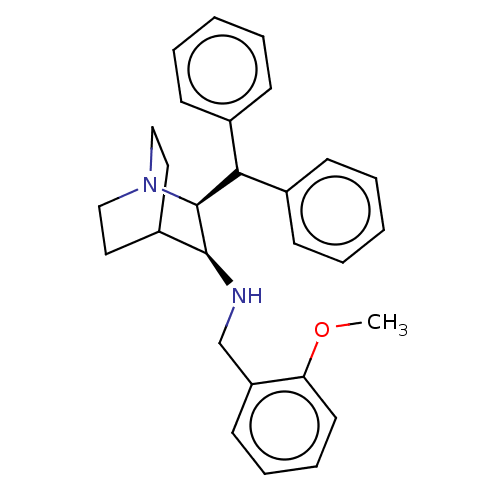
(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Tachykinin receptor 1 in guinea pig brain using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Tachykinin receptor 1 in guinea pig brain using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049342

(CHEMBL352176 | N-[1-{[Formyl-(2-methoxy-benzyl)-am...)Show SMILES COc1ccccc1CN(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(CC1)c1ccccc1)C=O Show InChI InChI=1S/C32H37N5O3/c1-40-31-14-8-5-9-25(31)21-36(24-38)22-27(19-26-20-33-30-13-7-6-12-29(26)30)34-32(39)23-35-15-17-37(18-16-35)28-10-3-2-4-11-28/h2-14,20,24,27,33H,15-19,21-23H2,1H3,(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049341
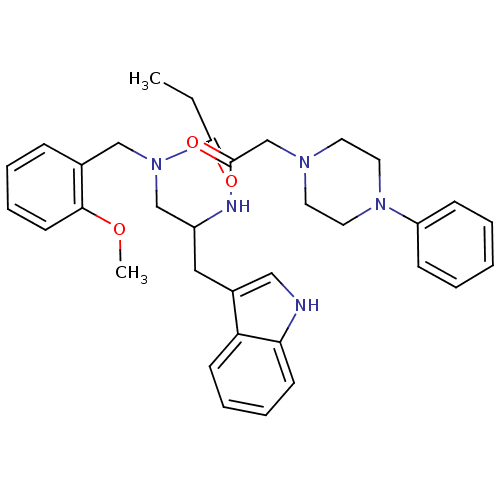
(CHEMBL350381 | N-{3-(1H-Indol-3-yl)-2-[2-(4-phenyl...)Show SMILES CCC(=O)N(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(CC1)c1ccccc1)Cc1ccccc1OC Show InChI InChI=1S/C34H41N5O3/c1-3-34(41)39(23-26-11-7-10-16-32(26)42-2)24-28(21-27-22-35-31-15-9-8-14-30(27)31)36-33(40)25-37-17-19-38(20-18-37)29-12-5-4-6-13-29/h4-16,22,28,35H,3,17-21,23-25H2,1-2H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
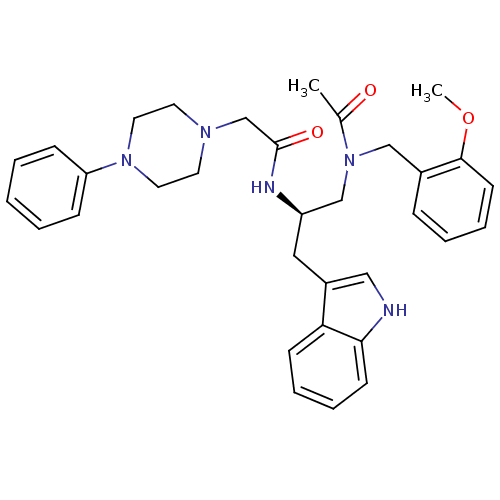
(Homo sapiens (Human)) | BDBM50049318
(CHEMBL351274 | N-[(R)-1-{[Acetyl-(2-methoxy-benzyl...)Show SMILES COc1ccccc1CN(C[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(CC1)c1ccccc1)C(C)=O Show InChI InChI=1S/C33H39N5O3/c1-25(39)38(22-26-10-6-9-15-32(26)41-2)23-28(20-27-21-34-31-14-8-7-13-30(27)31)35-33(40)24-36-16-18-37(19-17-36)29-11-4-3-5-12-29/h3-15,21,28,34H,16-20,22-24H2,1-2H3,(H,35,40)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50049338

(CHEMBL353093 | N-[1-{[Acetyl-(2-methoxy-benzyl)-am...)Show SMILES COc1ccccc1CN(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(Cc2ccccc2)CC1)C(C)=O Show InChI InChI=1S/C34H41N5O3/c1-26(40)39(23-28-12-6-9-15-33(28)42-2)24-30(20-29-21-35-32-14-8-7-13-31(29)32)36-34(41)25-38-18-16-37(17-19-38)22-27-10-4-3-5-11-27/h3-15,21,30,35H,16-20,22-25H2,1-2H3,(H,36,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Tachykinin receptor 1 in guinea pig brain using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049338

(CHEMBL353093 | N-[1-{[Acetyl-(2-methoxy-benzyl)-am...)Show SMILES COc1ccccc1CN(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(Cc2ccccc2)CC1)C(C)=O Show InChI InChI=1S/C34H41N5O3/c1-26(40)39(23-28-12-6-9-15-33(28)42-2)24-30(20-29-21-35-32-14-8-7-13-31(29)32)36-34(41)25-38-18-16-37(17-19-38)22-27-10-4-3-5-11-27/h3-15,21,30,35H,16-20,22-25H2,1-2H3,(H,36,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Tachykinin receptor 1 in guinea pig brain using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
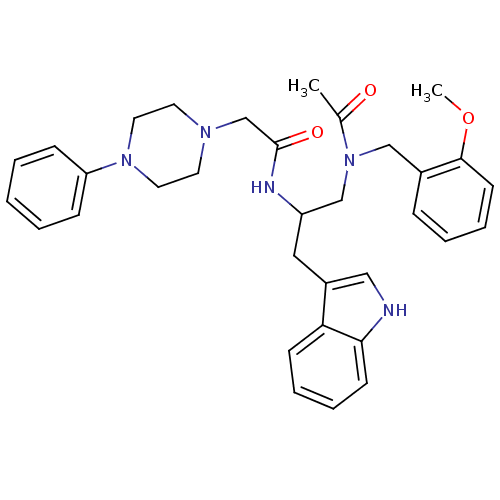
(Homo sapiens (Human)) | BDBM50049356
(CHEMBL554713 | N-[1-{[Acetyl-(2-methoxy-benzyl)-am...)Show SMILES COc1ccccc1CN(CC(Cc1c[nH]c2ccccc12)NC(=O)CN1CCN(CC1)c1ccccc1)C(C)=O Show InChI InChI=1S/C33H39N5O3/c1-25(39)38(22-26-10-6-9-15-32(26)41-2)23-28(20-27-21-34-31-14-8-7-13-30(27)31)35-33(40)24-36-16-18-37(19-17-36)29-11-4-3-5-12-29/h3-15,21,28,34H,16-20,22-24H2,1-2H3,(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049352
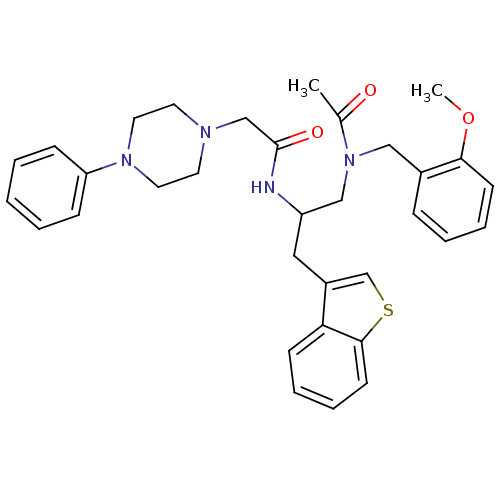
(CHEMBL165007 | N-(1-{[Acetyl-(2-methoxy-benzyl)-am...)Show SMILES COc1ccccc1CN(CC(Cc1csc2ccccc12)NC(=O)CN1CCN(CC1)c1ccccc1)C(C)=O Show InChI InChI=1S/C33H38N4O3S/c1-25(38)37(21-26-10-6-8-14-31(26)40-2)22-28(20-27-24-41-32-15-9-7-13-30(27)32)34-33(39)23-35-16-18-36(19-17-35)29-11-4-3-5-12-29/h3-15,24,28H,16-23H2,1-2H3,(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tachykinin receptor 1 in human IM-9 cells using [125I]-labeled Boltan-Hunter substance P as radioligand |
J Med Chem 39: 736-48 (1996)
Article DOI: 10.1021/jm950616c
BindingDB Entry DOI: 10.7270/Q20864C7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data