

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104934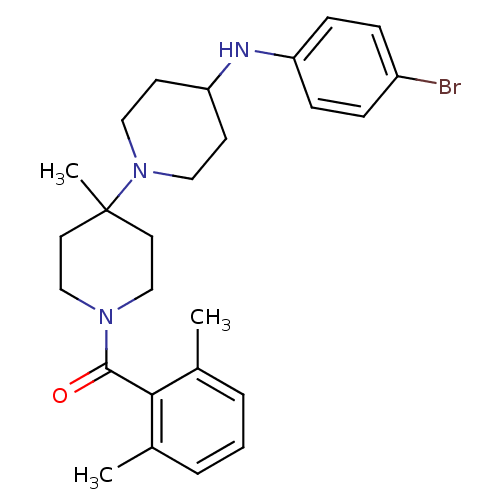 (CHEMBL292625 | [4-(4-Bromo-phenylamino)-4'-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143734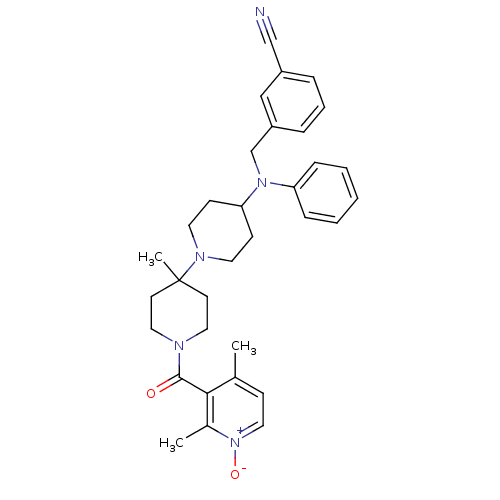 (3-({[1'-(2,4-Dimethyl-1-oxy-pyridine-3-carbonyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50047259 (4-(1-(1H-imidazol-1-yl)vinyl)benzonitrile | 4-(1-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human placental Cytochrome P450 19A1 | J Med Chem 36: 1393-400 (1993) BindingDB Entry DOI: 10.7270/Q2ZK5FR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143736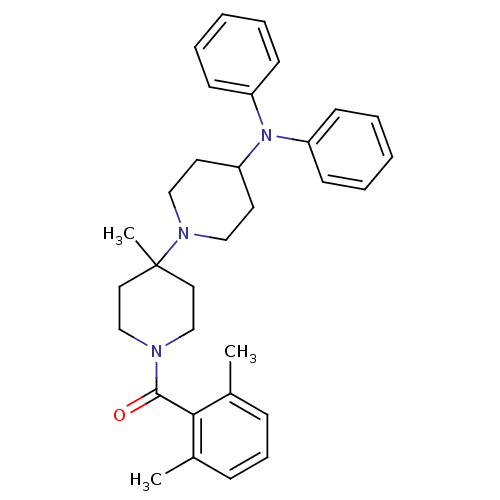 ((2,6-Dimethyl-phenyl)-(4-diphenylamino-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143762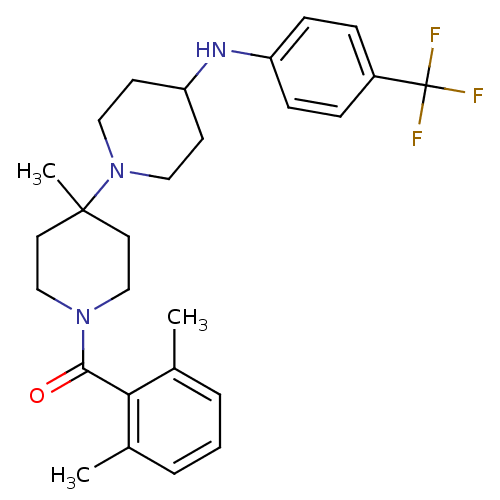 ((2,6-Dimethyl-phenyl)-[4'-methyl-4-(4-trifluoromet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143756 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-diphenylamino-4'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143762 ((2,6-Dimethyl-phenyl)-[4'-methyl-4-(4-trifluoromet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143757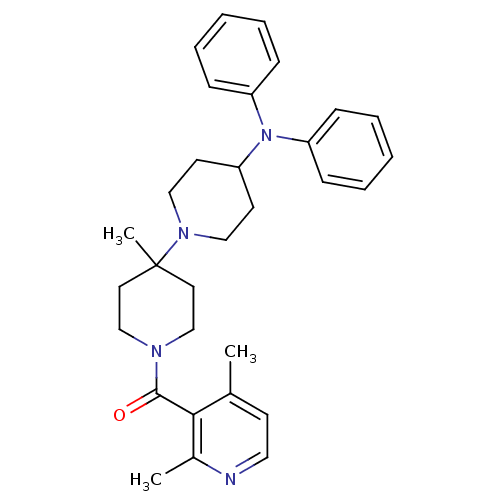 ((2,4-Dimethyl-pyridin-3-yl)-(4-diphenylamino-4'-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143740 ((4-(4-(benzyl(phenyl)amino)piperidin-1-yl)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143761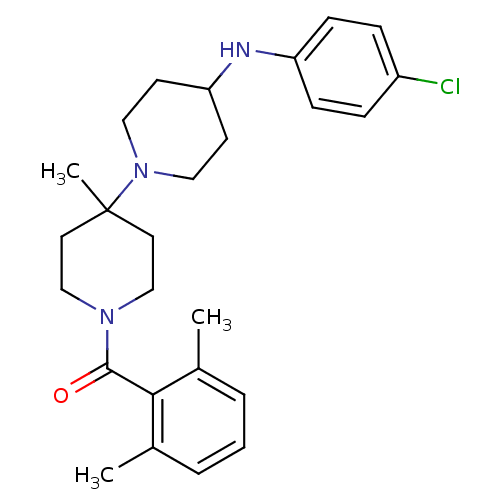 (CHEMBL61466 | [4-(4-Chloro-phenylamino)-4'-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743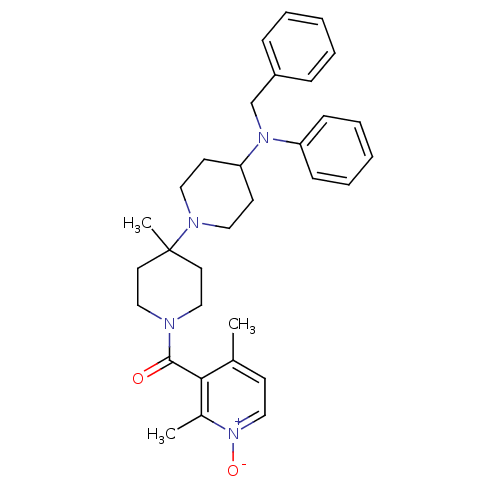 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104934 (CHEMBL292625 | [4-(4-Bromo-phenylamino)-4'-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143756 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-diphenylamino-4'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143757 ((2,4-Dimethyl-pyridin-3-yl)-(4-diphenylamino-4'-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143745 (CHEMBL60225 | {4-[Benzyl-(4-bromo-phenyl)-amino]-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143735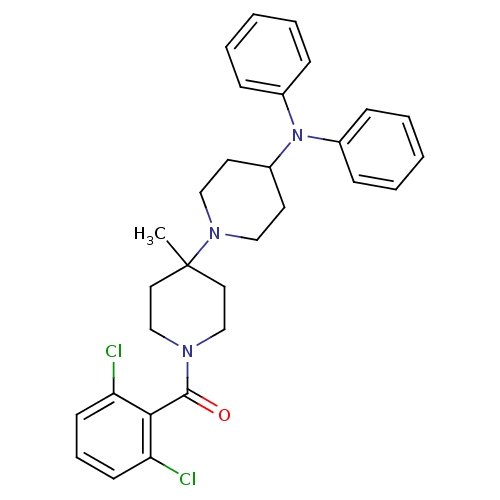 ((2,6-Dichloro-phenyl)-(4-diphenylamino-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143761 (CHEMBL61466 | [4-(4-Chloro-phenylamino)-4'-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143736 ((2,6-Dimethyl-phenyl)-(4-diphenylamino-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143747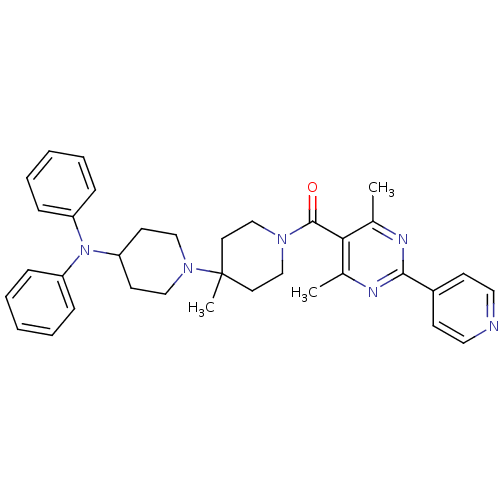 ((4,6-Dimethyl-2-pyridin-4-yl-pyrimidin-5-yl)-(4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314628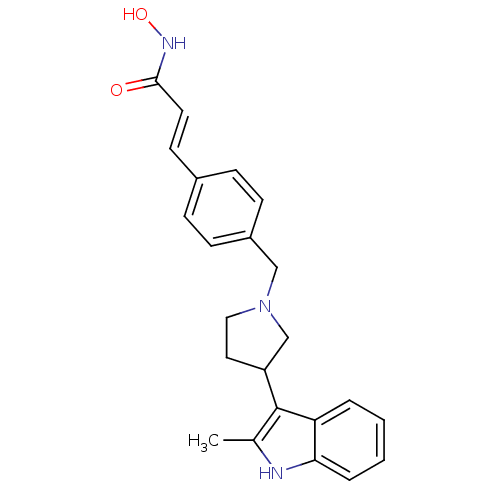 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143734 (3-({[1'-(2,4-Dimethyl-1-oxy-pyridine-3-carbonyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50047261 (5-(1H-imidazol-1-yl)-7,8-dihydronaphthalene-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human placental Cytochrome P450 19A1 | J Med Chem 36: 1393-400 (1993) BindingDB Entry DOI: 10.7270/Q2ZK5FR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143741 ((4,6-dimethyl-1-oxy-pyrimidin-5-yl)-(4-diphenylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314637 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314630 ((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50066269 (CHEMBL3401541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human renin by fluorescence based FRET assay in presence of plasma | Bioorg Med Chem Lett 25: 1782-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.039 BindingDB Entry DOI: 10.7270/Q2Z039TX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143740 ((4-(4-(benzyl(phenyl)amino)piperidin-1-yl)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143764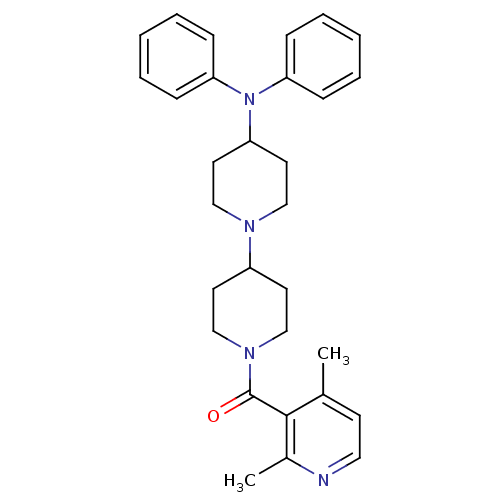 ((2,4-Dimethyl-pyridin-3-yl)-(4-diphenylamino-[1,4'...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9920 ((S)Fadrozole | 4-[(5S)-5,6,7,8-tetrahydroimidazo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human placental Cytochrome P450 19A1 | J Med Chem 36: 1393-400 (1993) BindingDB Entry DOI: 10.7270/Q2ZK5FR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143750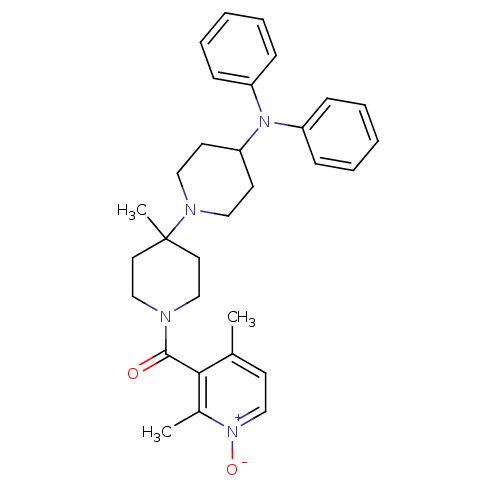 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-diphenylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143750 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-diphenylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143735 ((2,6-Dichloro-phenyl)-(4-diphenylamino-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50047260 (4,4'-((1H-imidazol-1-yl)methylene)dibenzonitrile |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human placental Cytochrome P450 19A1 | J Med Chem 36: 1393-400 (1993) BindingDB Entry DOI: 10.7270/Q2ZK5FR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143754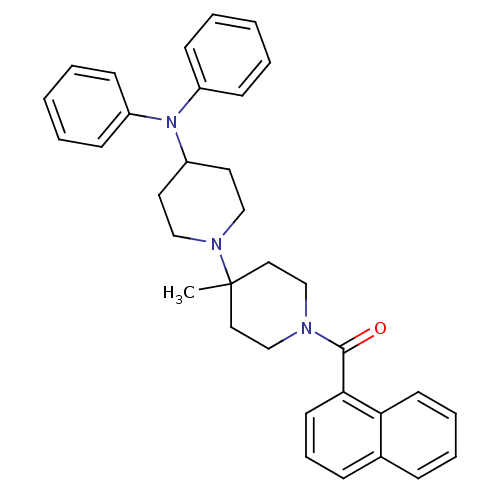 ((4-Diphenylamino-4'-methyl-[1,4']bipiperidinyl-1'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143737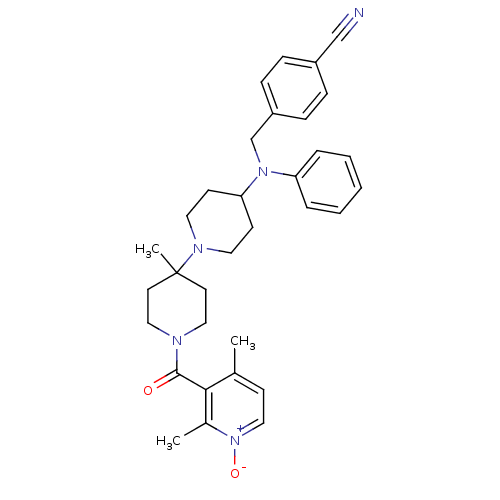 (4-({[1'-(2,4-Dimethyl-1-oxy-pyridine-3-carbonyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50427040 (CHEMBL2322207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using fluorescence-quenched (RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... | J Med Chem 56: 2196-206 (2013) Article DOI: 10.1021/jm301706j BindingDB Entry DOI: 10.7270/Q25X2B8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50427040 (CHEMBL2322207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of human plasma renin | J Med Chem 56: 2196-206 (2013) Article DOI: 10.1021/jm301706j BindingDB Entry DOI: 10.7270/Q25X2B8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314627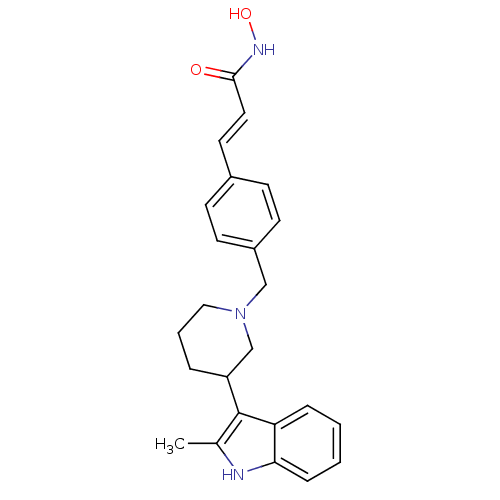 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143743 (CHEMBL61536 | [4-(Benzyl-phenyl-amino)-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143745 (CHEMBL60225 | {4-[Benzyl-(4-bromo-phenyl)-amino]-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143764 ((2,4-Dimethyl-pyridin-3-yl)-(4-diphenylamino-[1,4'...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50047264 (4-((4-bromophenylimino)(1H-imidazol-1-yl)methyl)be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human placental Cytochrome P450 19A1 | J Med Chem 36: 1393-400 (1993) BindingDB Entry DOI: 10.7270/Q2ZK5FR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143760 ((4-Diphenylamino-4'-methyl-[1,4']bipiperidinyl-1'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against human C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143747 ((4,6-Dimethyl-2-pyridin-4-yl-pyrimidin-5-yl)-(4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against human C-C chemokine receptor type 5 | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143747 ((4,6-Dimethyl-2-pyridin-4-yl-pyrimidin-5-yl)-(4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonistic activity against monkey C-C chemokine receptor type 5. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143756 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-diphenylamino-4'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Rises in intracellular [Ca2+] levels by using [Ca2+] sensitive Fluo4 dye in C-C chemokine receptor type 5-transfected CHO cells. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50143735 ((2,6-Dichloro-phenyl)-(4-diphenylamino-4'-methyl-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Rises in intracellular [Ca2+] levels by using [Ca2+] sensitive Fluo4 dye in C-C chemokine receptor type 5-transfected CHO cells. | J Med Chem 47: 1939-55 (2004) Article DOI: 10.1021/jm031046g BindingDB Entry DOI: 10.7270/Q2V12478 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50066269 (CHEMBL3401541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human renin by fluorescence based FRET assay | Bioorg Med Chem Lett 25: 1782-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.039 BindingDB Entry DOI: 10.7270/Q2Z039TX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50066269 (CHEMBL3401541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Inhibition of recombinant human renin by fluorescence based FRET assay | Bioorg Med Chem Lett 25: 1782-6 (2015) Article DOI: 10.1016/j.bmcl.2015.02.039 BindingDB Entry DOI: 10.7270/Q2Z039TX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 320 total ) | Next | Last >> |