

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50434903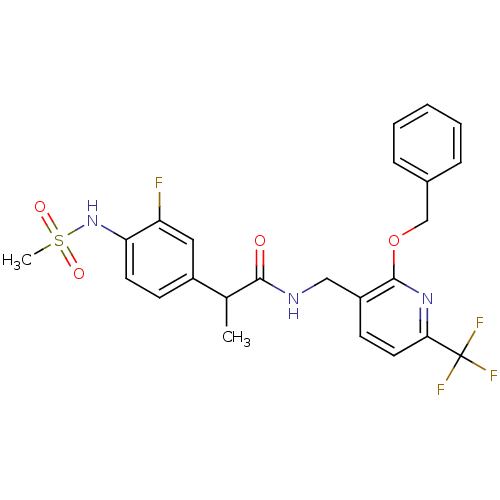 (CHEMBL2385223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... | Eur J Med Chem 64: 589-602 (2013) Article DOI: 10.1016/j.ejmech.2013.04.003 BindingDB Entry DOI: 10.7270/Q2BZ67FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494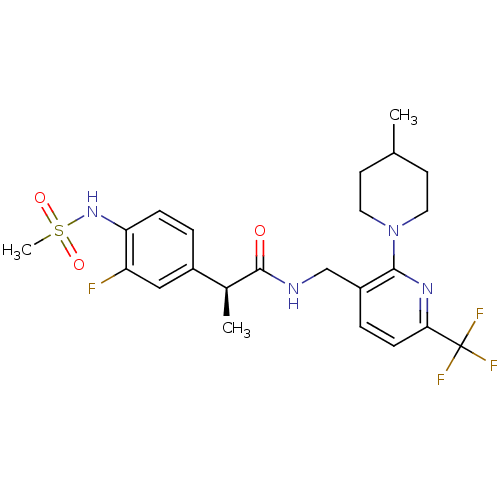 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | Bioorg Med Chem 21: 6657-64 (2013) Article DOI: 10.1016/j.bmc.2013.08.015 BindingDB Entry DOI: 10.7270/Q26Q1ZPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | J Med Chem 55: 8392-408 (2012) Article DOI: 10.1021/jm300780p BindingDB Entry DOI: 10.7270/Q2TX3GH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029704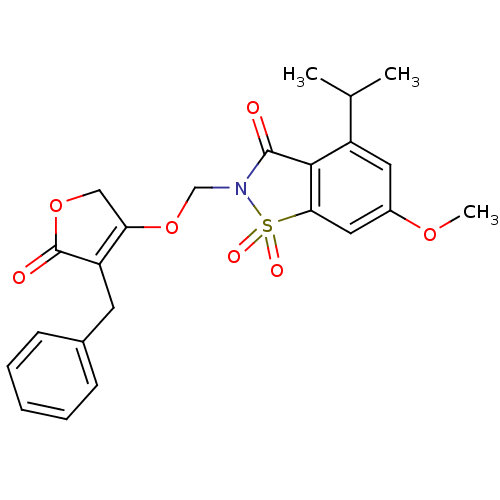 (2-(4-Benzyl-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029709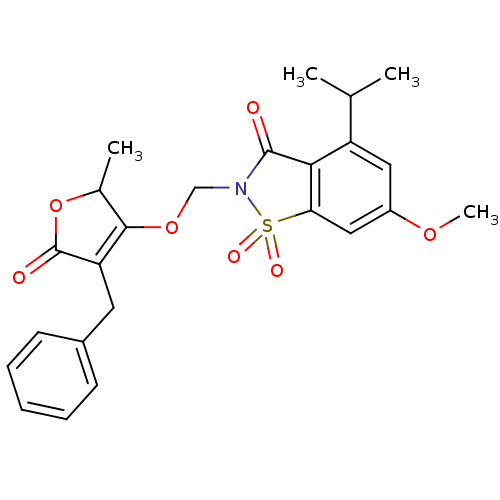 (2-(4-Benzyl-2-methyl-5-oxo-2,5-dihydro-furan-3-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553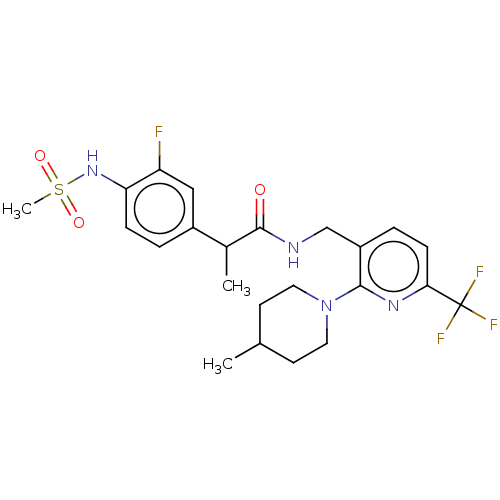 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activation by FLIPR assay | Bioorg Med Chem Lett 25: 2326-30 (2015) Article DOI: 10.1016/j.bmcl.2015.04.024 BindingDB Entry DOI: 10.7270/Q2Z60QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50061298 (CHEMBL3393837) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 assessed as inhibition of NADA-induced effect at 1 uM by FLIPR assay | Bioorg Med Chem Lett 25: 803-6 (2015) Article DOI: 10.1016/j.bmcl.2014.12.086 BindingDB Entry DOI: 10.7270/Q2JD4ZG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50049553 (CHEMBL2177428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50073160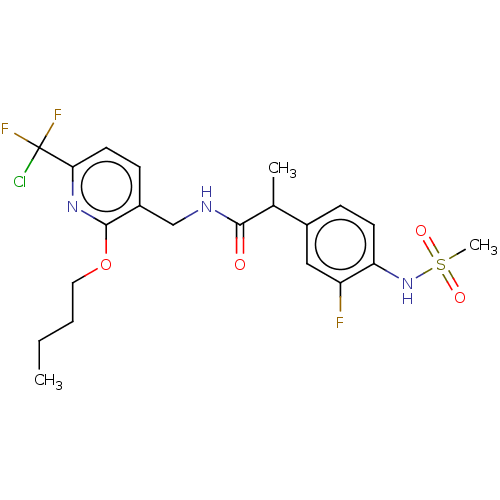 (CHEMBL3407762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029710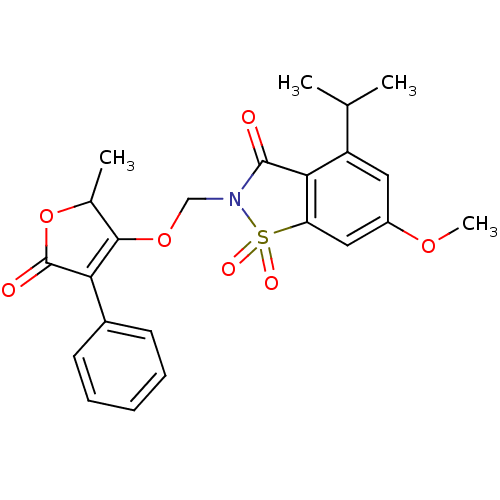 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-4-phenyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285289 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699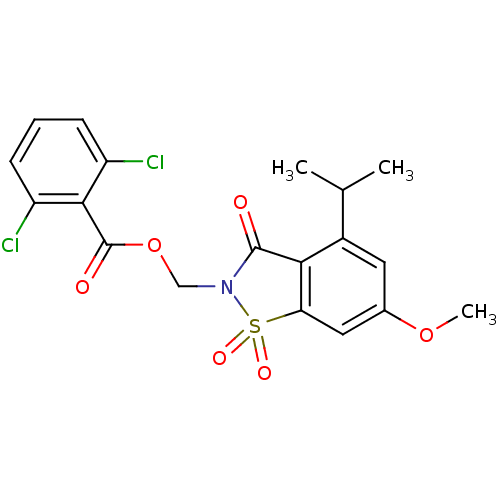 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029698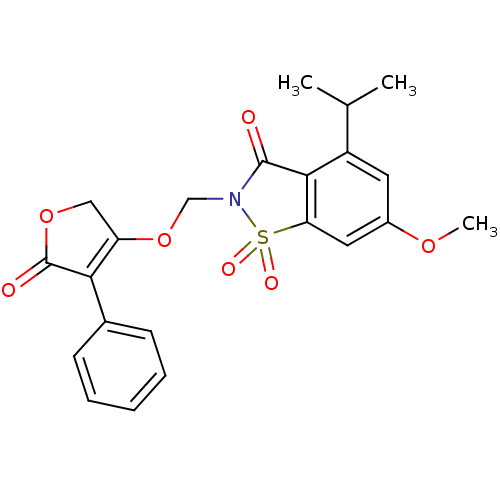 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-4-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029717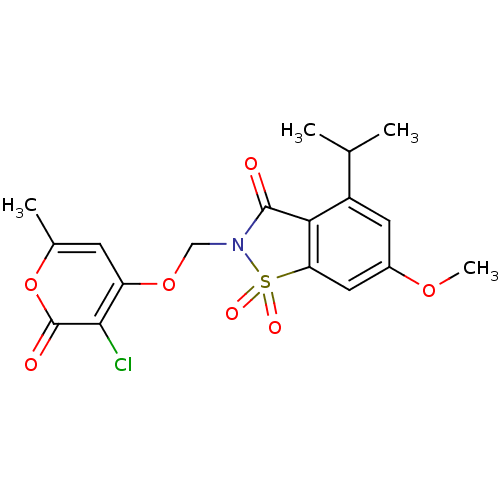 (2-(3-Chloro-6-methyl-2-oxo-2H-pyran-4-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029696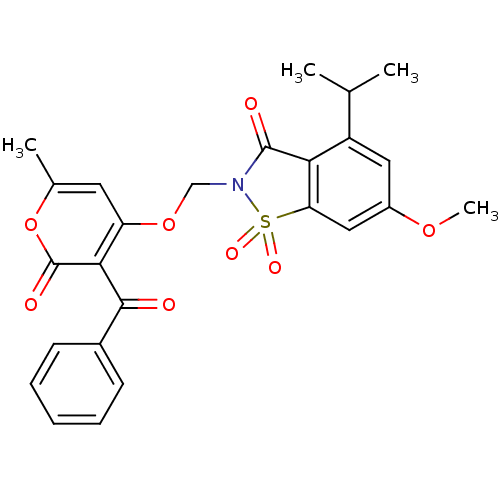 (2-(3-Benzoyl-6-methyl-2-oxo-2H-pyran-4-yloxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631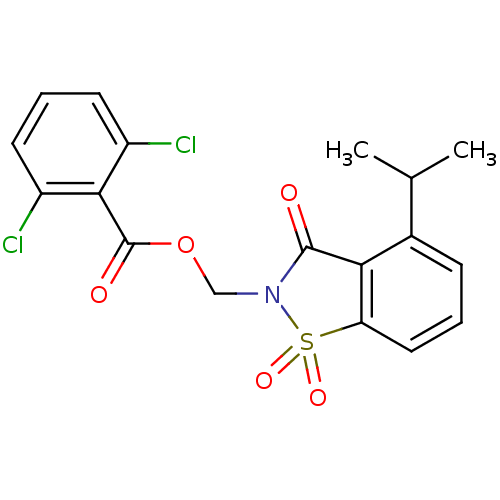 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ratio of Kreact to that of Kinact was determined on human leukocyte elastase(HLE) | Bioorg Med Chem Lett 5: 325-330 (1995) Article DOI: 10.1016/0960-894X(95)00029-S BindingDB Entry DOI: 10.7270/Q2XP74WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50128911 (CHEMBL3627723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay | Bioorg Med Chem 23: 6844-54 (2015) Article DOI: 10.1016/j.bmc.2015.10.001 BindingDB Entry DOI: 10.7270/Q2DZ0B4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50128922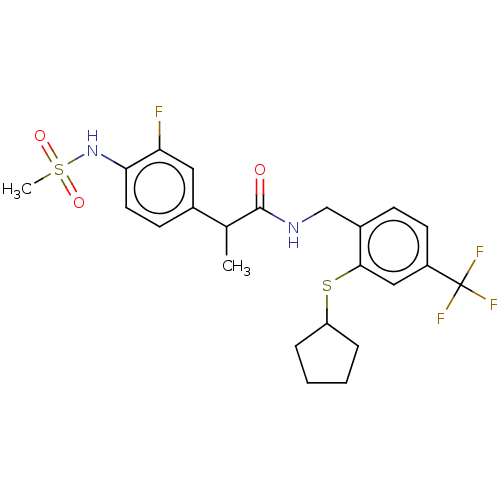 (CHEMBL3627950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay | Bioorg Med Chem 23: 6844-54 (2015) Article DOI: 10.1016/j.bmc.2015.10.001 BindingDB Entry DOI: 10.7270/Q2DZ0B4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029712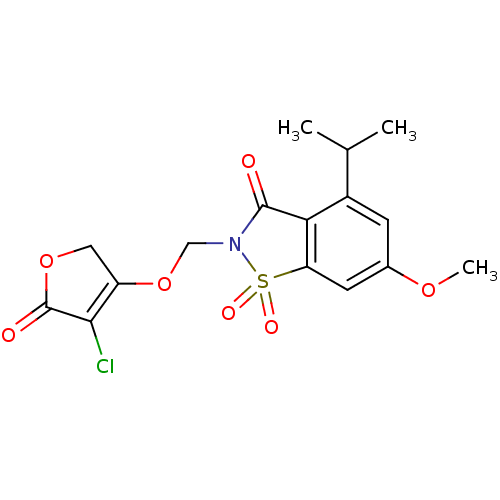 (2-(4-Chloro-5-oxo-2,5-dihydro-furan-3-yloxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286326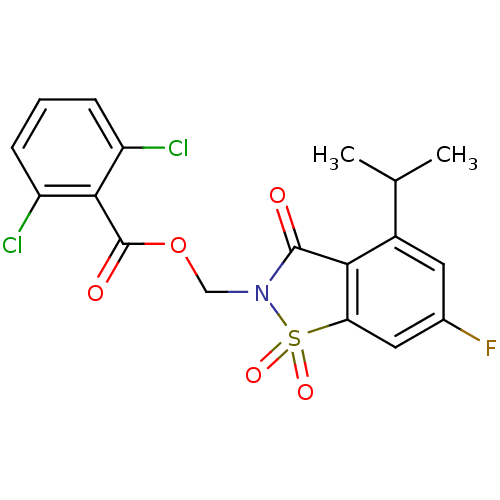 (2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Observed binding affinity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 325-330 (1995) Article DOI: 10.1016/0960-894X(95)00029-S BindingDB Entry DOI: 10.7270/Q2XP74WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029713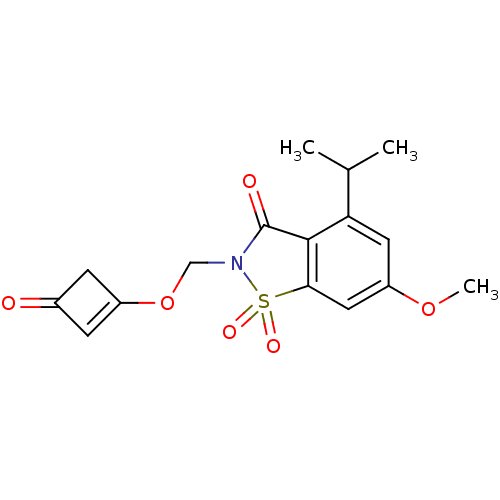 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-cyclobut-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029691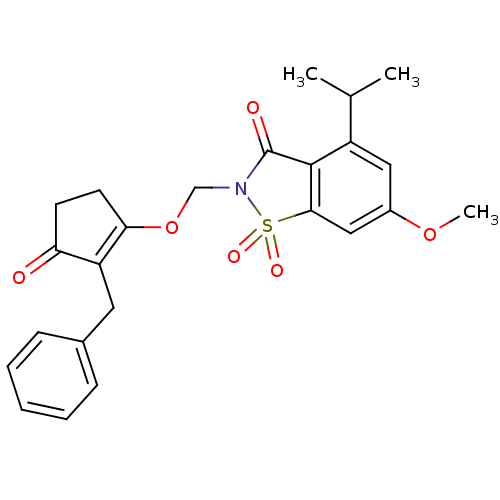 (2-(2-Benzyl-3-oxo-cyclopent-1-enyloxymethyl)-4-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034671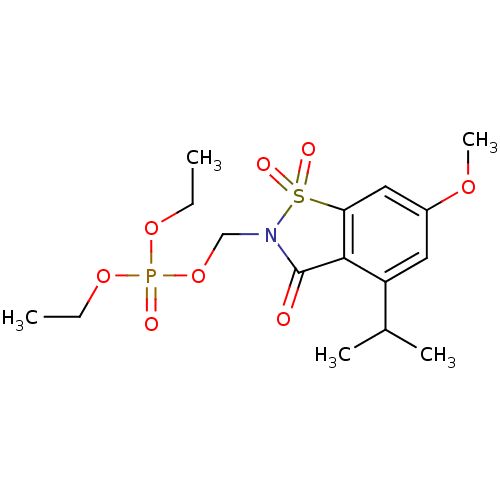 (CHEMBL41327 | Phosphoric acid diethyl ester 4-isop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50073159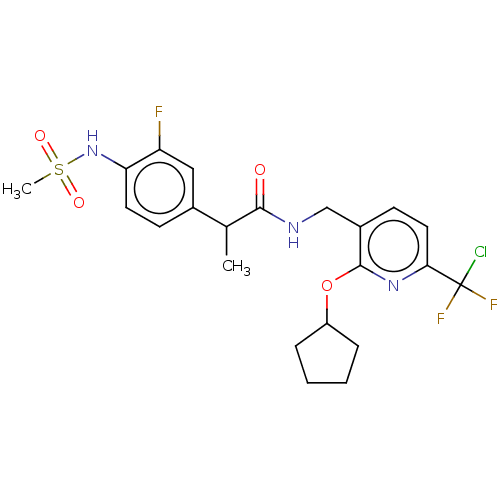 (CHEMBL3407765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of N-arachidonoyl dopamine-induced activity by FLIPR assay | Eur J Med Chem 93: 101-8 (2015) Article DOI: 10.1016/j.ejmech.2015.02.001 BindingDB Entry DOI: 10.7270/Q2N0188S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555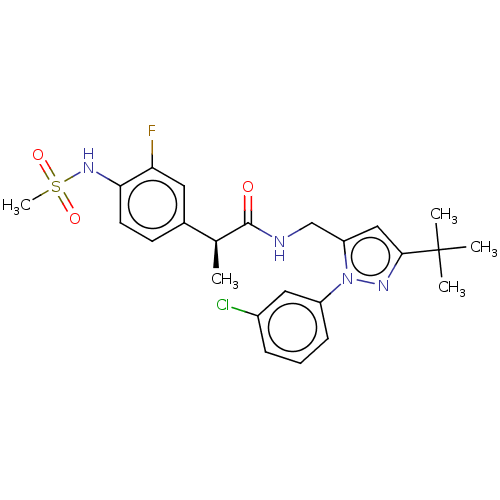 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of NADA-induced intracellular calcium level preincubated with cells ... | Bioorg Med Chem Lett 27: 4383-4388 (2017) Article DOI: 10.1016/j.bmcl.2017.08.020 BindingDB Entry DOI: 10.7270/Q2CF9SPD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285280 (2,6-Dichloro-benzoic acid 4-ethoxy-6-methoxy-1,1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50442379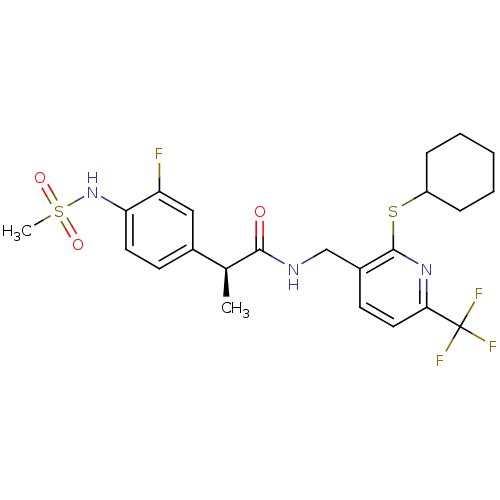 (CHEMBL2442912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | Bioorg Med Chem 21: 6657-64 (2013) Article DOI: 10.1016/j.bmc.2013.08.015 BindingDB Entry DOI: 10.7270/Q26Q1ZPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029692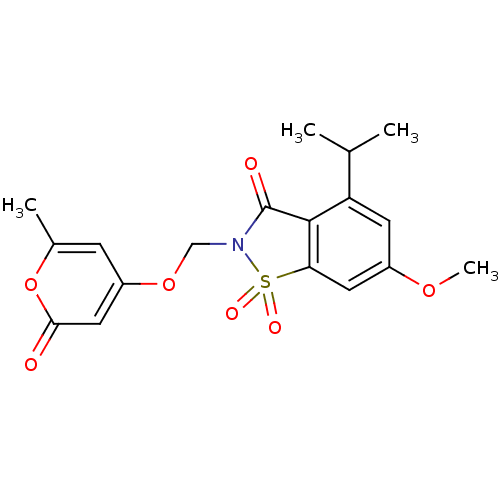 (4-Isopropyl-6-methoxy-2-(6-methyl-2-oxo-2H-pyran-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029695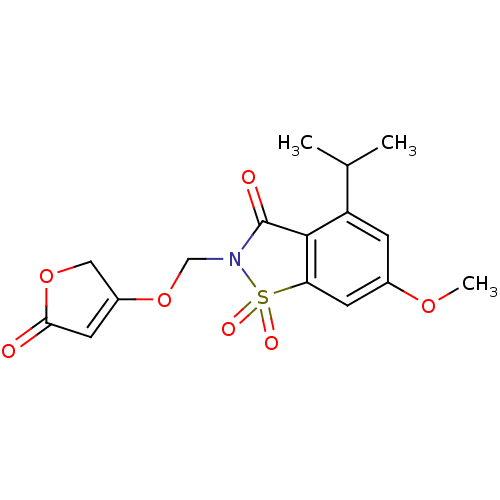 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(5-oxo-2,5-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029711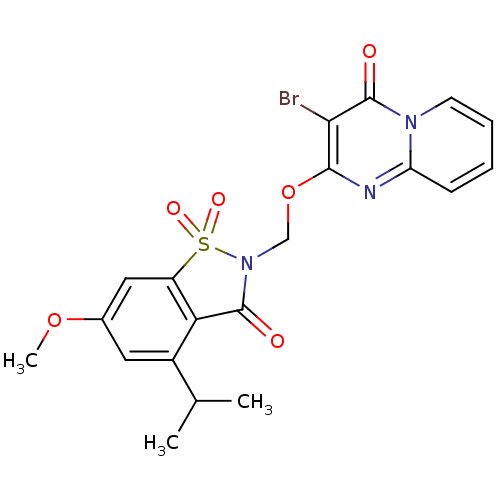 (3-Bromo-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029720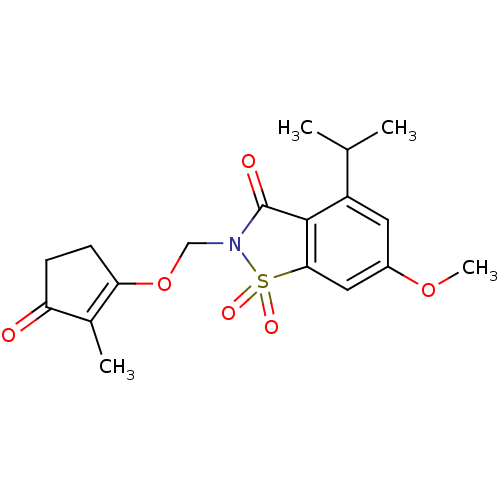 (4-Isopropyl-6-methoxy-2-(2-methyl-3-oxo-cyclopent-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029707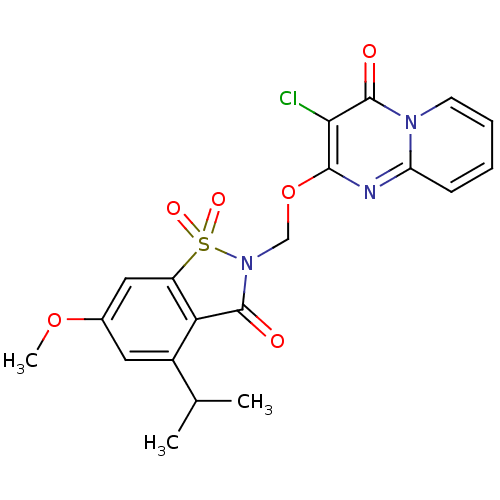 (3-Chloro-2-(4-isopropyl-6-methoxy-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034676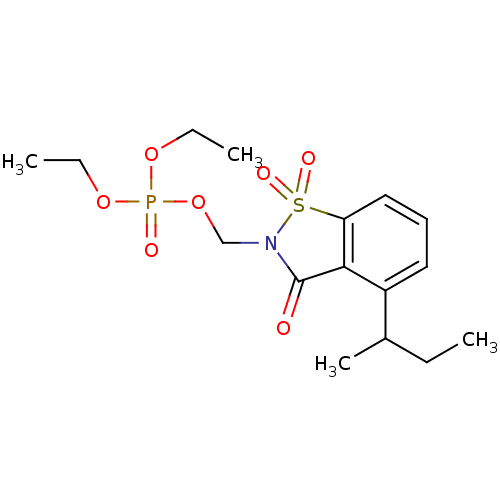 (CHEMBL41881 | Phosphoric acid 4-sec-butyl-1,1,3-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285286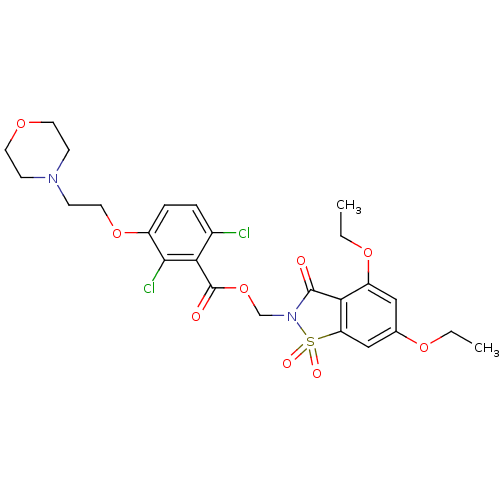 (2,6-Dichloro-3-(2-morpholin-4-yl-ethoxy)-benzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039642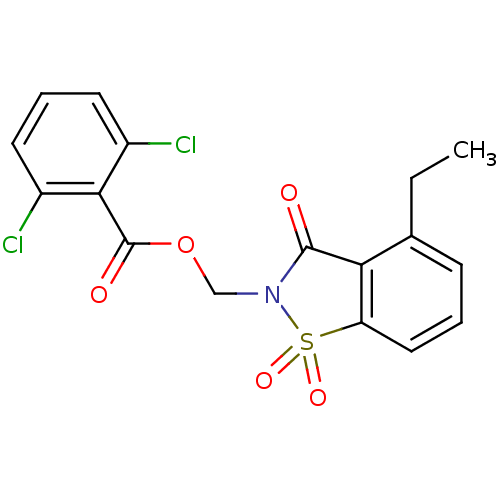 (2,6-Dichloro-benzoic acid 4-ethyl-1,1,3-trioxo-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Observed binding affinity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 325-330 (1995) Article DOI: 10.1016/0960-894X(95)00029-S BindingDB Entry DOI: 10.7270/Q2XP74WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029721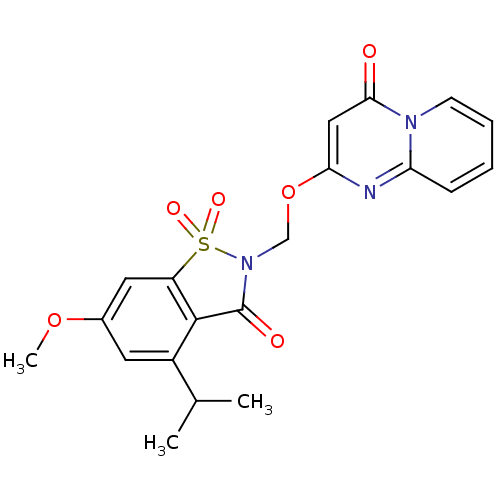 (2-(4-Isopropyl-6-methoxy-1,1,3-trioxo-1,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50070377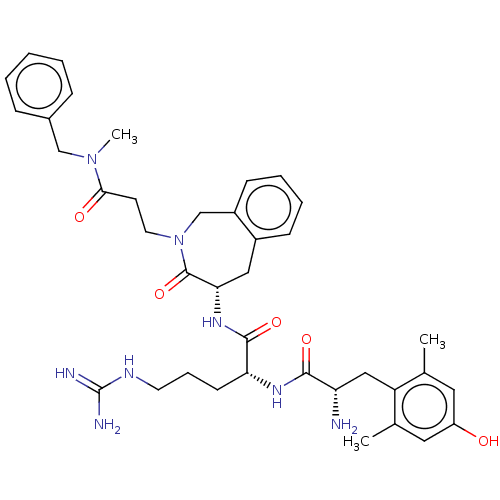 (CHEMBL3408519) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane after 2 hrs | ACS Med Chem Lett 6: 1209-14 (2015) Article DOI: 10.1021/acsmedchemlett.5b00359 BindingDB Entry DOI: 10.7270/Q2VH5QQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285284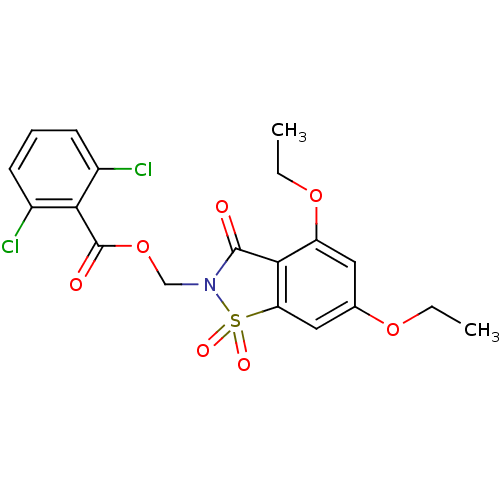 (2,6-Dichloro-benzoic acid 4,6-diethoxy-1,1,3-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50285283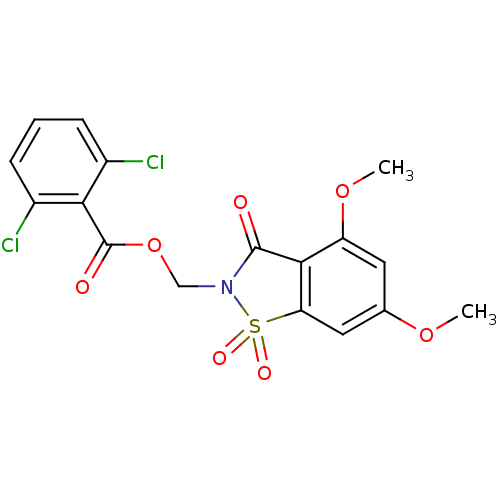 (2,6-Dichloro-benzoic acid 4,6-dimethoxy-1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Leukocyte Elastase (HLE) as apparent binding constant (kreact/kinact) | Bioorg Med Chem Lett 5: 105-109 (1995) Article DOI: 10.1016/0960-894X(94)00466-S BindingDB Entry DOI: 10.7270/Q2V69JJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029715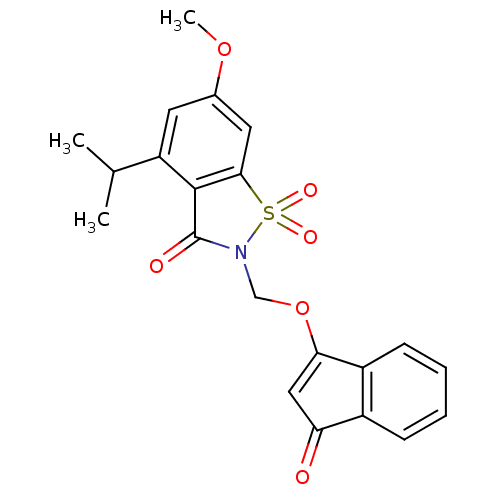 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(3-oxo-3H-inden-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029718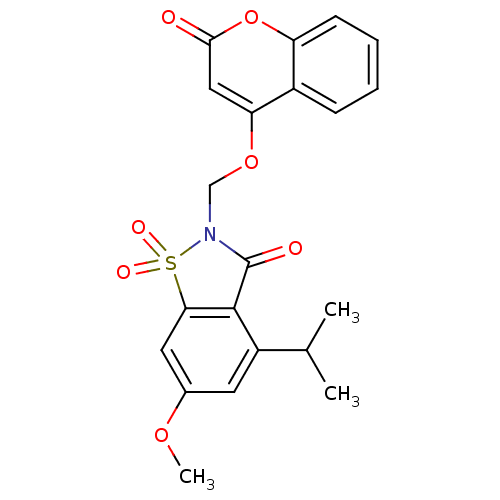 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(2-oxo-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50212159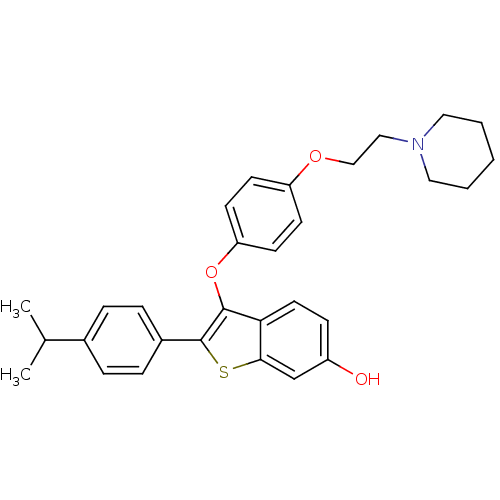 (2-(4-isopropylphenyl)-3-(4-(2-(piperidin-1-yl)etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human recombinant ERalpha | Bioorg Med Chem Lett 17: 3544-9 (2007) Article DOI: 10.1016/j.bmcl.2007.04.044 BindingDB Entry DOI: 10.7270/Q2ZS2W6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029700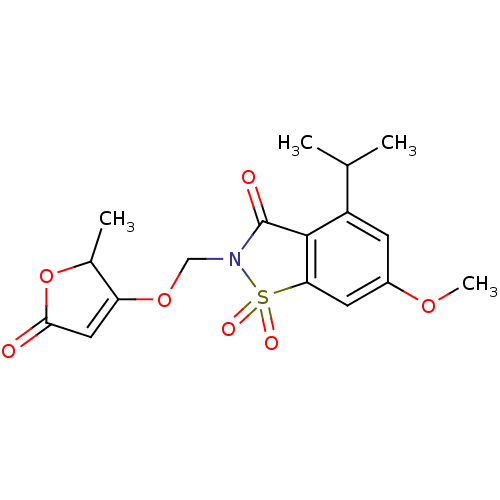 (4-Isopropyl-6-methoxy-2-(2-methyl-5-oxo-2,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Potency of inhibition of human leukocyte elastase is expressed as apparent binding constant | J Med Chem 38: 4687-92 (1995) BindingDB Entry DOI: 10.7270/Q2KP816X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50034677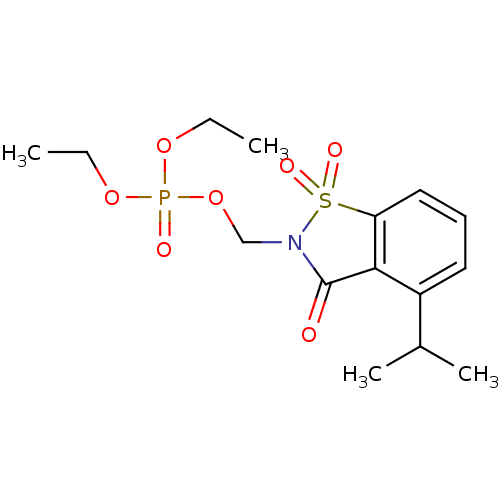 (CHEMBL288810 | Phosphoric acid diethyl ester 4-iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description In vitro inhibitory activity against Human leukocyte elastase | J Med Chem 38: 1571-4 (1995) BindingDB Entry DOI: 10.7270/Q2M32TS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50256547 (CHEMBL4104073) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of capsaicin induced calcium influx pretreated for 6 mins followed... | Bioorg Med Chem 25: 2451-2462 (2017) Article DOI: 10.1016/j.bmc.2017.03.004 BindingDB Entry DOI: 10.7270/Q2BZ68HP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127310 (US8791268, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6606 total ) | Next | Last >> |