

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM20875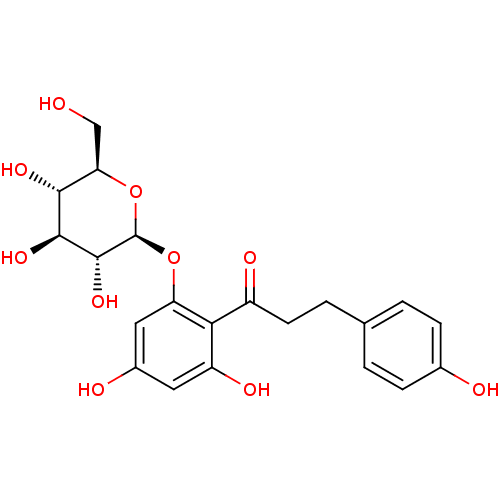 (1-(2,4-dihydroxy-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422357 (CHEMBL2303984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50087823 (1-[2-(4,5-Dihydroxy-6-hydroxymethyl-tetrahydro-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422353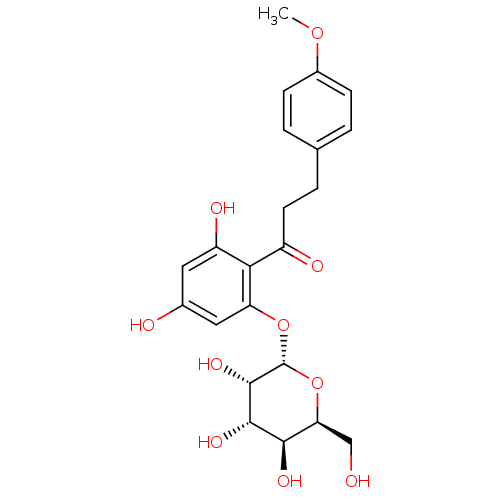 (CHEMBL2303988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422355 (CHEMBL2303989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422350 (CHEMBL2303987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422349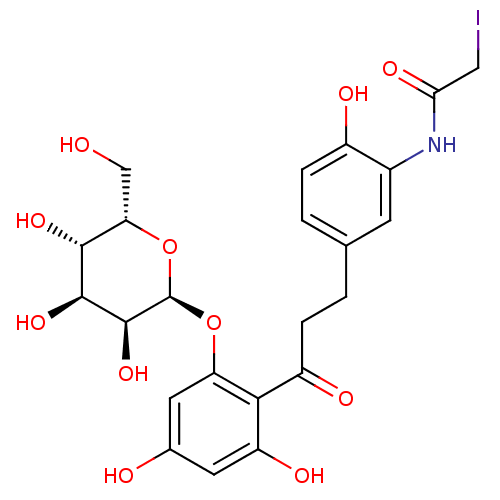 (CHEMBL2303986) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422348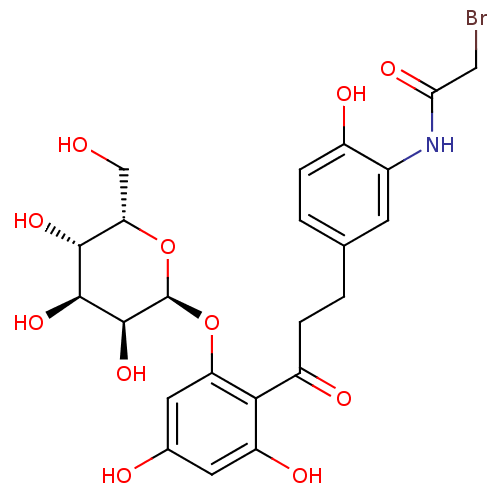 (CHEMBL2303985) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50409197 (CHEMBL2096900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422352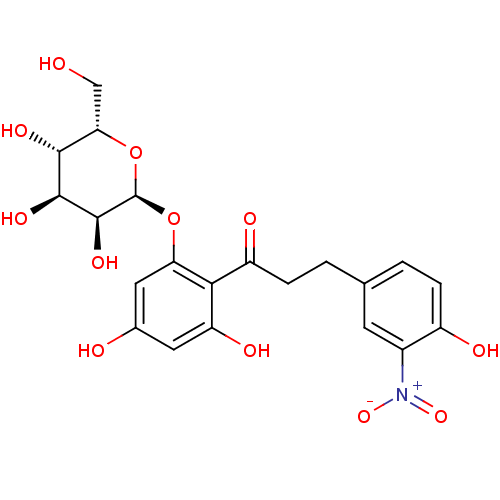 (CHEMBL2303990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422346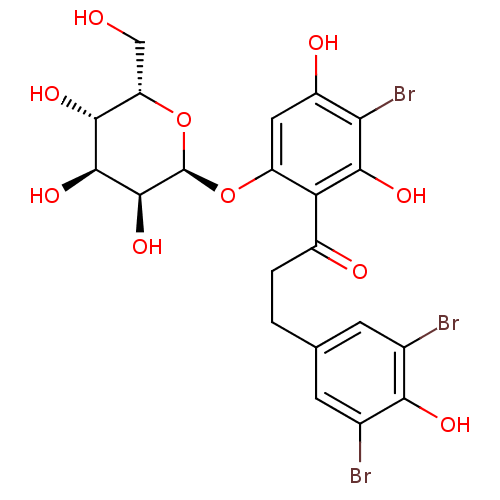 (CHEMBL2303991) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422351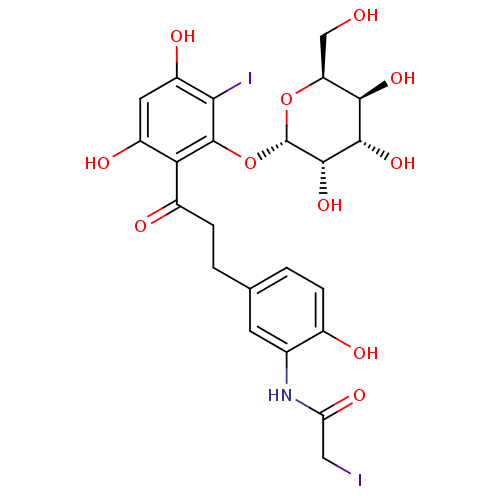 (CHEMBL2303981) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422356 (CHEMBL2303983) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM23446 (3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422345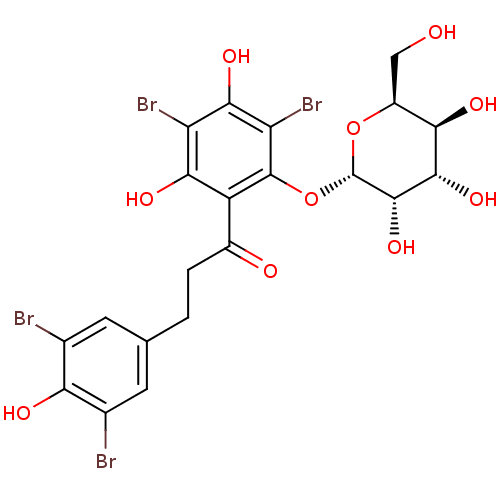 (CHEMBL2303982) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422358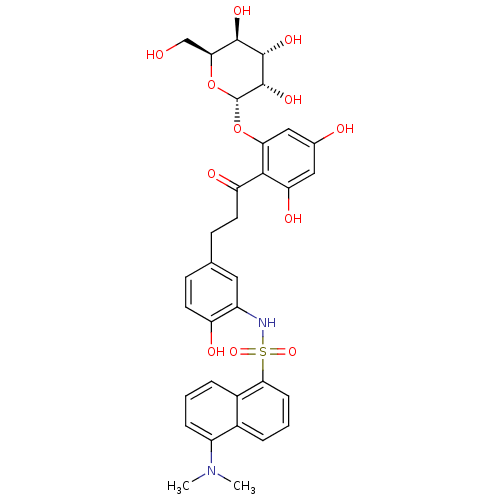 (CHEMBL2303994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422354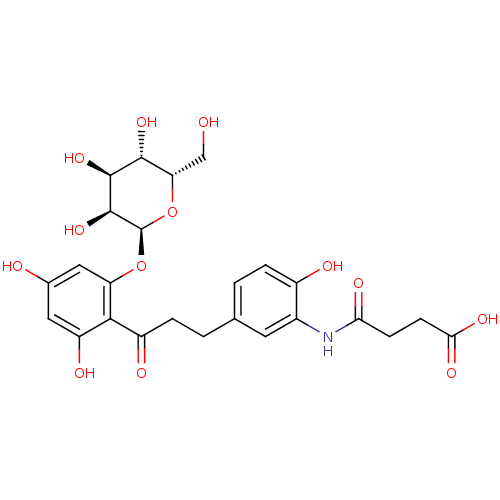 (CHEMBL2303993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50422347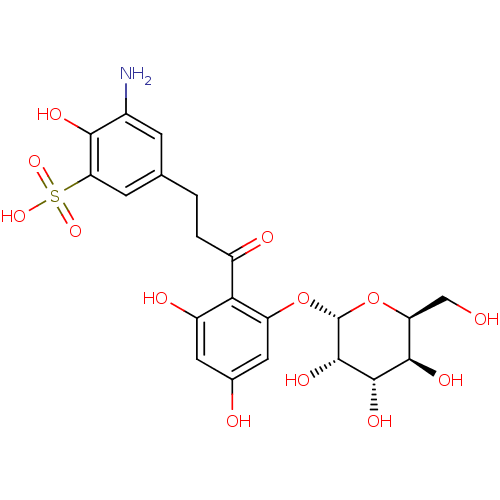 (CHEMBL2303992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50409196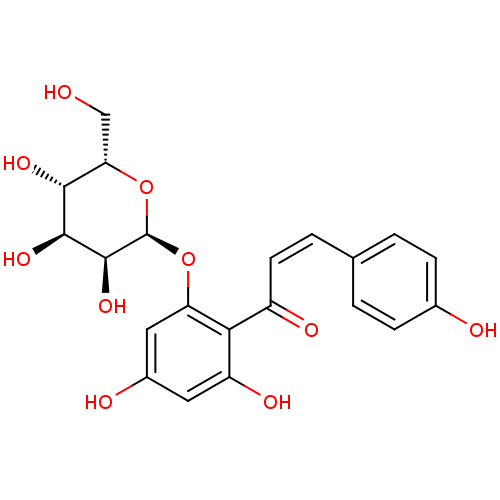 (CHEMBL2029165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institut f£r molekulare Physiologie Curated by ChEMBL | Assay Description Binding affinity against Sodium/glucose co-transporter of isolated renal brush border membranes. | J Med Chem 43: 1692-8 (2000) BindingDB Entry DOI: 10.7270/Q261112H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153906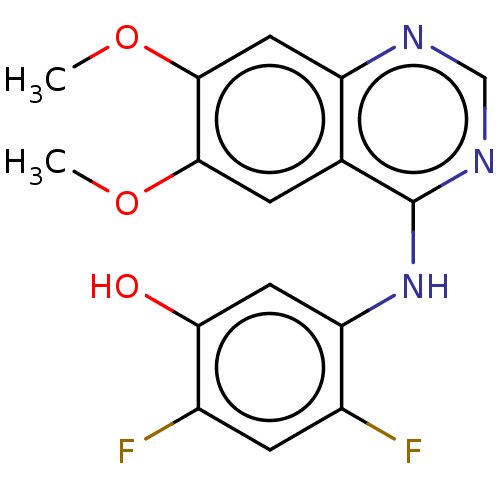 (CHEMBL3775169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM4627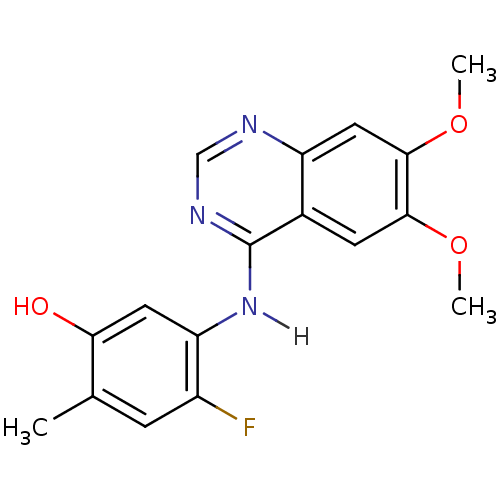 (5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153979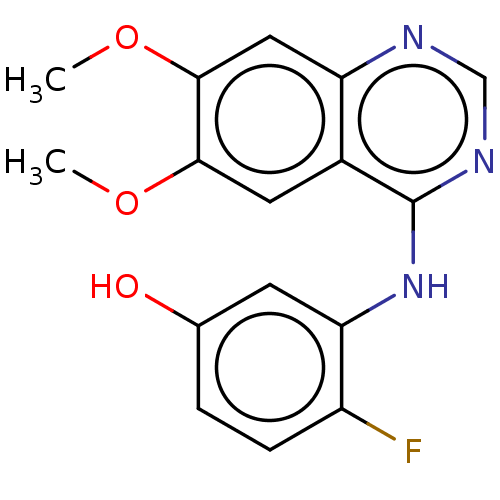 (CHEMBL3774904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153902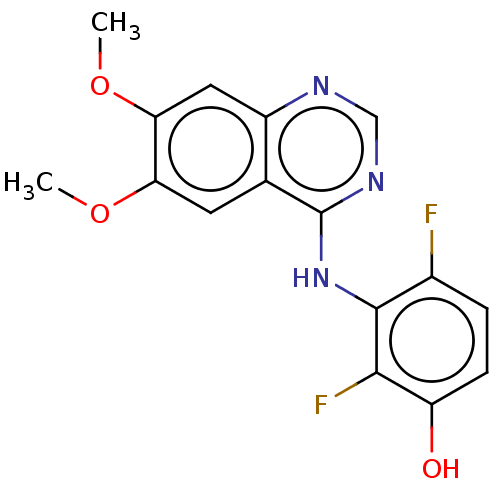 (CHEMBL3775557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153903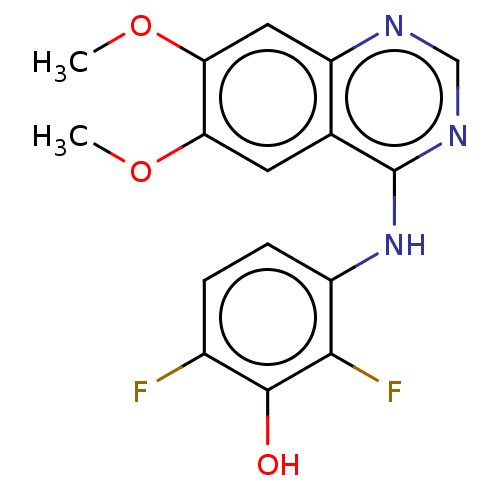 (CHEMBL3775336) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4627 (5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50154001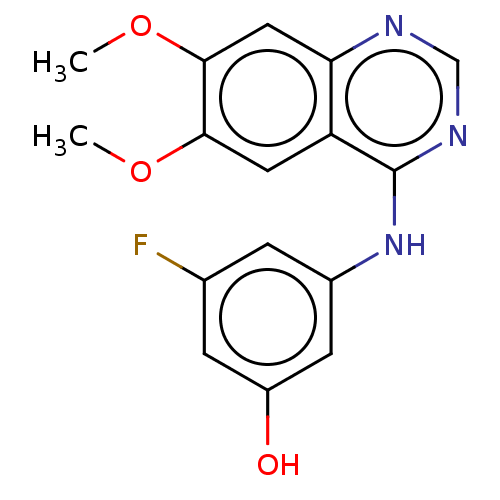 (CHEMBL3775934) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153908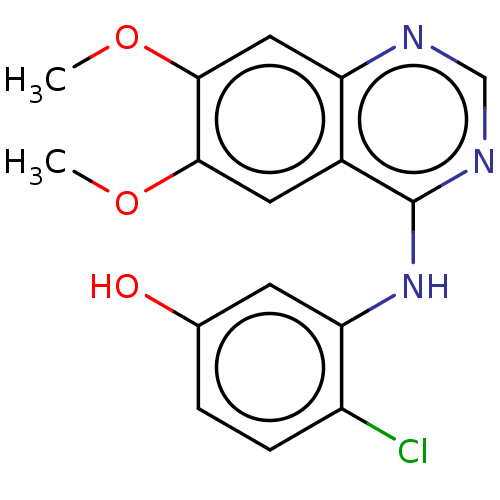 (CHEMBL3774489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50154249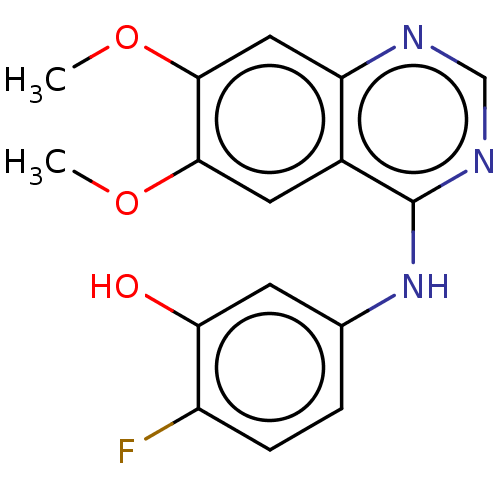 (CHEMBL3775879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50154246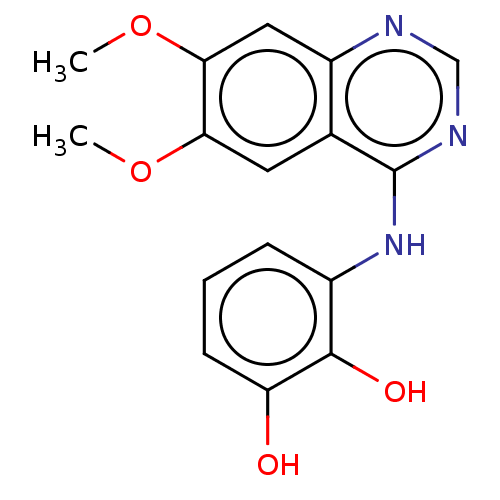 (CHEMBL3774580) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153895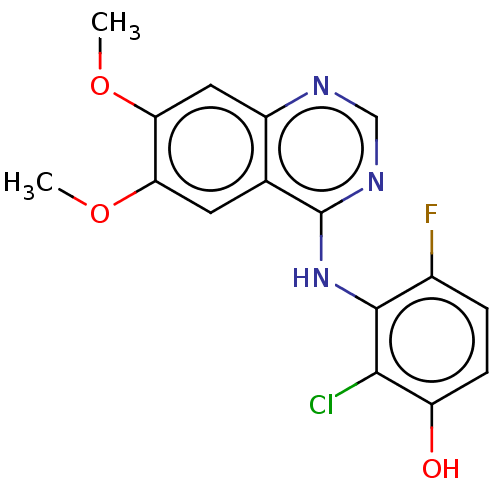 (CHEMBL3774953) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM4622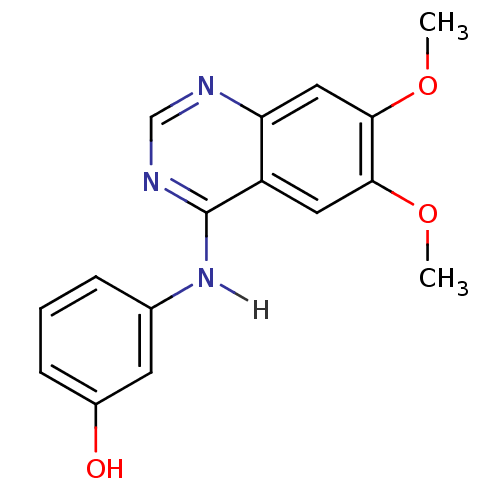 (3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | An...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM26477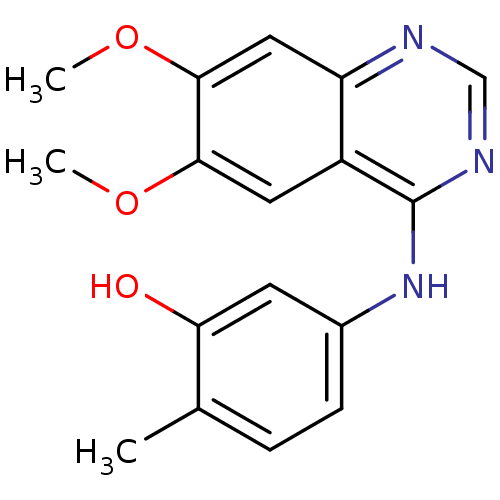 (5-[(6,7-dimethoxyquinazolin-4-yl)amino]-2-methylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153901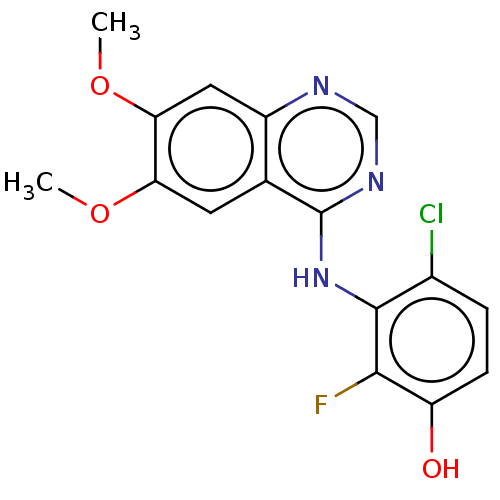 (CHEMBL3775511) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165968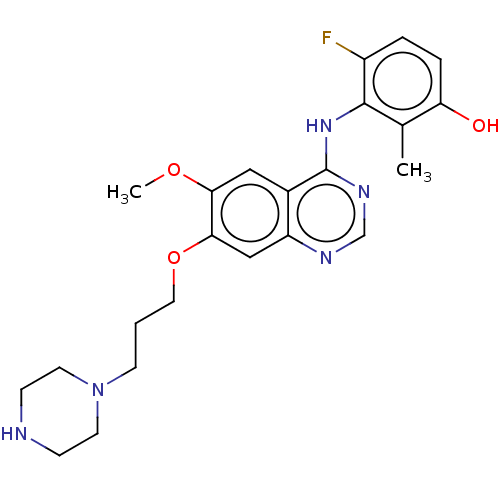 (CHEMBL3797261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153905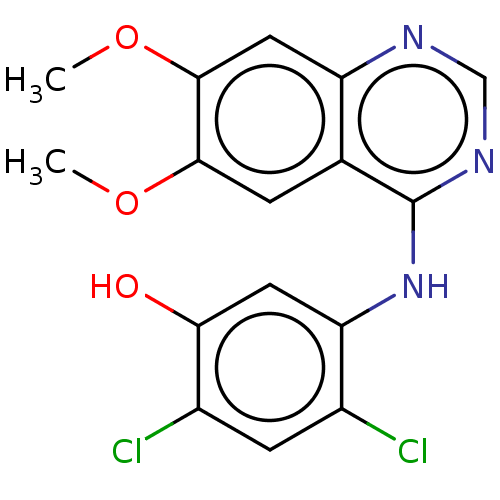 (CHEMBL3775190) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165922 (CHEMBL3799523) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153907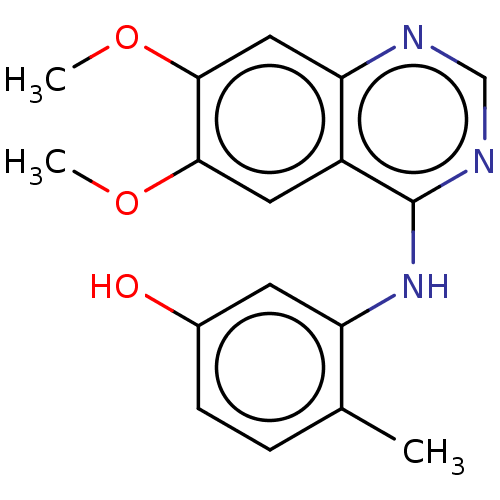 (CHEMBL3774951) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165977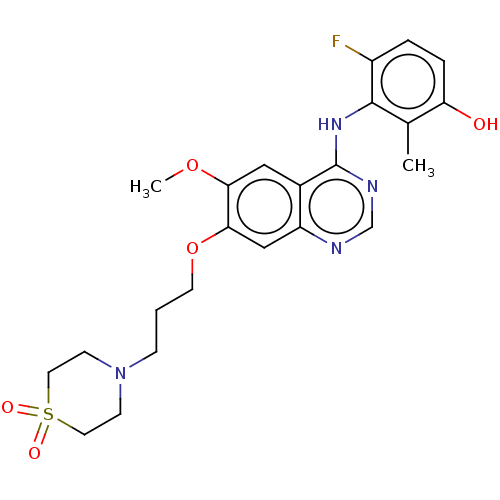 (CHEMBL3798584) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153897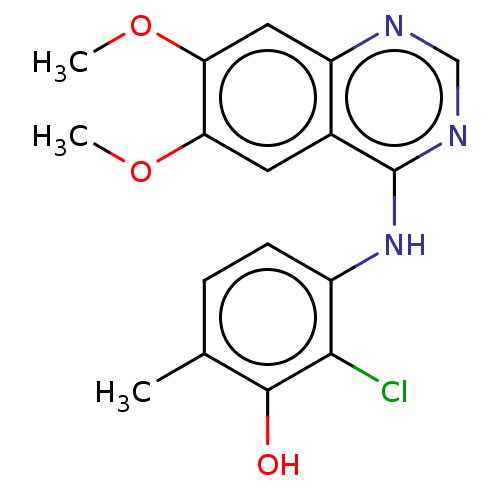 (CHEMBL3775560) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50153906 (CHEMBL3775169) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4627 (5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) expressed in mouse BA/F3 cells assessed as reduction in cell viability after 48 hrs by Cell titre glo-based lumine... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153896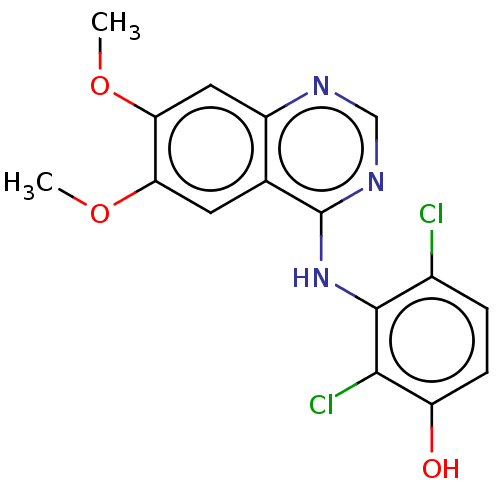 (CHEMBL3775585) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50154010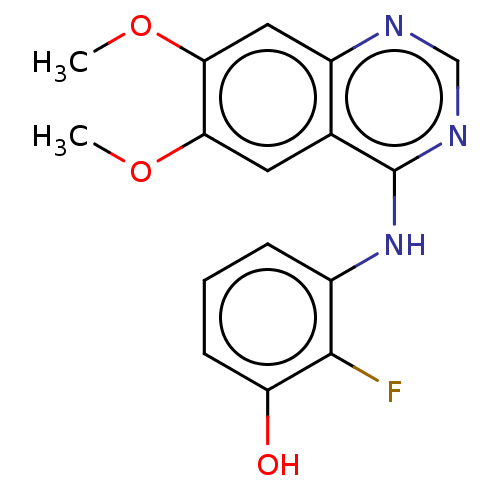 (CHEMBL3775903) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50154248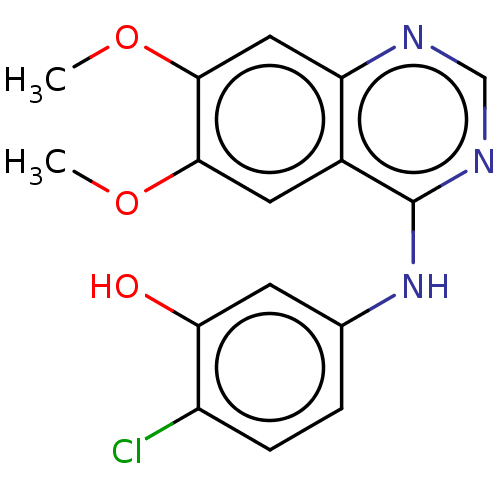 (CHEMBL235851) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165975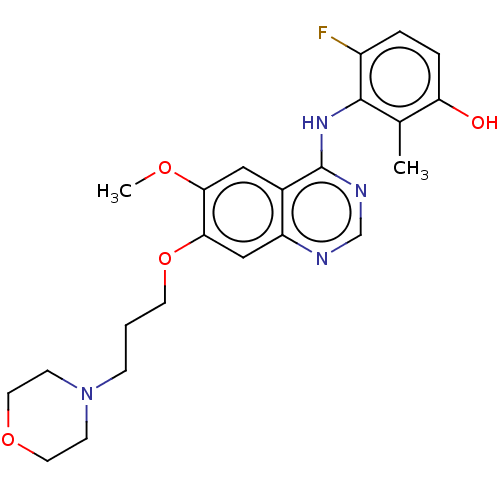 (CHEMBL3799933) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50154247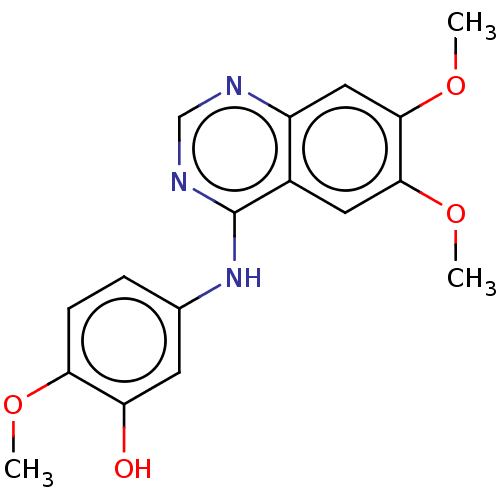 (CHEMBL3774563) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165970 (CHEMBL3799971) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165898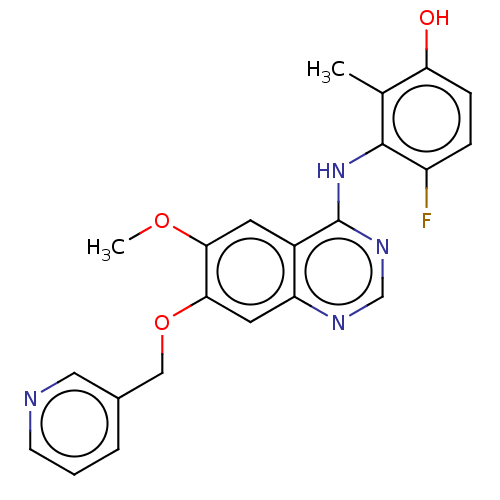 (CHEMBL3800346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153904 (CHEMBL3775415) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50165974 (CHEMBL3797688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of N-terminal GST fused human RET (658 to 1114 amino acid residue) using CSKtide as substrate expressed in baculovirus preincubated for 15... | Bioorg Med Chem Lett 26: 2724-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.100 BindingDB Entry DOI: 10.7270/Q2WM1G93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 295 total ) | Next | Last >> |