

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50335280 (CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335281 (CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335285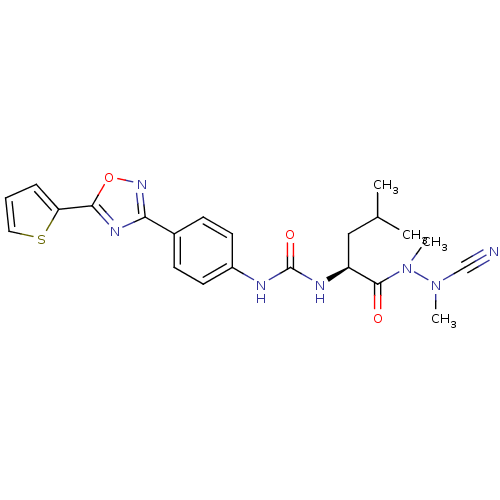 (CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335289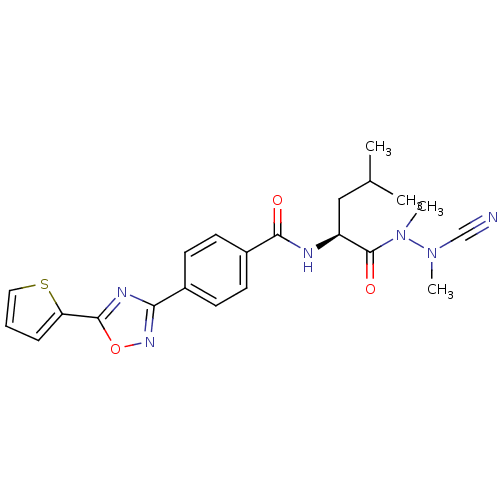 (CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50335281 (CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335284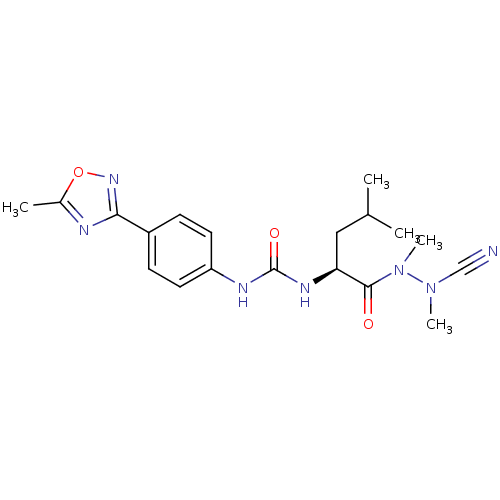 (CHEMBL1651349 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50304794 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335278 (CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335283 (CHEMBL1651357 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127171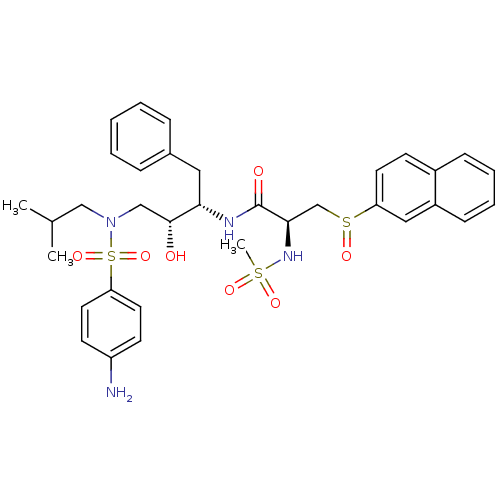 (CHEMBL299578 | N-{3-[(4-Amino-benzenesulfonyl)-iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335282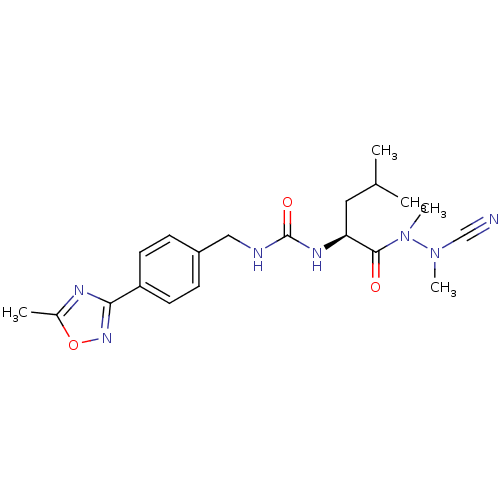 (CHEMBL1651356 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50304793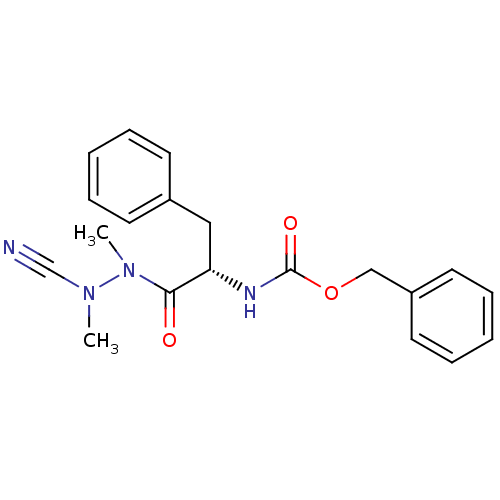 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335289 (CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335281 (CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335283 (CHEMBL1651357 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335279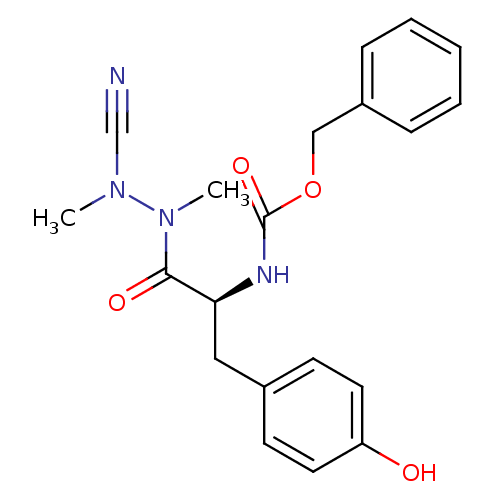 (CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335282 (CHEMBL1651356 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335284 (CHEMBL1651349 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335280 (CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335278 (CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50335289 (CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165949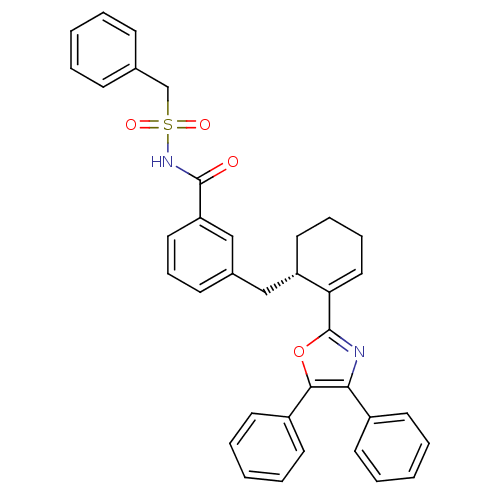 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335285 (CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50304794 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50335279 (CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50335289 (CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127172 (4-Amino-N-[3-benzyl-2-hydroxy-6-methanesulfonylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50335279 (CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50335280 (CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50335278 (CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50304794 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574836 (CHEMBL4871105) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335277 (CHEMBL1651351 | N-(Benzyloxycarbonyl)-isoleucyl-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50335278 (CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335290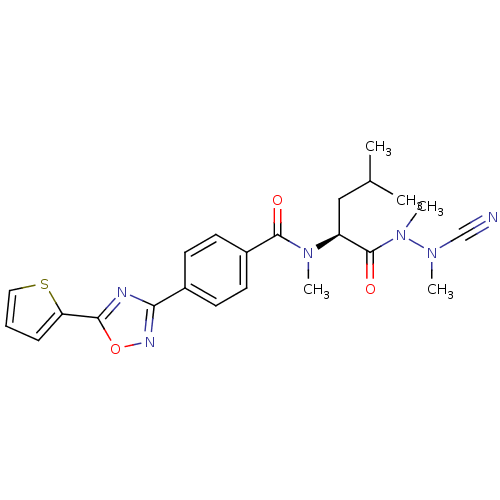 (CHEMBL1651362 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50335280 (CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50304793 ((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165945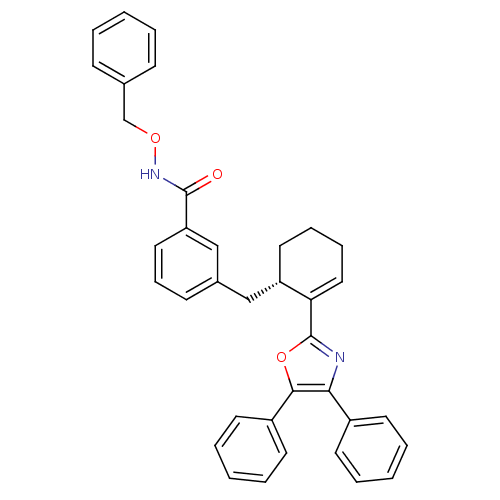 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Grupo Ferrer Curated by ChEMBL | Assay Description Binding affinity measured at the 5-hydroxytryptamine 2A receptor by the inhibition of [3H]-ketanserin binding to rat cortex using unlabeled mianserin... | J Med Chem 41: 5402-9 (1999) Article DOI: 10.1021/jm9810396 BindingDB Entry DOI: 10.7270/Q2QC02N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249779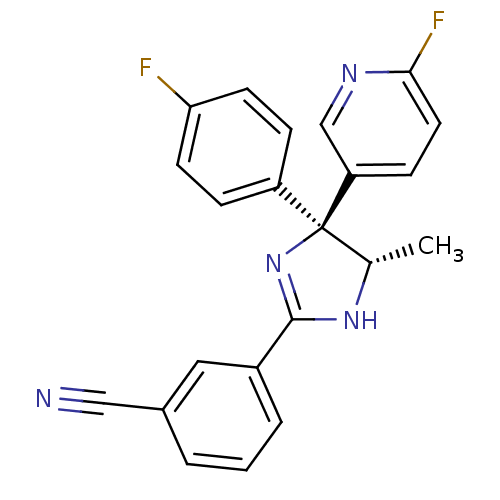 (3-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335277 (CHEMBL1651351 | N-(Benzyloxycarbonyl)-isoleucyl-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50335279 (CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50335276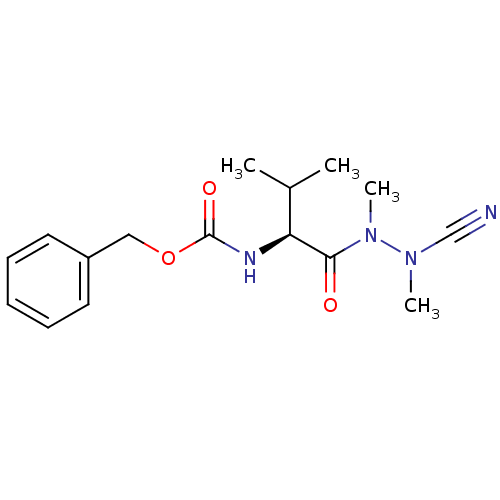 (CHEMBL1651242 | N-(Benzyloxycarbonyl)-valyl-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50335277 (CHEMBL1651351 | N-(Benzyloxycarbonyl)-isoleucyl-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B after 30 mins by spectrophotometric assay | J Med Chem 54: 396-400 (2011) Article DOI: 10.1021/jm101272p BindingDB Entry DOI: 10.7270/Q2PV6MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4383 total ) | Next | Last >> |