 Found 570 hits with Last Name = 'fukuda' and Initial = 'y'
Found 570 hits with Last Name = 'fukuda' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127501
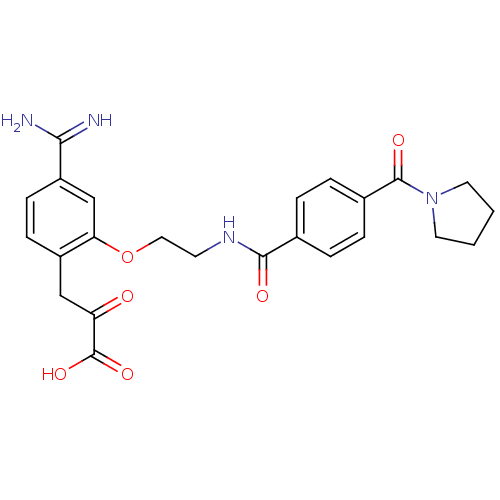
(3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C24H26N4O6/c25-21(26)18-8-7-17(13-19(29)24(32)33)20(14-18)34-12-9-27-22(30)15-3-5-16(6-4-15)23(31)28-10-1-2-11-28/h3-8,14H,1-2,9-13H2,(H3,25,26)(H,27,30)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127492
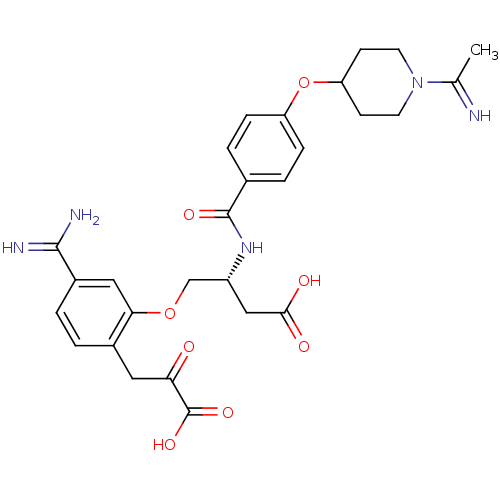
(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)N[C@@H](COc1cc(ccc1CC(=O)C(O)=O)C(N)=N)CC(O)=O Show InChI InChI=1S/C28H33N5O8/c1-16(29)33-10-8-22(9-11-33)41-21-6-4-17(5-7-21)27(37)32-20(14-25(35)36)15-40-24-13-19(26(30)31)3-2-18(24)12-23(34)28(38)39/h2-7,13,20,22,29H,8-12,14-15H2,1H3,(H3,30,31)(H,32,37)(H,35,36)(H,38,39)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127502
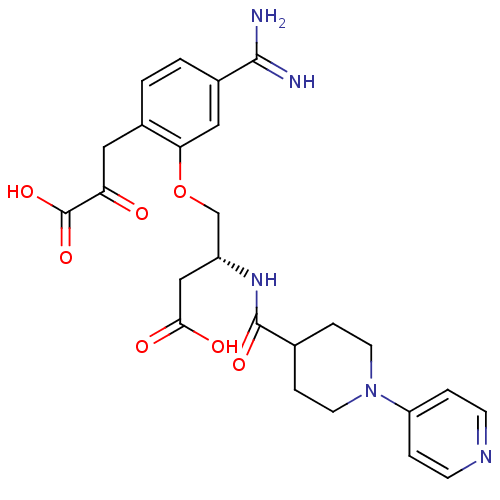
(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OC[C@@H](CC(O)=O)NC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C25H29N5O7/c26-23(27)17-2-1-16(11-20(31)25(35)36)21(12-17)37-14-18(13-22(32)33)29-24(34)15-5-9-30(10-6-15)19-3-7-28-8-4-19/h1-4,7-8,12,15,18H,5-6,9-11,13-14H2,(H3,26,27)(H,29,34)(H,32,33)(H,35,36)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127495

(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C26H31N5O6/c1-16(27)31-11-8-21(9-12-31)37-20-6-4-17(5-7-20)25(33)30-10-13-36-23-15-19(24(28)29)3-2-18(23)14-22(32)26(34)35/h2-7,15,21,27H,8-14H2,1H3,(H3,28,29)(H,30,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127504
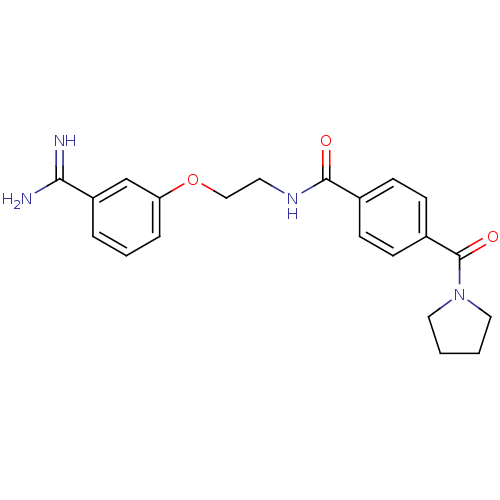
(CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C21H24N4O3/c22-19(23)17-4-3-5-18(14-17)28-13-10-24-20(26)15-6-8-16(9-7-15)21(27)25-11-1-2-12-25/h3-9,14H,1-2,10-13H2,(H3,22,23)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127494
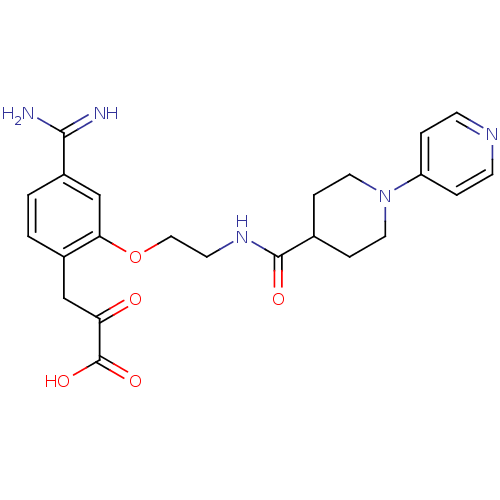
(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H27N5O5/c24-21(25)17-2-1-16(13-19(29)23(31)32)20(14-17)33-12-9-27-22(30)15-5-10-28(11-6-15)18-3-7-26-8-4-18/h1-4,7-8,14-15H,5-6,9-13H2,(H3,24,25)(H,27,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127498
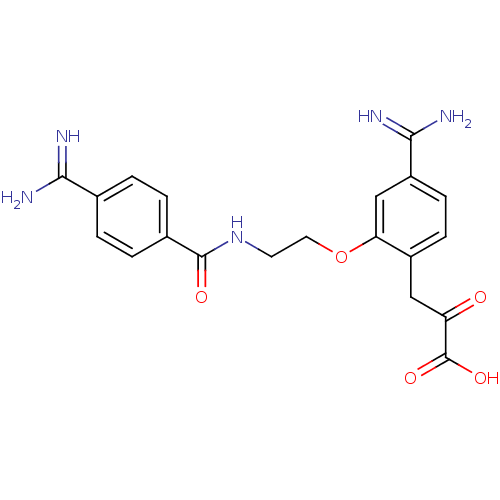
(3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C20H21N5O5/c21-17(22)11-1-3-12(4-2-11)19(27)25-7-8-30-16-10-14(18(23)24)6-5-13(16)9-15(26)20(28)29/h1-6,10H,7-9H2,(H3,21,22)(H3,23,24)(H,25,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127503
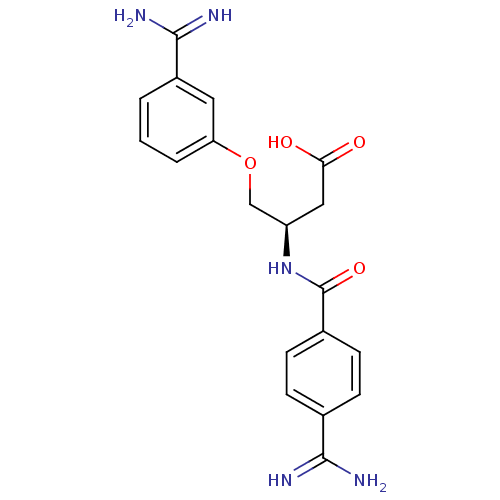
(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127493
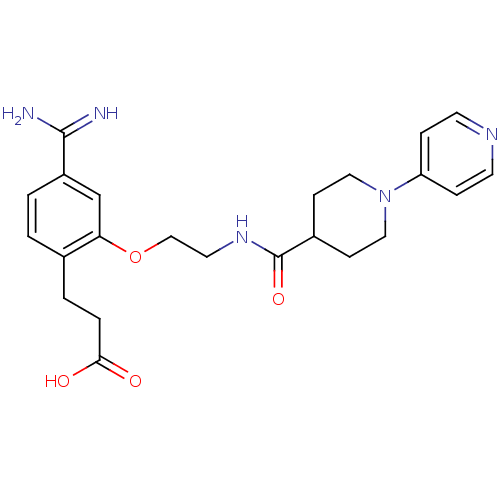
(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CCC(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H29N5O4/c24-22(25)18-2-1-16(3-4-21(29)30)20(15-18)32-14-11-27-23(31)17-7-12-28(13-8-17)19-5-9-26-10-6-19/h1-2,5-6,9-10,15,17H,3-4,7-8,11-14H2,(H3,24,25)(H,27,31)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127496
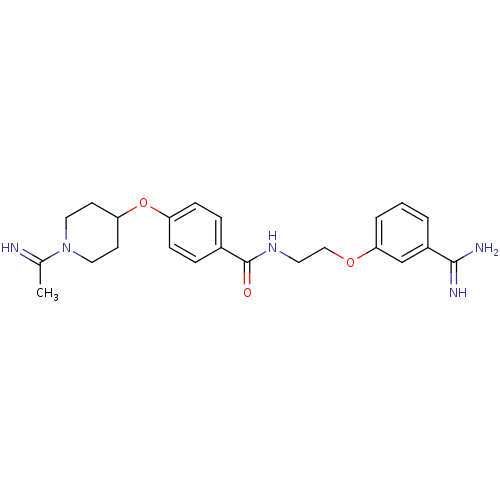
(CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cccc(c1)C(N)=N Show InChI InChI=1S/C23H29N5O3/c1-16(24)28-12-9-20(10-13-28)31-19-7-5-17(6-8-19)23(29)27-11-14-30-21-4-2-3-18(15-21)22(25)26/h2-8,15,20,24H,9-14H2,1H3,(H3,25,26)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127506
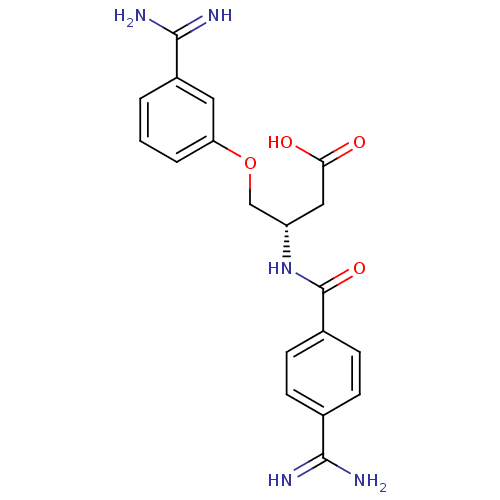
(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127497

(4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...)Show InChI InChI=1S/C17H19N5O2/c18-15(19)11-4-6-12(7-5-11)17(23)22-8-9-24-14-3-1-2-13(10-14)16(20)21/h1-7,10H,8-9H2,(H3,18,19)(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127500
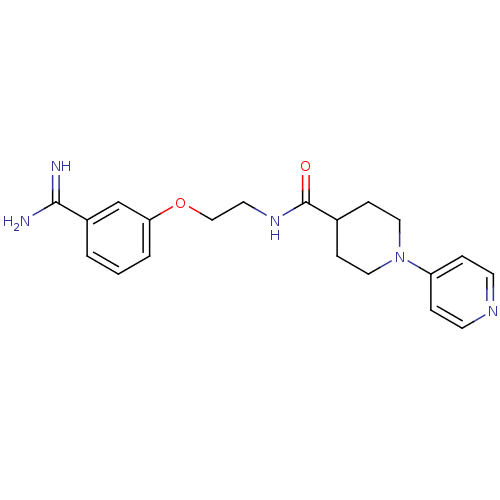
(3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C20H25N5O2/c21-19(22)16-2-1-3-18(14-16)27-13-10-24-20(26)15-6-11-25(12-7-15)17-4-8-23-9-5-17/h1-5,8-9,14-15H,6-7,10-13H2,(H3,21,22)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127499
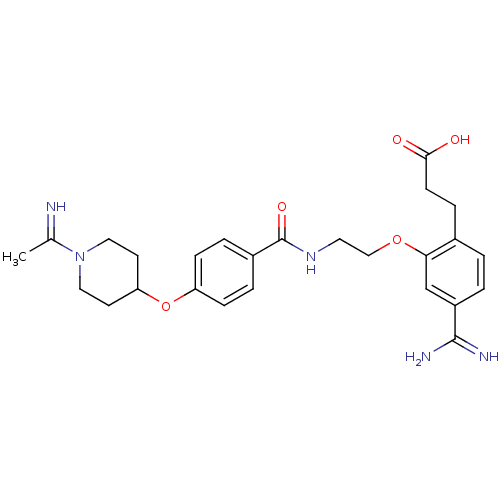
(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CCC(O)=O)C(N)=N Show InChI InChI=1S/C26H33N5O5/c1-17(27)31-13-10-22(11-14-31)36-21-7-4-19(5-8-21)26(34)30-12-15-35-23-16-20(25(28)29)3-2-18(23)6-9-24(32)33/h2-5,7-8,16,22,27H,6,9-15H2,1H3,(H3,28,29)(H,30,34)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50127505
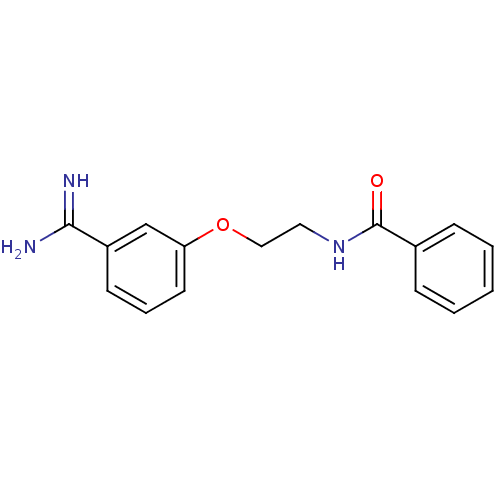
(CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show InChI InChI=1S/C16H17N3O2/c17-15(18)13-7-4-8-14(11-13)21-10-9-19-16(20)12-5-2-1-3-6-12/h1-8,11H,9-10H2,(H3,17,18)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X (fXa) |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127501

(3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C24H26N4O6/c25-21(26)18-8-7-17(13-19(29)24(32)33)20(14-18)34-12-9-27-22(30)15-3-5-16(6-4-15)23(31)28-10-1-2-11-28/h3-8,14H,1-2,9-13H2,(H3,25,26)(H,27,30)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127498

(3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C20H21N5O5/c21-17(22)11-1-3-12(4-2-11)19(27)25-7-8-30-16-10-14(18(23)24)6-5-13(16)9-15(26)20(28)29/h1-6,10H,7-9H2,(H3,21,22)(H3,23,24)(H,25,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127495

(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CC(=O)C(O)=O)C(N)=N Show InChI InChI=1S/C26H31N5O6/c1-16(27)31-11-8-21(9-12-31)37-20-6-4-17(5-7-20)25(33)30-10-13-36-23-15-19(24(28)29)3-2-18(23)14-22(32)26(34)35/h2-7,15,21,27H,8-14H2,1H3,(H3,28,29)(H,30,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127504

(CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)c2ccc(cc2)C(=O)N2CCCC2)c1 Show InChI InChI=1S/C21H24N4O3/c22-19(23)17-4-3-5-18(14-17)28-13-10-24-20(26)15-6-8-16(9-7-15)21(27)25-11-1-2-12-25/h3-9,14H,1-2,10-13H2,(H3,22,23)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127494

(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H27N5O5/c24-21(25)17-2-1-16(13-19(29)23(31)32)20(14-17)33-12-9-27-22(30)15-5-10-28(11-6-15)18-3-7-26-8-4-18/h1-4,7-8,14-15H,5-6,9-13H2,(H3,24,25)(H,27,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127496

(CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cccc(c1)C(N)=N Show InChI InChI=1S/C23H29N5O3/c1-16(24)28-12-9-20(10-13-28)31-19-7-5-17(6-8-19)23(29)27-11-14-30-21-4-2-3-18(15-21)22(25)26/h2-8,15,20,24H,9-14H2,1H3,(H3,25,26)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127492

(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)N[C@@H](COc1cc(ccc1CC(=O)C(O)=O)C(N)=N)CC(O)=O Show InChI InChI=1S/C28H33N5O8/c1-16(29)33-10-8-22(9-11-33)41-21-6-4-17(5-7-21)27(37)32-20(14-25(35)36)15-40-24-13-19(26(30)31)3-2-18(24)12-23(34)28(38)39/h2-7,13,20,22,29H,8-12,14-15H2,1H3,(H3,30,31)(H,32,37)(H,35,36)(H,38,39)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127502

(4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...)Show SMILES NC(=N)c1ccc(CC(=O)C(O)=O)c(OC[C@@H](CC(O)=O)NC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C25H29N5O7/c26-23(27)17-2-1-16(11-20(31)25(35)36)21(12-17)37-14-18(13-22(32)33)29-24(34)15-5-9-30(10-6-15)19-3-7-28-8-4-19/h1-4,7-8,12,15,18H,5-6,9-11,13-14H2,(H3,26,27)(H,29,34)(H,32,33)(H,35,36)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127497

(4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...)Show InChI InChI=1S/C17H19N5O2/c18-15(19)11-4-6-12(7-5-11)17(23)22-8-9-24-14-3-1-2-13(10-14)16(20)21/h1-7,10H,8-9H2,(H3,18,19)(H3,20,21)(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127505

(CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...)Show InChI InChI=1S/C16H17N3O2/c17-15(18)13-7-4-8-14(11-13)21-10-9-19-16(20)12-5-2-1-3-6-12/h1-8,11H,9-10H2,(H3,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127500

(3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...)Show SMILES NC(=N)c1cccc(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C20H25N5O2/c21-19(22)16-2-1-3-18(14-16)27-13-10-24-20(26)15-6-11-25(12-7-15)17-4-8-23-9-5-17/h1-5,8-9,14-15H,6-7,10-13H2,(H3,21,22)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127493

(3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...)Show SMILES NC(=N)c1ccc(CCC(O)=O)c(OCCNC(=O)C2CCN(CC2)c2ccncc2)c1 Show InChI InChI=1S/C23H29N5O4/c24-22(25)18-2-1-16(3-4-21(29)30)20(15-18)32-14-11-27-23(31)17-7-12-28(13-8-17)19-5-9-26-10-6-19/h1-2,5-6,9-10,15,17H,3-4,7-8,11-14H2,(H3,24,25)(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127503

(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127499

(3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1)C(=O)NCCOc1cc(ccc1CCC(O)=O)C(N)=N Show InChI InChI=1S/C26H33N5O5/c1-17(27)31-13-10-22(11-14-31)36-21-7-4-19(5-8-21)26(34)30-12-15-35-23-16-20(25(28)29)3-2-18(23)6-9-24(32)33/h2-5,7-8,16,22,27H,6,9-15H2,1H3,(H3,28,29)(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50127506

(3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N[C@H](COc1cccc(c1)C(N)=N)CC(O)=O Show InChI InChI=1S/C19H21N5O4/c20-17(21)11-4-6-12(7-5-11)19(27)24-14(9-16(25)26)10-28-15-3-1-2-13(8-15)18(22)23/h1-8,14H,9-10H2,(H3,20,21)(H3,22,23)(H,24,27)(H,25,26)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
J Med Chem 46: 1845-57 (2003)
Article DOI: 10.1021/jm020485x
BindingDB Entry DOI: 10.7270/Q2PZ586C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082445
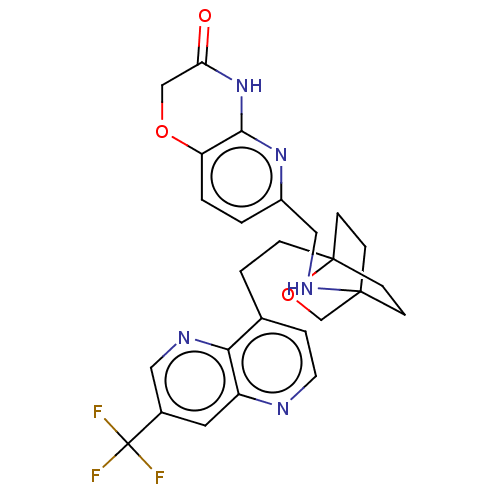
(CHEMBL3422978)Show SMILES FC(F)(F)c1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-5.09,-.93,;-4.02,-1.54,;-4.02,-2.78,;-5.09,-2.16,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)17-11-19-22(31-12-17)16(4-10-30-19)3-5-25-8-6-24(7-9-25,15-37-25)32-13-18-1-2-20-23(33-18)34-21(35)14-36-20/h1-2,4,10-12,32H,3,5-9,13-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4817

(N-[2-(dimethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo...)Show SMILES CN(C)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C20H23FN4O2/c1-11-17(23-12(2)18(11)20(27)22-7-8-25(3)4)10-15-14-9-13(21)5-6-16(14)24-19(15)26/h5-6,9-10,23H,7-8H2,1-4H3,(H,22,27)(H,24,26)/b15-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082380
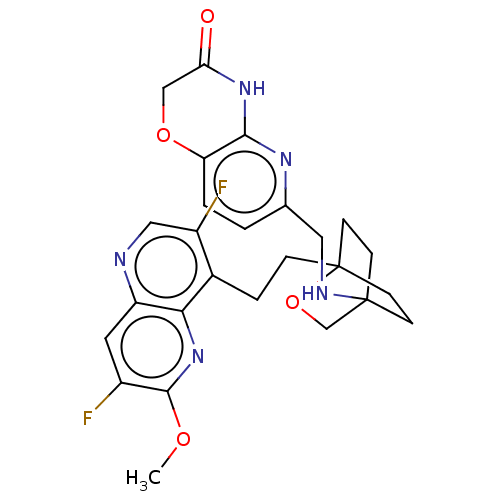
(CHEMBL3422952)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-24-17(27)10-19-22(33-24)16(18(28)12-29-19)4-5-26-8-6-25(7-9-26,14-37-26)30-11-15-2-3-20-23(31-15)32-21(34)13-36-20/h2-3,10,12,30H,4-9,11,13-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082429

(CHEMBL3422970)Show SMILES Cc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-3.75,1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-19(13-28)12-21-24(31-17)18(5-11-29-21)4-6-27-9-7-26(8-10-27,16-36-27)30-14-20-2-3-22-25(32-20)33-23(34)15-35-22/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082385
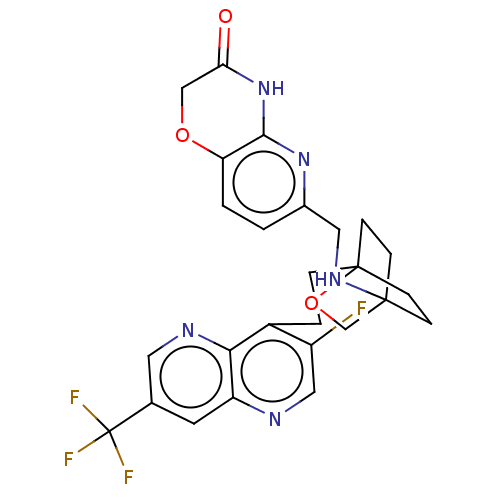
(CHEMBL3422959)Show SMILES Fc1cnc2cc(cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C(F)(F)F Show InChI InChI=1S/C26H25F4N5O3/c27-18-12-31-19-9-15(26(28,29)30)10-32-22(19)17(18)3-4-25-7-5-24(6-8-25,14-38-25)33-11-16-1-2-20-23(34-16)35-21(36)13-37-20/h1-2,9-10,12,33H,3-8,11,13-14H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082440

(CHEMBL3422977)Show SMILES FC(F)(F)c1ccc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,.93,;-4.02,1.54,;-4.02,2.78,;-5.09,2.16,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)20-4-2-18-22(33-20)16(6-12-30-18)5-7-25-10-8-24(9-11-25,15-37-25)31-13-17-1-3-19-23(32-17)34-21(35)14-36-19/h1-4,6,12,31H,5,7-11,13-15H2,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4821
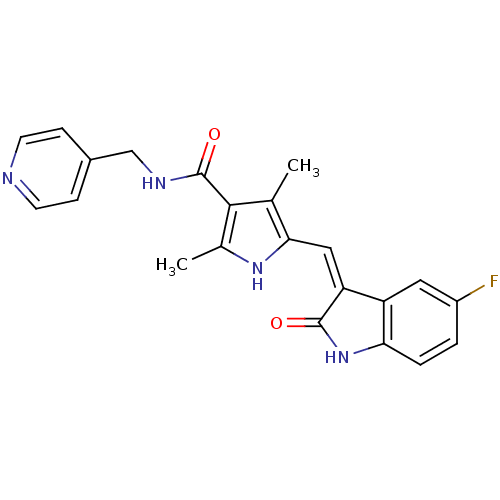
(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCc1ccncc1 Show InChI InChI=1S/C22H19FN4O2/c1-12-19(10-17-16-9-15(23)3-4-18(16)27-21(17)28)26-13(2)20(12)22(29)25-11-14-5-7-24-8-6-14/h3-10,26H,11H2,1-2H3,(H,25,29)(H,27,28)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4818

(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCCN1CCCC1 Show InChI InChI=1S/C22H25FN4O2/c1-13-19(12-17-16-11-15(23)5-6-18(16)26-21(17)28)25-14(2)20(13)22(29)24-7-10-27-8-3-4-9-27/h5-6,11-12,25H,3-4,7-10H2,1-2H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082435
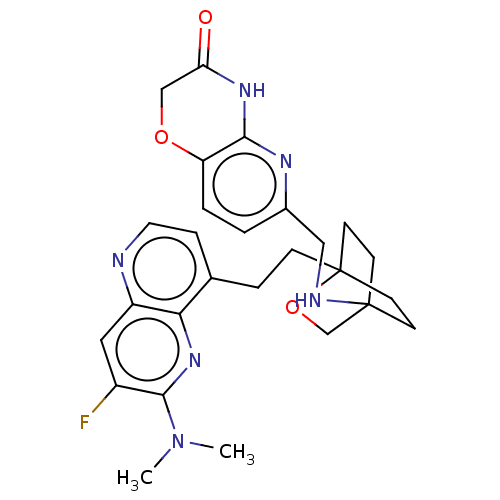
(CHEMBL3422976)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C27H31FN6O3/c1-34(2)25-19(28)13-20-23(33-25)17(6-12-29-20)5-7-27-10-8-26(9-11-27,16-37-27)30-14-18-3-4-21-24(31-18)32-22(35)15-36-21/h3-4,6,12-13,30H,5,7-11,14-16H2,1-2H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082382
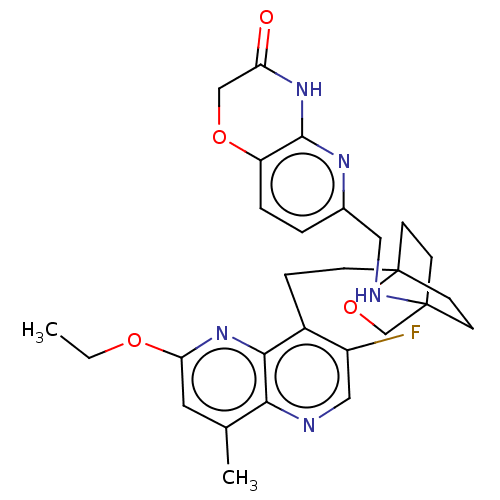
(CHEMBL3422954)Show SMILES CCOc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,3.71,;-4.02,3.09,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-3-36-23-12-17(2)24-25(34-23)19(20(29)14-30-24)6-7-28-10-8-27(9-11-28,16-38-28)31-13-18-4-5-21-26(32-18)33-22(35)15-37-21/h4-5,12,14,31H,3,6-11,13,15-16H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50437671
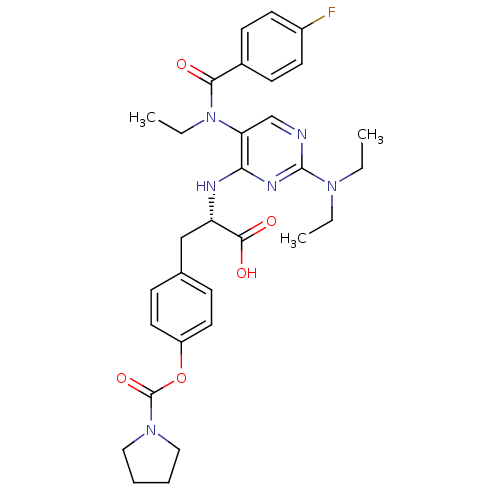
(CHEMBL2408057)Show SMILES CCN(CC)c1ncc(N(CC)C(=O)c2ccc(F)cc2)c(N[C@@H](Cc2ccc(OC(=O)N3CCCC3)cc2)C(O)=O)n1 |r| Show InChI InChI=1S/C31H37FN6O5/c1-4-36(5-2)30-33-20-26(38(6-3)28(39)22-11-13-23(32)14-12-22)27(35-30)34-25(29(40)41)19-21-9-15-24(16-10-21)43-31(42)37-17-7-8-18-37/h9-16,20,25H,4-8,17-19H2,1-3H3,(H,40,41)(H,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of alpha4beta1 integrin-mediated human jurkat cell adhesion to fibronectin in absence of human serum albumin |
Bioorg Med Chem Lett 23: 4370-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.076
BindingDB Entry DOI: 10.7270/Q22J6D91 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082390
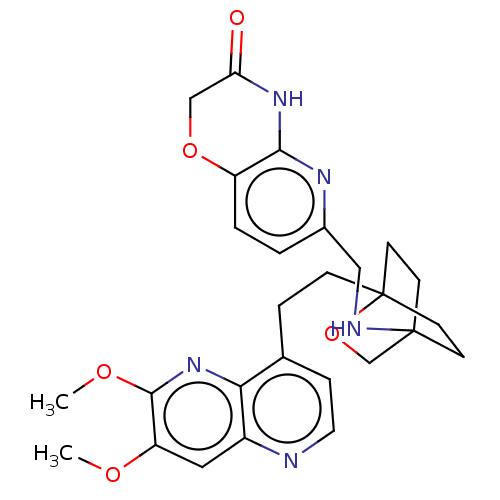
(CHEMBL3422964)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1OC |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,)| Show InChI InChI=1S/C27H31N5O5/c1-34-21-13-19-23(32-25(21)35-2)17(6-12-28-19)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-20-24(30-18)31-22(33)15-36-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50437674
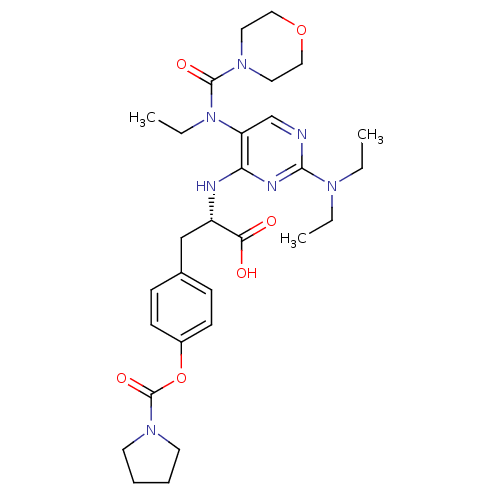
(CHEMBL2408066)Show SMILES CCN(CC)c1ncc(N(CC)C(=O)N2CCOCC2)c(N[C@@H](Cc2ccc(OC(=O)N3CCCC3)cc2)C(O)=O)n1 |r| Show InChI InChI=1S/C29H41N7O6/c1-4-33(5-2)27-30-20-24(36(6-3)28(39)34-15-17-41-18-16-34)25(32-27)31-23(26(37)38)19-21-9-11-22(12-10-21)42-29(40)35-13-7-8-14-35/h9-12,20,23H,4-8,13-19H2,1-3H3,(H,37,38)(H,30,31,32)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of alpha4beta1 integrin-mediated human jurkat cell adhesion to fibronectin in absence of human serum albumin |
Bioorg Med Chem Lett 23: 4370-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.076
BindingDB Entry DOI: 10.7270/Q22J6D91 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082394

(CHEMBL3422966)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-25-18(13-28)12-20-23(33-25)17(5-11-29-20)4-6-27-9-7-26(8-10-27,16-37-27)30-14-19-2-3-21-24(31-19)32-22(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50437660
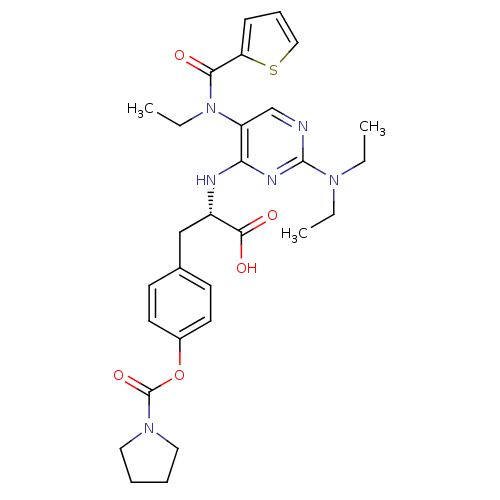
(CHEMBL2408058)Show SMILES CCN(CC)c1ncc(N(CC)C(=O)c2cccs2)c(N[C@@H](Cc2ccc(OC(=O)N3CCCC3)cc2)C(O)=O)n1 |r| Show InChI InChI=1S/C29H36N6O5S/c1-4-33(5-2)28-30-19-23(35(6-3)26(36)24-10-9-17-41-24)25(32-28)31-22(27(37)38)18-20-11-13-21(14-12-20)40-29(39)34-15-7-8-16-34/h9-14,17,19,22H,4-8,15-16,18H2,1-3H3,(H,37,38)(H,30,31,32)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of alpha4beta1 integrin-mediated human jurkat cell adhesion to fibronectin in absence of human serum albumin |
Bioorg Med Chem Lett 23: 4370-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.076
BindingDB Entry DOI: 10.7270/Q22J6D91 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082383

(CHEMBL3422957)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C27H27F4N5O4/c1-38-24-17(27(29,30)31)10-19-22(36-24)16(18(28)12-32-19)4-5-26-8-6-25(7-9-26,14-40-26)33-11-15-2-3-20-23(34-15)35-21(37)13-39-20/h2-3,10,12,33H,4-9,11,13-14H2,1H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082388
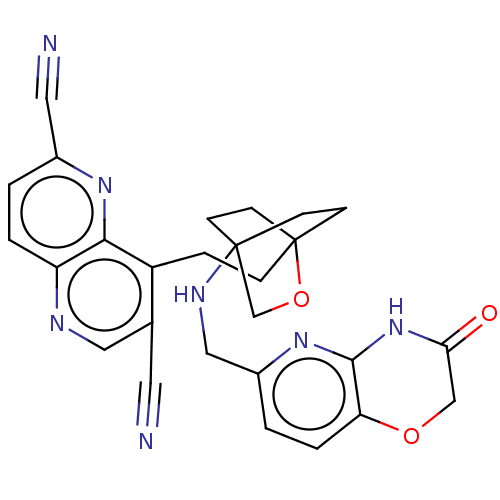
(CHEMBL3422962)Show SMILES O=C1COc2ccc(CNC34CCC(CCc5c(cnc6ccc(nc56)C#N)C#N)(CC3)OC4)nc2N1 |(10.96,15.37,;9.76,15.09,;8.71,16.22,;7.21,15.87,;6.76,14.39,;5.26,14.04,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.09,2.16,;4,1.54,;5.07,2.16,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,)| Show InChI InChI=1S/C27H25N7O3/c28-11-17-13-30-21-3-1-18(12-29)32-24(21)20(17)5-6-27-9-7-26(8-10-27,16-37-27)31-14-19-2-4-22-25(33-19)34-23(35)15-36-22/h1-4,13,31H,5-10,14-16H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082427
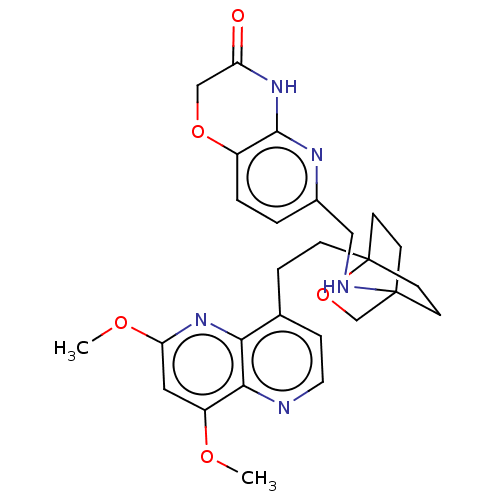
(CHEMBL3422968)Show SMILES COc1cc(OC)c2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-3.08,;-2.39,-3.71,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-20-13-22(35-2)32-23-17(6-12-28-24(20)23)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-19-25(30-18)31-21(33)15-36-19/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50437663
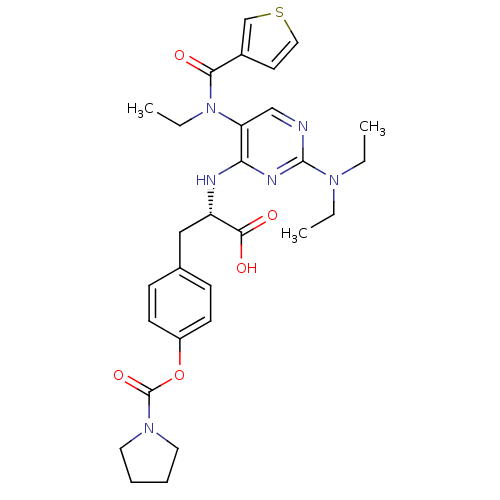
(CHEMBL2408059)Show SMILES CCN(CC)c1ncc(N(CC)C(=O)c2ccsc2)c(N[C@@H](Cc2ccc(OC(=O)N3CCCC3)cc2)C(O)=O)n1 |r| Show InChI InChI=1S/C29H36N6O5S/c1-4-33(5-2)28-30-18-24(35(6-3)26(36)21-13-16-41-19-21)25(32-28)31-23(27(37)38)17-20-9-11-22(12-10-20)40-29(39)34-14-7-8-15-34/h9-13,16,18-19,23H,4-8,14-15,17H2,1-3H3,(H,37,38)(H,30,31,32)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of alpha4beta1 integrin-mediated human jurkat cell adhesion to fibronectin in absence of human serum albumin |
Bioorg Med Chem Lett 23: 4370-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.076
BindingDB Entry DOI: 10.7270/Q22J6D91 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM4820
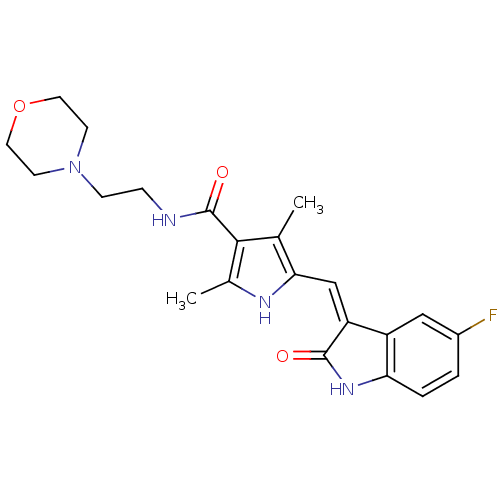
(5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C22H25FN4O3/c1-13-19(12-17-16-11-15(23)3-4-18(16)26-21(17)28)25-14(2)20(13)22(29)24-5-6-27-7-9-30-10-8-27/h3-4,11-12,25H,5-10H2,1-2H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc.
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... |
J Med Chem 46: 1116-9 (2003)
Article DOI: 10.1021/jm0204183
BindingDB Entry DOI: 10.7270/Q2D50K5B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data