 Found 545 hits with Last Name = 'gibbs' and Initial = 'b'
Found 545 hits with Last Name = 'gibbs' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50004744

(CHEMBL2370453 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCCCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:132.137,4.4,65.73,49.54,26.34,96.105,17.22,37.38,166.171,81.88,145.149,174.179,wD:107.108,75.79,57.62,8.13,154.158,114.125,136.140,2.2,(23.18,-7.9,;24.72,-8.01,;25.58,-6.74,;24.91,-5.35,;27.12,-6.85,;27.79,-8.23,;26.93,-9.51,;25.39,-9.4,;27.6,-10.89,;29.14,-11.01,;30,-9.73,;31.54,-9.84,;32.21,-11.23,;32.4,-8.57,;26.74,-12.17,;27.41,-13.55,;28.94,-13.67,;26.54,-14.83,;25.01,-14.72,;24.14,-15.99,;22.61,-15.88,;21.94,-14.5,;21.74,-17.16,;27.22,-16.22,;26.35,-17.49,;24.82,-17.38,;27.02,-18.88,;26.16,-20.15,;26.83,-21.54,;25.97,-22.81,;26.64,-24.2,;28.18,-24.31,;29.04,-23.03,;28.37,-21.65,;28.56,-18.99,;29.42,-17.71,;28.75,-16.33,;30.96,-17.82,;31.63,-19.21,;30.77,-20.48,;31.44,-21.87,;29.23,-20.37,;31.82,-16.55,;33.36,-16.66,;34.03,-18.04,;34.22,-15.38,;35.76,-15.49,;36.62,-14.22,;35.95,-12.83,;38.16,-14.33,;38.83,-15.72,;40.37,-15.83,;41.04,-17.21,;41.82,-15.3,;39.02,-13.06,;40.56,-13.17,;41.23,-14.55,;41.42,-11.89,;40.75,-10.51,;41.62,-9.23,;42.92,-8.42,;40.94,-7.85,;42.96,-12,;43.82,-10.73,;43.15,-9.34,;45.36,-10.84,;46.03,-12.22,;47.57,-12.34,;48.38,-13.64,;49.88,-13.27,;49.99,-11.74,;48.56,-11.16,;46.22,-9.56,;47.76,-9.67,;49.12,-10.4,;48.62,-8.4,;47.95,-7.01,;46.42,-6.9,;50.16,-8.51,;51.02,-7.24,;50.35,-5.85,;52.56,-7.35,;53.23,-8.73,;54.77,-8.84,;55.44,-10.23,;54.58,-11.5,;56.98,-10.34,;53.42,-6.07,;54.96,-6.18,;55.63,-7.57,;55.82,-4.91,;57.36,-5.02,;58.22,-3.74,;59.76,-3.86,;60.62,-2.58,;59.95,-1.19,;62.16,-2.69,;62.83,-4.08,;61.97,-5.35,;62.64,-6.74,;64.18,-6.85,;64.85,-8.23,;63.98,-9.51,;66.38,-8.34,;63.02,-1.42,;64.56,-1.53,;65.23,-2.91,;65.42,-.25,;64.9,1.2,;66.12,2.14,;67.39,1.28,;66.96,-.2,;67.91,-1.42,;67.33,-2.84,;69.43,-1.21,;70.01,.22,;71.54,.43,;72.48,-.79,;74.01,-.57,;74.59,.85,;73.64,2.07,;72.12,1.86,;70.38,-2.42,;71.9,-2.21,;72.85,-3.43,;73.21,-1.4,;27.98,-5.57,;29.52,-5.68,;27.31,-4.19,;25.8,-3.92,;25.58,-2.39,;26.97,-1.72,;28.04,-2.83,;29.56,-2.62,;30.51,-3.83,;30.14,-1.19,;31.67,-.98,;32.61,-2.2,;32.03,-3.62,;32.98,-4.84,;32.4,-6.27,;34.5,-4.63,;32.25,.45,;31.3,1.66,;33.77,.66,;34.35,2.08,;33.41,3.3,;31.88,3.09,;30.94,4.3,;31.52,5.73,;29.41,4.09,;35.88,2.29,;36.83,1.08,;36.46,3.72,;37.99,3.93,;38.93,2.72,;40.46,2.93,;41.4,1.71,;42.93,1.92,;43.51,3.35,;45.03,3.56,;42.56,4.56,;41.04,4.35,;38.57,5.36,;37.62,6.57,;40.09,5.57,;40.67,7,;39.73,8.21,;40.31,9.64,;39.36,10.85,;41.83,9.85,;42.2,7.21,;43.14,5.99,;42.78,8.63,;44.3,8.84,;44.88,10.27,;46.41,10.48,;46.99,11.91,;46.04,13.12,;48.51,12.12,;45.25,7.63,;44.67,6.2,;46.77,7.84,)| Show InChI InChI=1S/C115H162N28O40/c1-6-59(4)96(113(181)143-45-17-24-83(143)111(179)132-70(35-41-92(157)158)100(168)129-69(34-40-91(155)156)101(169)135-75(48-63-27-29-65(146)30-28-63)105(173)134-73(46-58(2)3)103(171)133-72(114(182)183)32-38-86(117)149)141-102(170)71(36-42-93(159)160)130-99(167)68(33-39-90(153)154)131-104(172)74(47-61-18-9-7-10-19-61)136-108(176)79(53-95(163)164)127-89(152)55-123-97(165)78(52-94(161)162)139-107(175)77(51-87(118)150)138-106(174)76(50-64-54-121-57-124-64)137-109(177)81(56-144)140-98(166)67(31-37-85(116)148)126-88(151)26-14-13-25-84(147)66(22-15-43-122-115(119)120)128-110(178)82-23-16-44-142(82)112(180)80(125-60(5)145)49-62-20-11-8-12-21-62/h7-12,18-21,27-30,54,57-59,66-83,96,144,146H,6,13-17,22-26,31-53,55-56H2,1-5H3,(H2,116,148)(H2,117,149)(H2,118,150)(H,121,124)(H,123,165)(H,125,145)(H,126,151)(H,127,152)(H,128,178)(H,129,168)(H,130,167)(H,131,172)(H,132,179)(H,133,171)(H,134,173)(H,135,169)(H,136,176)(H,137,177)(H,138,174)(H,139,175)(H,140,166)(H,141,170)(H,153,154)(H,155,156)(H,157,158)(H,159,160)(H,161,162)(H,163,164)(H,182,183)(H4,119,120,122)/t59-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,96-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004739
(CHEMBL2370455 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:130.135,4.4,105.106,65.73,49.54,26.34,94.103,17.22,37.38,112.123,81.88,143.147,172.177,wD:75.79,57.62,8.13,152.156,164.169,134.138,2.2,(23.16,-12.23,;24.7,-12.29,;25.52,-10.99,;24.81,-9.62,;27.06,-11.05,;27.78,-12.41,;26.96,-13.71,;25.42,-13.65,;27.68,-15.07,;29.22,-15.13,;30.04,-13.83,;31.58,-13.89,;32.29,-15.25,;32.4,-12.59,;26.86,-16.38,;27.57,-17.74,;29.11,-17.8,;26.75,-19.04,;25.22,-18.98,;24.39,-20.29,;22.86,-20.23,;22.14,-18.86,;22.03,-21.53,;27.47,-20.4,;26.65,-21.71,;25.11,-21.65,;27.37,-23.07,;26.55,-24.37,;27.27,-25.74,;28.81,-25.79,;29.52,-27.16,;28.7,-28.46,;27.16,-28.4,;26.45,-27.04,;28.91,-23.13,;29.73,-21.83,;29.01,-20.46,;31.27,-21.89,;31.99,-23.25,;31.17,-24.55,;31.88,-25.91,;29.63,-24.49,;32.09,-20.58,;33.63,-20.64,;34.35,-22,;34.45,-19.34,;35.99,-19.4,;36.81,-18.09,;36.09,-16.73,;38.35,-18.15,;39.07,-19.52,;40.6,-19.58,;42.03,-19,;41.32,-20.94,;39.17,-16.85,;40.71,-16.91,;41.42,-18.27,;41.53,-15.61,;40.81,-14.24,;41.63,-12.94,;40.91,-11.58,;42.91,-12.08,;43.07,-15.67,;43.89,-14.36,;43.17,-13,;45.43,-14.42,;46.14,-15.78,;47.68,-15.84,;48.54,-17.12,;50.02,-16.7,;50.08,-15.17,;48.64,-14.63,;46.25,-13.12,;47.79,-13.18,;49.17,-13.86,;48.61,-11.88,;47.89,-10.51,;46.35,-10.45,;50.14,-11.93,;50.97,-10.63,;50.25,-9.27,;52.5,-10.69,;53.22,-12.05,;54.76,-12.11,;55.48,-13.47,;54.66,-14.78,;57.02,-13.53,;53.33,-9.39,;54.86,-9.45,;55.58,-10.81,;55.68,-8.14,;57.22,-8.2,;58.04,-6.9,;57.33,-5.54,;59.58,-6.96,;60.3,-8.32,;59.48,-9.62,;60.2,-10.99,;61.74,-11.05,;62.46,-12.41,;61.63,-13.71,;63.99,-12.47,;60.4,-5.66,;61.94,-5.72,;62.66,-7.08,;62.76,-4.41,;64.3,-4.31,;64.68,-2.82,;63.37,-2,;62.19,-2.98,;60.7,-2.6,;59.63,-3.71,;60.28,-1.12,;61.35,-.02,;60.93,1.46,;59.44,1.84,;59.02,3.32,;60.1,4.43,;61.59,4.05,;62.01,2.57,;58.79,-.74,;58.37,.74,;56.87,1.11,;58.59,2.26,;27.88,-9.74,;29.42,-9.8,;27.16,-8.38,;25.64,-8.16,;25.38,-6.64,;26.74,-5.93,;27.84,-7,;29.36,-6.74,;30.35,-7.92,;29.89,-5.29,;31.41,-5.03,;32.4,-6.21,;31.87,-7.66,;32.85,-8.84,;34.37,-8.58,;32.32,-10.29,;31.94,-3.58,;30.96,-2.4,;33.46,-3.32,;33.99,-1.88,;33.01,-.69,;31.49,-.96,;30.5,.23,;31.04,1.67,;28.99,-.03,;35.51,-1.62,;36.5,-2.8,;36.04,-.17,;37.56,.09,;38.55,-1.09,;40.06,-.83,;41.05,-2.01,;42.57,-1.75,;43.1,-.31,;44.62,-.04,;42.11,.88,;40.59,.62,;38.09,1.54,;37.11,2.72,;39.61,1.8,;40.14,3.24,;41.66,3.51,;42.64,2.32,;44.16,2.59,;43.06,.84,;39.16,4.43,;37.64,4.17,;39.69,5.87,;41.2,6.13,;41.74,7.58,;43.25,7.84,;43.79,9.29,;45.3,9.55,;42.8,10.47,;42.19,4.95,;42.61,3.47,;43.71,5.21,)| Show InChI InChI=1S/C113H158N28O40/c1-6-57(4)94(111(179)141-43-15-22-81(141)109(177)130-68(31-39-90(155)156)98(166)127-67(30-38-89(153)154)99(167)133-73(46-61-23-25-63(144)26-24-61)103(171)132-71(44-56(2)3)101(169)131-70(112(180)181)28-35-84(115)147)139-100(168)69(32-40-91(157)158)128-97(165)66(29-37-88(151)152)129-102(170)72(45-59-16-9-7-10-17-59)134-106(174)77(51-93(161)162)125-87(150)53-121-95(163)76(50-92(159)160)137-105(173)75(49-85(116)148)136-104(172)74(48-62-52-119-55-122-62)135-107(175)79(54-142)138-96(164)65(27-34-83(114)146)124-86(149)36-33-82(145)64(20-13-41-120-113(117)118)126-108(176)80-21-14-42-140(80)110(178)78(123-58(5)143)47-60-18-11-8-12-19-60/h7-12,16-19,23-26,52,55-57,64-81,94,142,144H,6,13-15,20-22,27-51,53-54H2,1-5H3,(H2,114,146)(H2,115,147)(H2,116,148)(H,119,122)(H,121,163)(H,123,143)(H,124,149)(H,125,150)(H,126,176)(H,127,166)(H,128,165)(H,129,170)(H,130,177)(H,131,169)(H,132,171)(H,133,167)(H,134,174)(H,135,175)(H,136,172)(H,137,173)(H,138,164)(H,139,168)(H,151,152)(H,153,154)(H,155,156)(H,157,158)(H,159,160)(H,161,162)(H,180,181)(H4,117,118,120)/t57-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,94-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004745
(CHEMBL2370450 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:75.79,57.62,95.104,8.13,153.157,135.139,wD:131.136,4.4,106.107,65.73,49.54,26.34,17.22,37.38,165.170,113.124,81.88,144.148,173.178,2.2,(30.53,-12.45,;32.01,-12.05,;32.41,-10.56,;31.32,-9.47,;33.9,-10.16,;34.29,-8.67,;33.2,-7.58,;31.72,-7.98,;33.6,-6.1,;35.09,-5.7,;35.48,-4.21,;36.97,-3.81,;37.37,-2.32,;38.06,-4.89,;32.51,-5.01,;32.91,-3.52,;34.39,-3.12,;31.82,-2.43,;30.33,-2.83,;29.93,-4.32,;28.44,-4.72,;27.35,-3.63,;28.05,-6.21,;32.21,-.94,;31.12,.14,;29.63,-.26,;31.52,1.63,;30.43,2.72,;30.82,4.21,;32.31,4.61,;32.71,6.09,;31.62,7.18,;30.13,6.78,;29.73,5.29,;33,2.03,;34.1,.94,;33.7,-.54,;35.58,1.34,;35.98,2.83,;34.89,3.92,;33.4,3.52,;35.28,5.41,;36.67,.26,;38.16,.66,;38.56,2.15,;39.25,-.43,;40.74,-.03,;41.83,-1.12,;41.43,-2.6,;43.31,-.72,;43.71,.77,;45.2,1.17,;45.59,2.66,;46.72,.93,;44.4,-1.8,;45.89,-1.4,;46.29,.09,;46.98,-2.49,;46.59,-3.98,;47.68,-5.07,;49.11,-5.62,;47.28,-6.55,;48.47,-2.09,;49.56,-3.18,;49.16,-4.67,;51.05,-2.78,;51.44,-1.29,;52.93,-.89,;54.13,-1.86,;55.42,-1.01,;55.02,.47,;53.48,.55,;52.14,-3.86,;53.62,-3.46,;54.82,-2.49,;54.71,-4.55,;54.32,-6.04,;52.83,-6.44,;56.2,-4.15,;57.29,-5.24,;56.9,-6.73,;58.78,-4.84,;59.18,-3.35,;58.08,-2.26,;58.48,-.77,;59.97,-.37,;57.39,.31,;59.87,-5.92,;61.36,-5.52,;61.75,-4.04,;62.45,-6.61,;63.93,-6.21,;65.02,-7.3,;66.51,-6.9,;66.91,-5.41,;67.6,-7.98,;67.21,-9.47,;68.3,-10.56,;67.9,-12.05,;68.99,-13.14,;68.59,-14.62,;67.11,-15.02,;69.68,-15.71,;69.09,-7.58,;69.49,-6.1,;68.39,-5.01,;70.97,-5.7,;71.52,-4.26,;73.06,-4.34,;73.46,-5.82,;72.17,-6.66,;72.09,-8.2,;70.72,-8.9,;73.38,-9.04,;74.76,-8.34,;76.05,-9.17,;75.97,-10.71,;77.26,-11.55,;78.63,-10.85,;78.71,-9.31,;77.42,-8.47,;73.31,-10.58,;74.6,-11.41,;74.52,-12.95,;76.12,-11.65,;34.99,-11.25,;36.47,-10.85,;34.59,-12.74,;33.15,-13.29,;33.24,-14.83,;34.72,-15.22,;35.56,-13.93,;37.1,-13.85,;37.8,-12.47,;37.94,-15.14,;39.48,-15.06,;40.17,-13.68,;39.33,-12.39,;40.03,-11.02,;39.19,-9.73,;41.57,-10.94,;40.32,-16.35,;39.62,-17.72,;41.86,-16.26,;42.7,-17.55,;42,-18.93,;40.46,-19.01,;39.76,-20.38,;40.6,-21.67,;38.23,-20.47,;44.23,-17.47,;44.93,-16.1,;45.07,-18.76,;46.61,-18.68,;47.31,-17.31,;48.85,-17.22,;49.69,-18.51,;51.23,-18.43,;51.92,-17.06,;53.46,-16.97,;51.08,-15.77,;49.54,-15.85,;47.45,-19.97,;46.76,-21.34,;48.99,-19.89,;49.83,-21.18,;49.13,-22.55,;49.97,-23.84,;49.28,-25.21,;51.51,-23.76,;51.37,-21.09,;52.07,-19.72,;52.21,-22.38,;53.75,-22.3,;54.59,-23.59,;56.13,-23.51,;56.97,-24.8,;56.27,-26.17,;58.5,-24.72,;54.44,-20.93,;53.6,-19.64,;55.98,-20.85,)| Show InChI InChI=1S/C114H160N28O40/c1-6-58(4)95(112(180)142-44-16-23-82(142)110(178)131-69(34-40-91(156)157)99(167)128-68(33-39-90(154)155)100(168)134-74(47-62-26-28-64(145)29-27-62)104(172)133-72(45-57(2)3)102(170)132-71(113(181)182)31-37-85(116)148)140-101(169)70(35-41-92(158)159)129-98(166)67(32-38-89(152)153)130-103(171)73(46-60-17-9-7-10-18-60)135-107(175)78(52-94(162)163)126-88(151)54-122-96(164)77(51-93(160)161)138-106(174)76(50-86(117)149)137-105(173)75(49-63-53-120-56-123-63)136-108(176)80(55-143)139-97(165)66(30-36-84(115)147)125-87(150)25-13-24-83(146)65(21-14-42-121-114(118)119)127-109(177)81-22-15-43-141(81)111(179)79(124-59(5)144)48-61-19-11-8-12-20-61/h7-12,17-20,26-29,53,56-58,65-82,95,143,145H,6,13-16,21-25,30-52,54-55H2,1-5H3,(H2,115,147)(H2,116,148)(H2,117,149)(H,120,123)(H,122,164)(H,124,144)(H,125,150)(H,126,151)(H,127,177)(H,128,167)(H,129,166)(H,130,171)(H,131,178)(H,132,170)(H,133,172)(H,134,168)(H,135,175)(H,136,176)(H,137,173)(H,138,174)(H,139,165)(H,140,169)(H,152,153)(H,154,155)(H,156,157)(H,158,159)(H,160,161)(H,162,163)(H,181,182)(H4,118,119,121)/t58-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,95-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004743

(Ac-(D)Phe-Pro-Arg.Pro.Gln.Ser-H~s.Asn-AspGly-Asp-P...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C92H132N24O28/c1-7-49(4)75(90(142)115-35-17-25-65(115)84(136)109-62(91(143)144)37-48(2)3)111-85(137)66-26-15-33-113(66)86(138)55(29-31-72(122)123)104-78(130)56(38-51-19-10-8-11-20-51)105-81(133)60(43-74(126)127)102-71(121)45-99-76(128)59(42-73(124)125)108-80(132)58(41-70(94)120)107-79(131)57(40-53-44-97-47-100-53)106-82(134)63(46-117)110-77(129)54(28-30-69(93)119)103-83(135)64-24-16-34-114(64)89(141)67(23-14-32-98-92(95)96)112(6)88(140)68-27-18-36-116(68)87(139)61(101-50(5)118)39-52-21-12-9-13-22-52/h8-13,19-22,44,47-49,54-68,75,117H,7,14-18,23-43,45-46H2,1-6H3,(H2,93,119)(H2,94,120)(H,97,100)(H,99,128)(H,101,118)(H,102,121)(H,103,135)(H,104,130)(H,105,133)(H,106,134)(H,107,131)(H,108,132)(H,109,136)(H,110,129)(H,111,137)(H,122,123)(H,124,125)(H,126,127)(H,143,144)(H4,95,96,98)/t49-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,75-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004742
(CHEMBL385670 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)C\C=C\CNC(=O)C\C=C\CNC(=O)C\C=C\CNC(=O)CCCCC(=O)C(CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C82H118N16O20/c1-6-52(4)72(80(116)98-46-24-31-63(98)76(112)94-61(81(117)118)47-51(2)3)95-77(113)64-32-23-44-96(64)78(114)57(38-39-70(105)106)92-73(109)58(48-54-25-9-7-10-26-54)93-74(110)59(50-71(107)108)90-69(104)37-17-20-42-87-68(103)36-16-19-41-86-67(102)35-15-18-40-85-66(101)34-14-13-33-65(100)56(29-21-43-88-82(83)84)91-75(111)62-30-22-45-97(62)79(115)60(89-53(5)99)49-55-27-11-8-12-28-55/h7-12,15-20,25-28,51-52,56-64,72H,6,13-14,21-24,29-50H2,1-5H3,(H,85,101)(H,86,102)(H,87,103)(H,89,99)(H,90,104)(H,91,111)(H,92,109)(H,93,110)(H,94,112)(H,95,113)(H,105,106)(H,107,108)(H,117,118)(H4,83,84,88)/b18-15+,19-16+,20-17+/t52-,56?,57-,58-,59-,60-,61-,62-,63-,64-,72-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004741
(CHEMBL427978 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)C\C=C\CNC(=O)C\C=C\CNC(=O)CCCCC(=O)C(CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C77H111N15O19/c1-6-48(4)67(75(109)92-42-22-29-59(92)71(105)88-57(76(110)111)43-47(2)3)89-72(106)60-30-21-40-90(60)73(107)53(35-36-65(98)99)86-68(102)54(44-50-23-9-7-10-24-50)87-69(103)55(46-66(100)101)84-64(97)34-16-18-38-81-63(96)33-15-17-37-80-62(95)32-14-13-31-61(94)52(27-19-39-82-77(78)79)85-70(104)58-28-20-41-91(58)74(108)56(83-49(5)93)45-51-25-11-8-12-26-51/h7-12,15-18,23-26,47-48,52-60,67H,6,13-14,19-22,27-46H2,1-5H3,(H,80,95)(H,81,96)(H,83,93)(H,84,97)(H,85,104)(H,86,102)(H,87,103)(H,88,105)(H,89,106)(H,98,99)(H,100,101)(H,110,111)(H4,78,79,82)/b17-15+,18-16+/t48-,52?,53-,54-,55-,56-,57-,58-,59-,60-,67-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004738
(CHEMBL2370451 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:129.134,104.105,75.79,57.62,93.102,8.13,163.168,111.122,142.146,171.176,wD:4.4,65.73,49.54,26.34,17.22,37.38,151.155,81.88,133.137,2.2,(44.24,-11.55,;45.73,-11.18,;46.16,-9.7,;45.09,-8.59,;47.66,-9.33,;48.08,-7.85,;47.02,-6.74,;45.52,-7.11,;47.44,-5.26,;48.94,-4.89,;49.36,-3.41,;50.86,-3.04,;51.29,-1.56,;51.93,-4.15,;46.37,-4.15,;46.8,-2.67,;48.3,-2.3,;45.73,-1.56,;44.24,-1.93,;43.81,-3.41,;42.32,-3.78,;41.25,-2.67,;41.89,-5.26,;46.16,-.08,;45.09,1.03,;43.6,.66,;45.52,2.51,;44.45,3.62,;44.88,5.1,;46.37,5.47,;46.8,6.94,;45.73,8.05,;44.24,7.68,;43.81,6.2,;47.02,2.88,;48.08,1.77,;47.66,.29,;49.58,2.14,;50.01,3.62,;48.94,4.73,;47.44,4.36,;49.36,6.2,;50.65,1.03,;52.14,1.4,;52.57,2.88,;53.21,.29,;54.7,.66,;55.77,-.45,;55.34,-1.93,;57.27,-.08,;57.69,1.4,;59.19,1.77,;59.62,3.25,;60.7,1.5,;58.33,-1.19,;59.83,-.82,;60.26,.66,;60.9,-1.93,;60.47,-3.41,;61.54,-4.52,;61.11,-6,;62.96,-5.1,;62.39,-1.56,;63.46,-2.67,;63.03,-4.15,;64.95,-2.3,;65.38,-.82,;66.88,-.45,;68.05,-1.44,;69.36,-.63,;68.99,.86,;67.46,.97,;66.02,-3.41,;67.52,-3.04,;68.73,-2.1,;68.58,-4.15,;68.16,-5.63,;66.66,-6,;70.08,-3.78,;71.15,-4.89,;70.72,-6.37,;72.64,-4.52,;73.07,-3.04,;72,-1.93,;72.43,-.45,;71.36,.66,;73.92,-.08,;73.71,-5.63,;75.21,-5.26,;75.63,-3.78,;76.27,-6.37,;77.77,-6,;78.2,-4.52,;78.84,-7.11,;78.41,-8.59,;79.48,-9.7,;79.05,-11.18,;80.12,-12.29,;79.69,-13.77,;78.2,-14.14,;80.76,-14.88,;80.33,-6.74,;80.76,-5.26,;79.69,-4.15,;82.25,-4.89,;83.43,-5.88,;84.74,-5.07,;84.37,-3.58,;82.83,-3.47,;82.02,-2.16,;80.48,-2.21,;82.74,-.8,;84.28,-.75,;85.01,.61,;86.55,.66,;87.27,2.02,;86.46,3.33,;84.92,3.28,;84.19,1.92,;81.93,.51,;82.66,1.87,;83.87,2.81,;81.84,3.17,;48.72,-10.44,;48.3,-11.92,;50.22,-10.07,;50.8,-8.64,;52.33,-8.75,;52.7,-10.25,;51.4,-11.06,;51.29,-12.6,;49.9,-13.27,;52.56,-13.46,;52.45,-15,;51.07,-15.67,;49.79,-14.81,;48.41,-15.48,;47.13,-14.62,;48.3,-17.02,;53.73,-15.86,;55.11,-15.19,;53.62,-17.4,;54.89,-18.26,;56.28,-17.59,;56.39,-16.05,;57.77,-15.38,;59.05,-16.24,;57.88,-13.84,;54.78,-19.8,;53.4,-20.47,;56.06,-20.66,;55.95,-22.2,;54.56,-22.87,;54.45,-24.4,;53.07,-25.08,;52.96,-26.61,;54.23,-27.48,;54.12,-29.01,;55.62,-26.8,;55.73,-25.27,;57.22,-23.06,;58.61,-22.39,;57.11,-24.6,;58.39,-25.46,;59.77,-24.79,;61.05,-25.65,;62.43,-24.98,;60.94,-27.19,;58.28,-27,;56.89,-27.67,;59.55,-27.86,;59.44,-29.39,;60.72,-30.26,;60.61,-31.79,;61.88,-32.66,;63.27,-31.99,;61.77,-34.19,;58.06,-30.07,;56.78,-29.2,;57.95,-31.6,)| Show InChI InChI=1S/C112H156N28O40/c1-6-56(4)93(110(178)140-41-15-22-80(140)108(176)129-67(31-37-89(154)155)97(165)126-66(30-36-88(152)153)98(166)132-72(44-60-23-25-62(143)26-24-60)102(170)131-70(42-55(2)3)100(168)130-69(111(179)180)28-34-83(114)146)138-99(167)68(32-38-90(156)157)127-96(164)65(29-35-87(150)151)128-101(169)71(43-58-16-9-7-10-17-58)133-105(173)76(49-92(160)161)124-86(149)52-120-94(162)75(48-91(158)159)136-104(172)74(47-84(115)147)135-103(171)73(46-61-51-118-54-121-61)134-106(174)78(53-141)137-95(163)64(27-33-82(113)145)123-85(148)50-81(144)63(20-13-39-119-112(116)117)125-107(175)79-21-14-40-139(79)109(177)77(122-57(5)142)45-59-18-11-8-12-19-59/h7-12,16-19,23-26,51,54-56,63-80,93,141,143H,6,13-15,20-22,27-50,52-53H2,1-5H3,(H2,113,145)(H2,114,146)(H2,115,147)(H,118,121)(H,120,162)(H,122,142)(H,123,148)(H,124,149)(H,125,175)(H,126,165)(H,127,164)(H,128,169)(H,129,176)(H,130,168)(H,131,170)(H,132,166)(H,133,173)(H,134,174)(H,135,171)(H,136,172)(H,137,163)(H,138,167)(H,150,151)(H,152,153)(H,154,155)(H,156,157)(H,158,159)(H,160,161)(H,179,180)(H4,116,117,119)/t56-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,93-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059860
(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Assay using farnesyl-ras-CVLS as the protein acceptor substrate. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
FPTase activity was assayed in the biosynthetically forward direction at 30C. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004740

(CHEMBL2370449 | Hirudin analogue)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]\[#6]=[#6]\[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O Show InChI InChI=1S/C72H104N14O18/c1-6-44(4)62(70(102)86-38-20-27-55(86)66(98)82-53(71(103)104)39-43(2)3)83-67(99)56-28-19-36-84(56)68(100)49(32-33-60(91)92)80-63(95)50(40-46-21-9-7-10-22-46)81-64(96)51(42-61(93)94)78-59(90)31-15-16-34-75-58(89)30-14-13-29-57(88)48(25-17-35-76-72(73)74)79-65(97)54-26-18-37-85(54)69(101)52(77-45(5)87)41-47-23-11-8-12-24-47/h7-12,15-16,21-24,43-44,48-56,62H,6,13-14,17-20,25-42H2,1-5H3,(H,75,89)(H,77,87)(H,78,90)(H,79,97)(H,80,95)(H,81,96)(H,82,98)(H,83,99)(H,91,92)(H,93,94)(H,103,104)(H4,73,74,76)/b16-15+/t44-,48-,49-,50-,51-,52-,53-,54-,55-,56-,62-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059865
(4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6]=[#6]P([#8])(=O)[#6]P([#8])([#8])=O |w:14.13| Show InChI InChI=1S/C17H32O5P2/c1-15(2)8-5-9-16(3)10-6-11-17(4)12-7-13-23(18,19)14-24(20,21)22/h7-8,10,13,17H,5-6,9,11-12,14H2,1-4H3,(H,18,19)(H2,20,21,22)/b13-7?,16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
FPTase activity was assayed in the biosynthetically forward direction at 30C. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059865

(4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6]=[#6]P([#8])(=O)[#6]P([#8])([#8])=O |w:14.13| Show InChI InChI=1S/C17H32O5P2/c1-15(2)8-5-9-16(3)10-6-11-17(4)12-7-13-23(18,19)14-24(20,21)22/h7-8,10,13,17H,5-6,9,11-12,14H2,1-4H3,(H,18,19)(H2,20,21,22)/b13-7?,16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Assay using farnesyl-ras-CVLS as the protein acceptor substrate. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079961
((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-2-ylmet...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C40H47N5O4S/c1-4-28(2)37(43-38(46)21-34-22-41-27-45(34)23-29-16-17-30-10-5-6-12-32(30)20-29)25-44(26-39(47)42-36(40(48)49)18-19-50-3)24-33-14-9-13-31-11-7-8-15-35(31)33/h5-17,20,22,27-28,36-37H,4,18-19,21,23-26H2,1-3H3,(H,42,47)(H,43,46)(H,48,49)/t28?,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079974
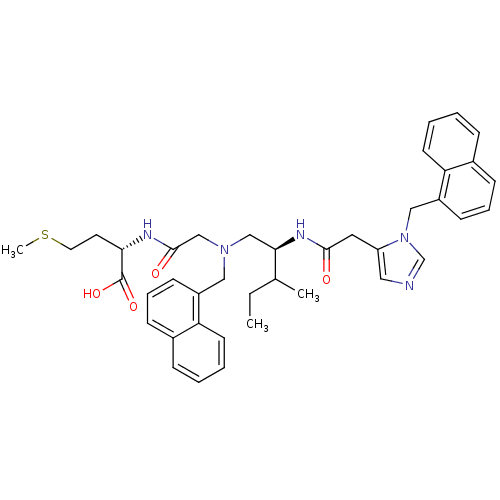
((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-1-ylmet...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1cccc2ccccc12 Show InChI InChI=1S/C40H47N5O4S/c1-4-28(2)37(43-38(46)21-33-22-41-27-45(33)24-32-16-10-14-30-12-6-8-18-35(30)32)25-44(26-39(47)42-36(40(48)49)19-20-50-3)23-31-15-9-13-29-11-5-7-17-34(29)31/h5-18,22,27-28,36-37H,4,19-21,23-26H2,1-3H3,(H,42,47)(H,43,46)(H,48,49)/t28?,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369371
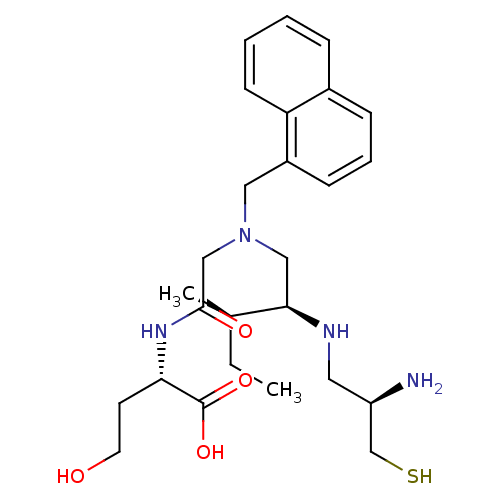
(CHEMBL1790750)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCO)C(O)=O)Cc1cccc2ccccc12)NC[C@@H](N)CS Show InChI InChI=1S/C26H40N4O4S/c1-3-18(2)24(28-13-21(27)17-35)15-30(16-25(32)29-23(11-12-31)26(33)34)14-20-9-6-8-19-7-4-5-10-22(19)20/h4-10,18,21,23-24,28,31,35H,3,11-17,27H2,1-2H3,(H,29,32)(H,33,34)/t18-,21-,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079956

((S)-2-[2-({(S)-2-[2-(3-Benzyl-3H-imidazol-4-yl)-ac...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccccc1 Show InChI InChI=1S/C36H45N5O4S/c1-4-26(2)33(39-34(42)19-30-20-37-25-41(30)21-27-11-6-5-7-12-27)23-40(24-35(43)38-32(36(44)45)17-18-46-3)22-29-15-10-14-28-13-8-9-16-31(28)29/h5-16,20,25-26,32-33H,4,17-19,21-24H2,1-3H3,(H,38,43)(H,39,42)(H,44,45)/t26?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369443
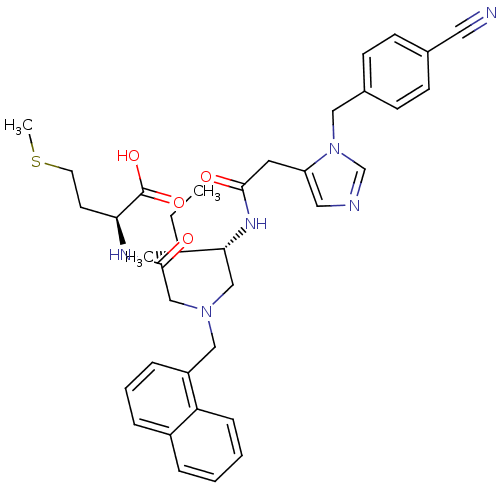
(CHEMBL252953)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C37H44N6O4S/c1-4-26(2)34(41-35(44)18-31-20-39-25-43(31)21-28-14-12-27(19-38)13-15-28)23-42(24-36(45)40-33(37(46)47)16-17-48-3)22-30-10-7-9-29-8-5-6-11-32(29)30/h5-15,20,25-26,33-34H,4,16-18,21-24H2,1-3H3,(H,40,45)(H,41,44)(H,46,47)/t26-,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079966
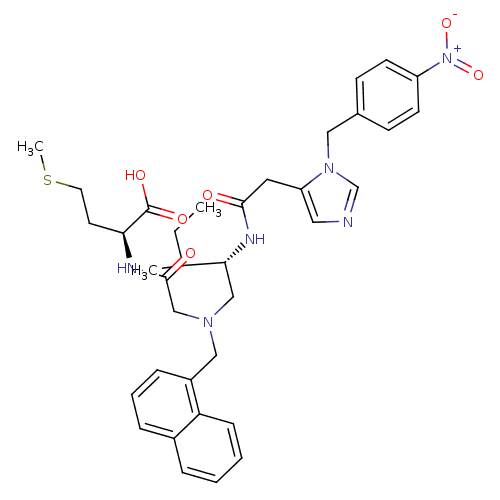
((S)-2-{2-[((S)-3-Methyl-2-{2-[3-(4-nitro-benzyl)-3...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C36H44N6O6S/c1-4-25(2)33(39-34(43)18-30-19-37-24-41(30)20-26-12-14-29(15-13-26)42(47)48)22-40(23-35(44)38-32(36(45)46)16-17-49-3)21-28-10-7-9-27-8-5-6-11-31(27)28/h5-15,19,24-25,32-33H,4,16-18,20-23H2,1-3H3,(H,38,44)(H,39,43)(H,45,46)/t25?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115916

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Oc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H24F3N5O3/c26-25(27,28)36-23-4-2-1-3-21(23)17-35-24(34)32-11-9-31(10-12-32)16-22-14-30-18-33(22)15-20-7-5-19(13-29)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50292415

(CHEMBL504845 | Zaragozic Acid B)Show SMILES C\C=C\CCCC\C=C\CCCCC(=O)O[C@@H]1[C@@H](O)[C@]2(CCC(C)C(O)C(C)C\C=C\c3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C39H54O13/c1-4-5-6-7-8-9-10-11-12-13-17-23-29(40)50-32-31(42)37(51-33(34(43)44)38(49,35(45)46)39(32,52-37)36(47)48)25-24-27(3)30(41)26(2)19-18-22-28-20-15-14-16-21-28/h4-5,10-11,14-16,18,20-22,26-27,30-33,41-42,49H,6-9,12-13,17,19,23-25H2,1-3H3,(H,43,44)(H,45,46)(H,47,48)/b5-4+,11-10+,22-18+/t26?,27?,30?,31-,32-,33-,37+,38-,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50083595
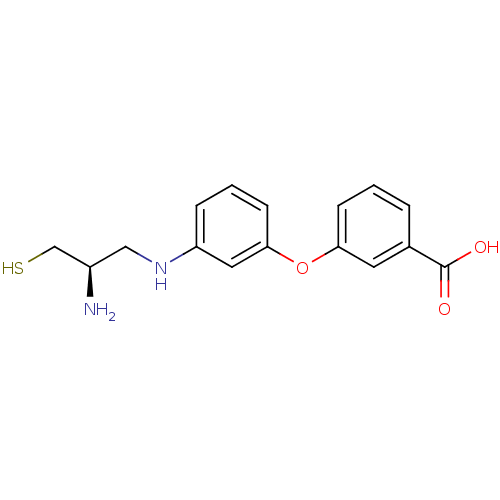
(3-[3-((R)-2-Amino-3-mercapto-propylamino)-phenoxy]...)Show InChI InChI=1S/C16H18N2O3S/c17-12(10-22)9-18-13-4-2-6-15(8-13)21-14-5-1-3-11(7-14)16(19)20/h1-8,12,18,22H,9-10,17H2,(H,19,20)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Farnesyltransferase in vitro. |
Bioorg Med Chem Lett 9: 3301-6 (2000)
BindingDB Entry DOI: 10.7270/Q2Q52NT7 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115911
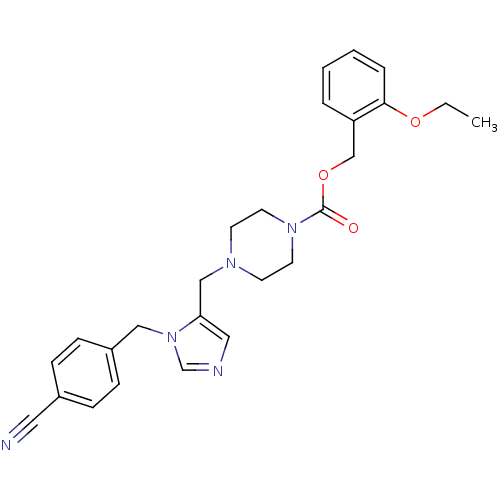
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES CCOc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C26H29N5O3/c1-2-33-25-6-4-3-5-23(25)19-34-26(32)30-13-11-29(12-14-30)18-24-16-28-20-31(24)17-22-9-7-21(15-27)8-10-22/h3-10,16,20H,2,11-14,17-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115919
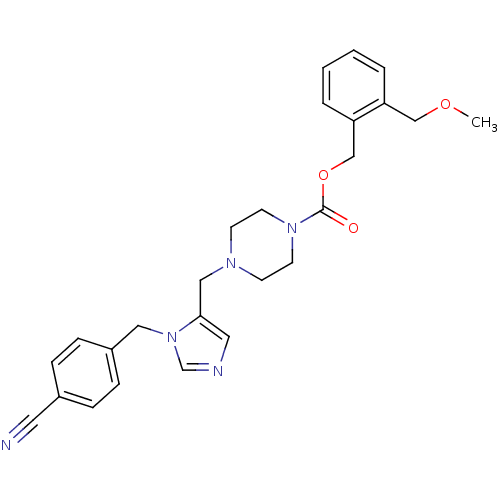
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES COCc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C26H29N5O3/c1-33-18-23-4-2-3-5-24(23)19-34-26(32)30-12-10-29(11-13-30)17-25-15-28-20-31(25)16-22-8-6-21(14-27)7-9-22/h2-9,15,20H,10-13,16-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115927

(4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...)Show SMILES Cc1ncc(CN2CCN(CC2)C(=O)OCc2ccccc2OC(F)(F)F)n1Cc1ccc(cc1)C#N Show InChI InChI=1S/C26H26F3N5O3/c1-19-31-15-23(34(19)16-21-8-6-20(14-30)7-9-21)17-32-10-12-33(13-11-32)25(35)36-18-22-4-2-3-5-24(22)37-26(27,28)29/h2-9,15H,10-13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyl protein transferase radiolabel [1-3H] incorporation |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115927

(4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...)Show SMILES Cc1ncc(CN2CCN(CC2)C(=O)OCc2ccccc2OC(F)(F)F)n1Cc1ccc(cc1)C#N Show InChI InChI=1S/C26H26F3N5O3/c1-19-31-15-23(34(19)16-21-8-6-20(14-30)7-9-21)17-32-10-12-33(13-11-32)25(35)36-18-22-4-2-3-5-24(22)37-26(27,28)29/h2-9,15H,10-13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled farnesyl transferase inhibitor |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115916

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Oc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H24F3N5O3/c26-25(27,28)36-23-4-2-1-3-21(23)17-35-24(34)32-11-9-31(10-12-32)16-22-14-30-18-33(22)15-20-7-5-19(13-29)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitor of Farnesyl protein transferase(FTPase) required to reduce radiolabel [1-3H] incorporation by 50% |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50292333
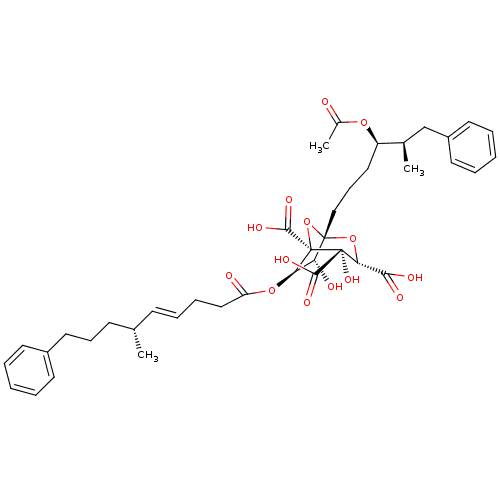
(CHEMBL505374 | zaragozic acid C)Show SMILES C[C@H](CCCc1ccccc1)\C=C\CCC(=O)O[C@@H]1[C@@H](O)[C@]2(CCC[C@@H](OC(C)=O)[C@H](C)Cc3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C40H50O14/c1-25(15-12-20-28-16-6-4-7-17-28)14-10-11-22-31(42)52-33-32(43)38(53-34(35(44)45)39(50,36(46)47)40(33,54-38)37(48)49)23-13-21-30(51-27(3)41)26(2)24-29-18-8-5-9-19-29/h4-10,14,16-19,25-26,30,32-34,43,50H,11-13,15,20-24H2,1-3H3,(H,44,45)(H,46,47)(H,48,49)/b14-10+/t25-,26+,30+,32+,33+,34+,38-,39+,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50083589
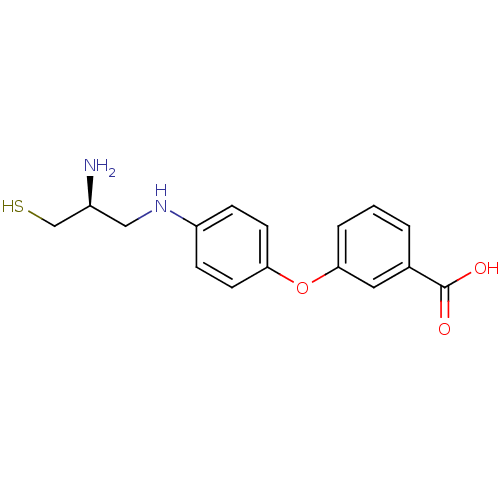
(3-[4-((S)-2-Amino-3-mercapto-propylamino)-phenoxy]...)Show InChI InChI=1S/C16H18N2O3S/c17-12(10-22)9-18-13-4-6-14(7-5-13)21-15-3-1-2-11(8-15)16(19)20/h1-8,12,18,22H,9-10,17H2,(H,19,20)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Farnesyltransferase in vitro. |
Bioorg Med Chem Lett 9: 3301-6 (2000)
BindingDB Entry DOI: 10.7270/Q2Q52NT7 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079978
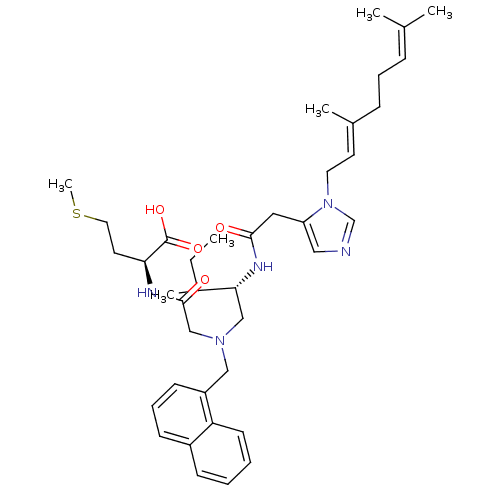
((S)-2-{2-[((S)-2-{2-[3-((E)-3,7-Dimethyl-octa-2,6-...)Show SMILES [#6]-[#6]-[#6](-[#6])-[#6@@H](-[#6]-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)-[#6]-c1cccc2ccccc12)-[#7]-[#6](=O)-[#6]-c1cncn1-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C39H55N5O4S/c1-7-30(5)36(42-37(45)22-33-23-40-27-44(33)20-18-29(4)13-10-12-28(2)3)25-43(26-38(46)41-35(39(47)48)19-21-49-6)24-32-16-11-15-31-14-8-9-17-34(31)32/h8-9,11-12,14-18,23,27,30,35-36H,7,10,13,19-22,24-26H2,1-6H3,(H,41,46)(H,42,45)(H,47,48)/b29-18+/t30?,35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079667
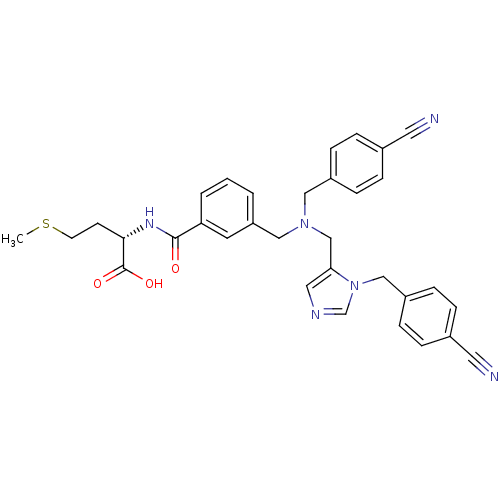
((S)-2-[3-({(4-Cyano-benzyl)-[3-(4-cyano-benzyl)-3H...)Show SMILES CSCC[C@H](NC(=O)c1cccc(CN(Cc2cncn2Cc2ccc(cc2)C#N)Cc2ccc(cc2)C#N)c1)C(O)=O Show InChI InChI=1S/C33H32N6O3S/c1-43-14-13-31(33(41)42)37-32(40)29-4-2-3-28(15-29)20-38(19-26-9-5-24(16-34)6-10-26)22-30-18-36-23-39(30)21-27-11-7-25(17-35)8-12-27/h2-12,15,18,23,31H,13-14,19-22H2,1H3,(H,37,40)(H,41,42)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Reduction in the FTase-catalyzed (enzyme purified from bovine brain) incorporation of [3H]-FPP into recombinant Ha-Ras. |
Bioorg Med Chem Lett 9: 1991-6 (1999)
BindingDB Entry DOI: 10.7270/Q2ZG6RFB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50083575
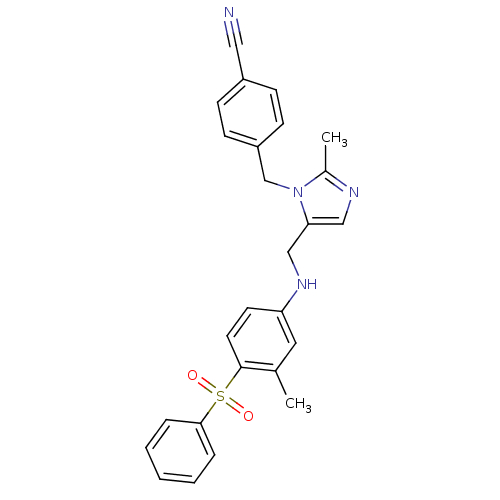
(4-{5-[(4-Benzenesulfonyl-3-methyl-phenylamino)-met...)Show SMILES Cc1ncc(CNc2ccc(c(C)c2)S(=O)(=O)c2ccccc2)n1Cc1ccc(cc1)C#N Show InChI InChI=1S/C26H24N4O2S/c1-19-14-23(12-13-26(19)33(31,32)25-6-4-3-5-7-25)29-17-24-16-28-20(2)30(24)18-22-10-8-21(15-27)9-11-22/h3-14,16,29H,17-18H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Farnesyltransferase in vitro. |
Bioorg Med Chem Lett 9: 3301-6 (2000)
BindingDB Entry DOI: 10.7270/Q2Q52NT7 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079971

((S)-2-{2-[((S)-2-{2-[3-(4-Fluoro-benzyl)-3H-imidaz...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(F)cc1 Show InChI InChI=1S/C36H44FN5O4S/c1-4-25(2)33(40-34(43)18-30-19-38-24-42(30)20-26-12-14-29(37)15-13-26)22-41(23-35(44)39-32(36(45)46)16-17-47-3)21-28-10-7-9-27-8-5-6-11-31(27)28/h5-15,19,24-25,32-33H,4,16-18,20-23H2,1-3H3,(H,39,44)(H,40,43)(H,45,46)/t25?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50051873
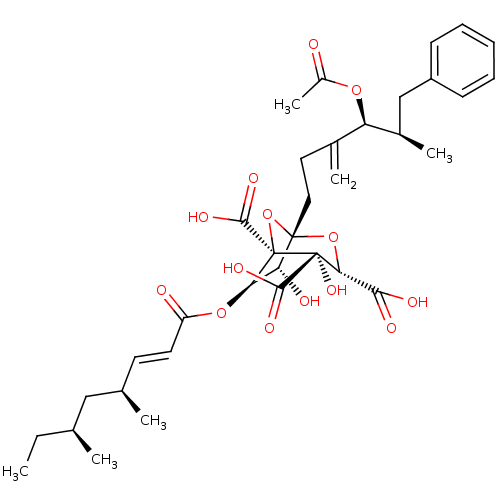
((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...)Show SMILES CC[C@H](C)C[C@H](C)\C=C\C(=O)O[C@@H]1[C@@H](O)[C@]2(CCC(=C)[C@@H](OC(C)=O)[C@H](C)Cc3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@H](O2)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C35H46O14/c1-7-19(2)17-20(3)13-14-25(37)47-28-27(38)33(48-29(30(39)40)34(45,31(41)42)35(28,49-33)32(43)44)16-15-21(4)26(46-23(6)36)22(5)18-24-11-9-8-10-12-24/h8-14,19-20,22,26-29,38,45H,4,7,15-18H2,1-3,5-6H3,(H,39,40)(H,41,42)(H,43,44)/b14-13+/t19-,20+,22+,26+,27+,28+,29+,33-,34+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat liver squalene synthase by liqiud scintillation counting |
J Nat Prod 56: 1923-1929 (1993)
Article DOI: 10.1021/np50101a009
BindingDB Entry DOI: 10.7270/Q2M908Q1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16179
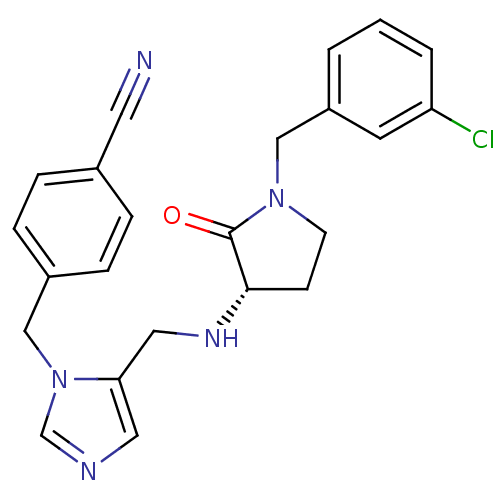
(4-{[5-({[(3S)-1-(3-chlorobenzyl)-2-oxopyrrolidin-3...)Show SMILES Clc1cccc(CN2CC[C@H](NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 |r| Show InChI InChI=1S/C23H22ClN5O/c24-20-3-1-2-19(10-20)15-28-9-8-22(23(28)30)27-13-21-12-26-16-29(21)14-18-6-4-17(11-25)5-7-18/h1-7,10,12,16,22,27H,8-9,13-15H2/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079968
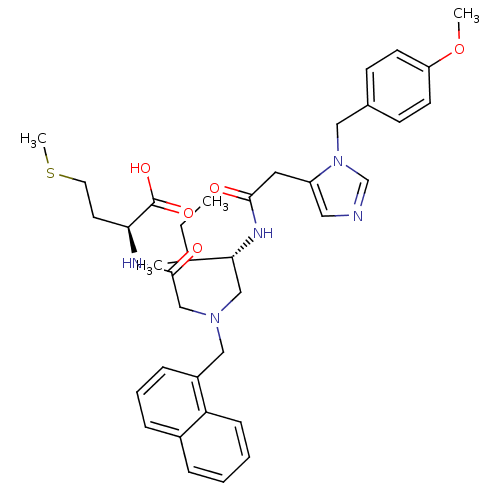
((S)-2-{2-[((S)-2-{2-[3-(4-Methoxy-benzyl)-3H-imida...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(OC)cc1 Show InChI InChI=1S/C37H47N5O5S/c1-5-26(2)34(40-35(43)19-30-20-38-25-42(30)21-27-13-15-31(47-3)16-14-27)23-41(24-36(44)39-33(37(45)46)17-18-48-4)22-29-11-8-10-28-9-6-7-12-32(28)29/h6-16,20,25-26,33-34H,5,17-19,21-24H2,1-4H3,(H,39,44)(H,40,43)(H,45,46)/t26?,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
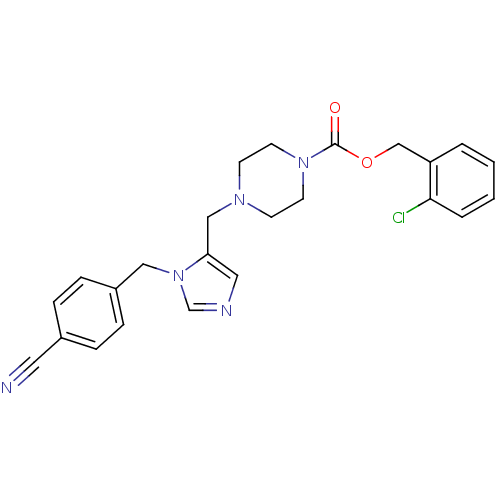
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115914
(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES Clc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C24H24ClN5O2/c25-23-4-2-1-3-21(23)17-32-24(31)29-11-9-28(10-12-29)16-22-14-27-18-30(22)15-20-7-5-19(13-26)6-8-20/h1-8,14,18H,9-12,15-17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of geranyl-geranylation of C-terminal CAAX sequence of Rap 1a in PSN-1 cells |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50083578

(4-{5-[(4-Benzenesulfonyl-phenylamino)-methyl]-imid...)Show SMILES O=S(=O)(c1ccccc1)c1ccc(NCc2cncn2Cc2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C24H20N4O2S/c25-14-19-6-8-20(9-7-19)17-28-18-26-15-22(28)16-27-21-10-12-24(13-11-21)31(29,30)23-4-2-1-3-5-23/h1-13,15,18,27H,16-17H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Farnesyltransferase in vitro. |
Bioorg Med Chem Lett 9: 3301-6 (2000)
BindingDB Entry DOI: 10.7270/Q2Q52NT7 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50083580

(4-{5-[(4-Benzoyl-phenylamino)-methyl]-imidazol-1-y...)Show SMILES O=C(c1ccccc1)c1ccc(NCc2cncn2Cc2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C25H20N4O/c26-14-19-6-8-20(9-7-19)17-29-18-27-15-24(29)16-28-23-12-10-22(11-13-23)25(30)21-4-2-1-3-5-21/h1-13,15,18,28H,16-17H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Farnesyltransferase in vitro. |
Bioorg Med Chem Lett 9: 3301-6 (2000)
BindingDB Entry DOI: 10.7270/Q2Q52NT7 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115925

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES COc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C25H27N5O3/c1-32-24-5-3-2-4-22(24)18-33-25(31)29-12-10-28(11-13-29)17-23-15-27-19-30(23)16-21-8-6-20(14-26)7-9-21/h2-9,15,19H,10-13,16-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [1-3H]-GGPP incorporation into biotinylated K4B-Ras peptide by geranylgeranyl transferase in the presence of 5 mM ATP |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115928

(4-[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmethyl]-pipe...)Show SMILES FC(F)(F)Cc1ccccc1COC(=O)N1CCN(Cc2cncn2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C26H26F3N5O2/c27-26(28,29)13-22-3-1-2-4-23(22)18-36-25(35)33-11-9-32(10-12-33)17-24-15-31-19-34(24)16-21-7-5-20(14-30)6-8-21/h1-8,15,19H,9-13,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
The concentration required to displace 50% of a highly potent radiolabeled FPTase inhibitor |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50083591
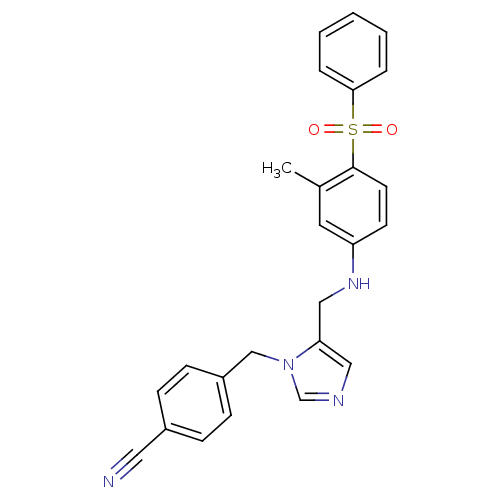
(4-{5-[(4-Benzenesulfonyl-3-methyl-phenylamino)-met...)Show SMILES Cc1cc(NCc2cncn2Cc2ccc(cc2)C#N)ccc1S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C25H22N4O2S/c1-19-13-22(11-12-25(19)32(30,31)24-5-3-2-4-6-24)28-16-23-15-27-18-29(23)17-21-9-7-20(14-26)8-10-21/h2-13,15,18,28H,16-17H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Farnesyltransferase in vitro. |
Bioorg Med Chem Lett 9: 3301-6 (2000)
BindingDB Entry DOI: 10.7270/Q2Q52NT7 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50051825

(CHEMBL27141 | [(S)-4-((R)-2-Amino-3-mercapto-propy...)Show SMILES N[C@@H](CS)CN1CCN(C[C@@H]1CCOC1CC1)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H31N3O2S/c24-18(16-29)14-25-11-12-26(15-19(25)10-13-28-20-8-9-20)23(27)22-7-3-5-17-4-1-2-6-21(17)22/h1-7,18-20,29H,8-16,24H2/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into Ha-Ras by Farnesyltransferase |
J Med Chem 39: 1345-8 (1996)
Article DOI: 10.1021/jm9508090
BindingDB Entry DOI: 10.7270/Q2B8576Z |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50051826
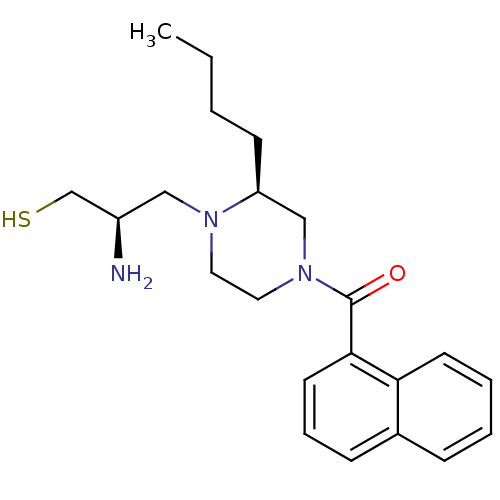
(CHEMBL27486 | [(S)-4-((R)-2-Amino-3-mercapto-propy...)Show SMILES CCCC[C@H]1CN(CCN1C[C@@H](N)CS)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H31N3OS/c1-2-3-9-19-15-25(13-12-24(19)14-18(23)16-27)22(26)21-11-6-8-17-7-4-5-10-20(17)21/h4-8,10-11,18-19,27H,2-3,9,12-16,23H2,1H3/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into Ha-Ras by Farnesyltransferase |
J Med Chem 39: 1345-8 (1996)
Article DOI: 10.1021/jm9508090
BindingDB Entry DOI: 10.7270/Q2B8576Z |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14025

((1R,2R,5R)-30-oxo-19,24-dioxa-2,6,10,12-tetraazahe...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCOc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O3/c26-11-17-2-1-16-9-24(17)33-19-3-4-23-20(10-19)22(6-8-32-23)30-7-5-21(25(30)31)28-13-18-12-27-15-29(18)14-16/h1-4,9-10,12,15,21-22,28H,5-8,13-14H2/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14018
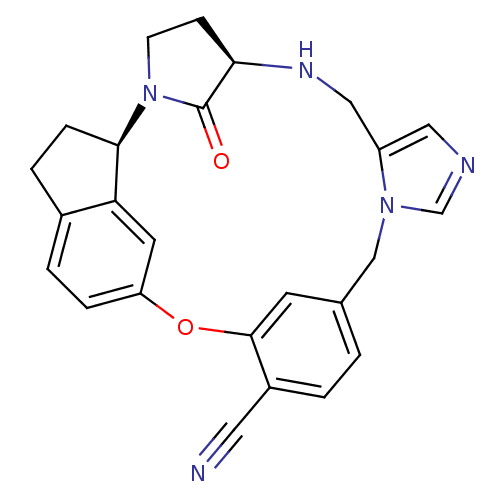
((17R, 20R)-19,20,21,22-Tetrahydro-19-oxo-17H-15,-1...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C25H23N5O2/c26-11-18-2-1-16-9-24(18)32-20-5-3-17-4-6-23(21(17)10-20)30-8-7-22(25(30)31)28-13-19-12-27-15-29(19)14-16/h1-3,5,9-10,12,15,22-23,28H,4,6-8,13-14H2/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14023

((1R,2R,5R)-30-oxo-19-oxa-2,6,10,12-tetraazahexacyc...)Show SMILES O=C1[C@H]2CCN1[C@@H]1CCCc3ccc(Oc4cc(Cn5cncc5CN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C26H25N5O2/c27-12-19-5-4-17-10-25(19)33-21-7-6-18-2-1-3-24(22(18)11-21)31-9-8-23(26(31)32)29-14-20-13-28-16-30(20)15-17/h4-7,10-11,13,16,23-24,29H,1-3,8-9,14-15H2/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14014
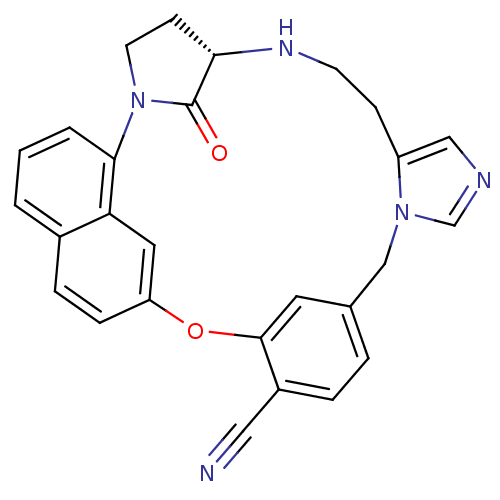
((5S)-31-oxo-20-oxa-2,6,11,13-tetraazahexacyclo[19....)Show SMILES O=C1[C@@H]2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5CCN2)ccc4C#N)cc13 |r| Show InChI InChI=1S/C27H23N5O2/c28-14-20-5-4-18-12-26(20)34-22-7-6-19-2-1-3-25(23(19)13-22)32-11-9-24(27(32)33)30-10-8-21-15-29-17-31(21)16-18/h1-7,12-13,15,17,24,30H,8-11,16H2/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50103360
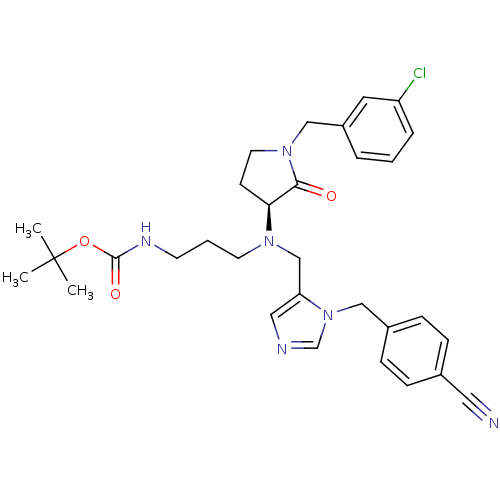
((3-{[1-(3-Chloro-benzyl)-2-oxo-pyrrolidin-3-yl]-[3...)Show SMILES CC(C)(C)OC(=O)NCCCN(Cc1cncn1Cc1ccc(cc1)C#N)[C@H]1CCN(Cc2cccc(Cl)c2)C1=O Show InChI InChI=1S/C31H37ClN6O3/c1-31(2,3)41-30(40)35-13-5-14-36(28-12-15-37(29(28)39)20-25-6-4-7-26(32)16-25)21-27-18-34-22-38(27)19-24-10-8-23(17-33)9-11-24/h4,6-11,16,18,22,28H,5,12-15,19-21H2,1-3H3,(H,35,40)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells |
J Med Chem 44: 2933-49 (2001)
BindingDB Entry DOI: 10.7270/Q2FF3RNW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50115913

(4-[3-(4-Cyano-benzyl)-2-methyl-3H-imidazol-4-ylmet...)Show SMILES CCOc1ccccc1COC(=O)N1CCN(Cc2cnc(C)n2Cc2ccc(cc2)C#N)CC1 Show InChI InChI=1S/C27H31N5O3/c1-3-34-26-7-5-4-6-24(26)20-35-27(33)31-14-12-30(13-15-31)19-25-17-29-21(2)32(25)18-23-10-8-22(16-28)9-11-23/h4-11,17H,3,12-15,18-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
The concentration required to displace 50% of a highly potent radiolabeled FPTase inhibitor |
Bioorg Med Chem Lett 12: 2027-30 (2002)
BindingDB Entry DOI: 10.7270/Q2TQ60VS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14017
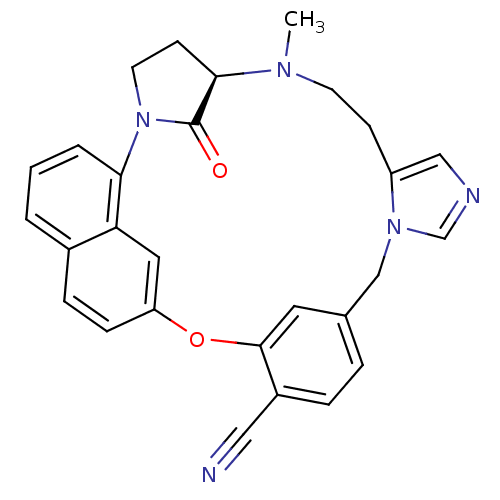
((5R)-6-methyl-31-oxo-20-oxa-2,6,11,13-tetraazahexa...)Show SMILES CN1CCc2cncn2Cc2ccc(C#N)c(Oc3ccc4cccc(N5CC[C@@H]1C5=O)c4c3)c2 |r| Show InChI InChI=1S/C28H25N5O2/c1-31-11-9-22-16-30-18-32(22)17-19-5-6-21(15-29)27(13-19)35-23-8-7-20-3-2-4-25(24(20)14-23)33-12-10-26(31)28(33)34/h2-8,13-14,16,18,26H,9-12,17H2,1H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Compounds were tested as inhibitors of FTase in vitro using purified recombinant human enzyme to catalyze the reaction between [3H]FPP and a recombin... |
J Med Chem 45: 2388-409 (2002)
Article DOI: 10.1021/jm010531d
BindingDB Entry DOI: 10.7270/Q2T72FPK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data